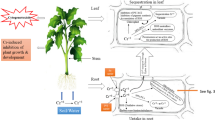
Abstract
In soil, chromium can be found in two main valence forms: hexavalent Cr (VI) and trivalent Cr (III). In terms of toxicity, the most toxic form to plants is Cr (VI). In the present study, we investigated the impact of Cr (VI) (0, 25, 50, 75 and 100 ppm) on growth, physiological parameters and the translocation kinetics of Cr (VI) in the faba bean plant (Vicia faba L.). The results showed that Cr (VI) negatively affects growth parameters (− 15% to − 72%), tolerance index (− 34.05% to − 64.7%), and reduce the total chlorophyll content (until 40%) compared to control plants without Cr (VI). However, the increase of Cr (VI) concentration in the soil, stimulated the synthesis of sugars (max 6,97 mg/g FM), proteins (max 62.89 µg/mg FM) and proline (max 98.57 µg/mg FM) and increased the electrolyte leakage (+ 2.5% to + 9%) compared to control plants. Cr (VI) concentrations in shoots and roots increased significantly for all Cr (VI) doses applied. The translocation factor results showed that the majority of the Cr (VI) absorbed by the plant is stored in the roots, with a very low bioaccumulation factor, which does not exceed 0.4. The findings show that Cr (VI) negatively affects the morpho-physiological parameters of Vicia faba, the bioaccumulation of organic solutes and the low bioaccumulation factor of Cr (VI) can be considered as a strategy of tolerance to Cr(V).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chromium is a chemical element that occurs naturally in the environment. It is mainly present in nature in two forms: trivalent (III) and hexavalent (VI). Cr (VI) is used in many industries activities such as cooling tower water treatment, metal plating, wood preservation and hide tanning. The hexavalent form is most mobile in soil and has mutagenic and carcinogenic properties (Zhitkovich 2011).
The harmful effects of Cr (VI) on crops is widely studied in many species: maize at 50 ppm (Mohammed et al. 2021a), faba bean at 50 and 100 ppm (Bouhadi et al. 2024), cyamopsis at 0.5, 1, 2, and 4 ppm (Sangwan et al. 2014), mint at 10 ppm (Bouhadi et al. 2021a), chickpea (Wani et al. 2010), radish at 150 ppm (Mohammed et al. 2021b), and common sorghum at 2 and 4 ppm (Kumar et al. 2019). The high concentration of Cr (VI) in soils has a negative impact on plants. For example, exposure to Cr (VI) at unsafe levels (more than 0.1 ppm in agricultural soils according to WHO) limits photosynthesis and chlorophyll pigment synthesis, and inactivates plant protein synthesis, thereby decreasing crop yields (Shukla et al. 2022). Plant tissues containing too much Cr are thought to undergo various morpho-physiological and biochemical alterations (Bouhadi et al. 2021b, 2024). Many studies have shown that Cr (VI) toxicity is mainly related to its ability to establish interactions with genetic processes, cellular macromolecules, and signal transduction and transmission channels (Kumari et al. 2016; Paithankar et al. 2021).
According to several researchers, Cr (VI) induces plant growth inhibition by causing ultrastructural changes in the cell membrane and chloroplasts, and consequently, the occurrence of leaf chlorosis and pigment reduction. Also, this metal disturbs the water and mineral balance as a result of the alteration of transpiration and absorption of nitrogen and other mineral elements (Ali et al. 2015a; Farooq et al. 2016; Reale et al. 2016). In addition, Cr (VI) induces the occurrence of several oxidative damages, such as the production of reactive oxygen species (ROS) that disrupt the redox balance of plants (Anjum et al. 2017).
Faced with anthropogenic pressure such as metal stress, plants synthesize stress-related proteins such as antioxidant enzymes, heat shock proteins, heavy metal chelating proteins and other types of no-enzymatic proteins to neutralize and detoxify oxidative damage (Sharma et al. 2019). In addition to proteins, plants produce organic solutes such as sugars and proline, which help them to tolerate stress and maintain cell turgidity.
According to Moroccan decree no. 1276-01 on the use of irrigation water, total chromium concentration must not exceed 1 mg/l. However, when available water resources are insufficient, the basin agency may allow the use for irrigation of water whose limit values for salinity, toxic ions and other effects do not meet the standards already mentioned in the decree.
In this context, the objective of this work is to study the effects of Cr (VI) on the morpho-physiological parameters of Vicia faba L. and the evaluation of its absorption and translocation according to its concentration applied in the soil. We chose Vicia faba L. due to its high sensitivity to metal stress and its wide use by researchers as a stress biomarker.
Materials and Methods
Seeds of Vicia faba L. (var. Defes) were disinfected with a 10% sodium hypochlorite solution for 15 min, rinsed thoroughly several times and germinated in Petri dishes on filter paper in an oven at 28°C. After germination (approx. 5 days), the young seedlings were transplanted into plastic pots (20 × 15 cm) (3 seedlings per pot). The pots, containing 2 kg of soil (the physico-chemicals analysis of soil used are attached in the supplemental data), were perforated at the base, watered with distilled water and placed in the greenhouse with a temperature ranging from 16–19 to 24–26°C and humidity ranging from 75% to 85% with a photoperiod (16 h/8 h). After 40 days of transplanting, the pots are divided into 5 groups and each group is treated differently: G1: control, irrigated only with distilled water 50 ml twice per week. G2: irrigated with a 25 ppm Cr (VI) solution. G3: irrigated with a 50 ppm Cr (VI) solution. G4: irrigated by a 75 ppm Cr (VI) solution. G5: irrigated by a solution of 100 ppm of Cr (VI). The selected chemical form of Cr used in this research is Cr (VI) as potassium dichromate (K2Cr2O7) (Sigma Aldrich, India).
The study was conducted over 4 weeks. Every 7 days, samples were taken from each group to determine the Cr (VI) content in the roots and shoots of Vicia faba.
Four weeks after treatment, Vicia faba plants were harvested to evaluate the effect of Cr (VI) on different morpho-physiological and biochemical parameters. The plants of each treatment were divided into two groups: the 1st is used to determine the length and dry weight of the root and shoot part (dry weight is determined after 48 h oven drying at 80°C). The 2nd is used to perform additional analyses as described below:
From the dry matter yield of control and Cr (VI) treated plants, the tolerance index was calculated using the formula given by Wilkins (1978):
The percentage of electrolyte leakage was determined according to the protocol of Ghoulam et al. (2002).
Pigment contents determined using the equations below from Lichtenthaler (1987):
The sugar content was determined by the phenol-sulfuric acid colorimetric method described by Dubois et al. (1956).
Quantification of the proline content was performed according to the ninhydrin-acetic acid method as described Bergman and Loxley (1970).
Proteins are assayed according to the method of Bradford (1976) (also known as the Coomassie protein assay).
The colorimetric method of Clesceri et al. (1998) was used to determine the concentration of Cr (VI) in shoots and roots of Vicia faba L. A 0.5 g sample of dry plant material was incinerated in an oven at 600°C for 5 h, and the ash is recovered in 3 ml hydrochloric acid (6 N), then diluted in distilled water and filtered. The reaction mixture consists of 5 ml filtrate, 0.8 ml distilled water, 0.2 ml 1,5-diphenylcarbazone (25 mg in 5 ml acetone) and 4 ml sulfuric acid (2 N). Chromium (VI), if present, reacts with 1,5-diphenylcarbazide to form a red-violet chromium-1,5-diphenylcarbazone complex. Extracts were measured spectrophotometrically at 540 nm using a double beam UV-6300PC spectrophotometer (VWR, Radnor PA).
The translocation factor and the bioaccumulation factor (BAF) of Cr (VI) was calculated According to (Bouhadi et al. 2021a),
All analysis in this research were done in three replicates and the analysis of variance is carried out with SPSS version 24.0. The significance of variations was determined by the ANOVA test and the groups by the Tukey post-hoc test (Khadraji et al. 2020).
Results
Plant growth, estimated by length (cm) and dry weight (g/plant) of aerial and root part, decreased significantly (p < 0.05) according to Cr (VI) concentrations (25, 50, 75, and 100 ppm) compared to the control (Table 1). After Cr (VI) exposure, root length decreased by 15%, 50%, 58% and 69% and aerial length by 16%, 37%, 39% and 68%, respectively for the 25, 50, 75 and 100 ppm Cr (VI) treatment compared to the control. The dry weight of both root and aerial parts decreased significantly (p < 0.05) according to Cr (VI) concentrations (Table 1). This decrease in shoot and root dry biomass ranged from 17% to 58% and 50%–72%, respectively, compared to the control. Roots of plants exposed to Cr (VI) also showed black necroses several centimeters long.
Figure 1 shows that the tolerance index (TI) decreased with increasing applied Cr (VI) concentration, i.e., the dry matter yield of Cr (VI)-treated plants decreased compared to control plants. The TI varied with increasing Cr (VI) concentration. The maximum TI (64.7%) was recorded at the low dose (25ppm), and the minimum TI (34.05%) was noted in plants exposed to 100 ppm Cr (VI).
The results in Fig. 2 show that the progressive increase in Cr (VI) concentration adjusted electrolyte leakage in Vicia faba L. leaves. The increase observed, compared with the control, varied from 2.5% to 9% depending on the Cr (VI) concentration applied.
Analyses of the pigment content in leaves of Vicia faba L. exposed to Cr (VI) showed a decrease with increasing Cr (VI) concentration in the soil (Fig. 3A). The maximum reduction (until 40%) is observed at a concentration of 100 ppm Cr (VI). Concerning the carotenoid content, there is no significant difference between the different Cr (VI) doses tested.
The results in Fig. 3B show that exposure to chromium VI induced a significant accumulation of TSS in the leaves of Vicia faba L. This accumulation increased progressively with the increase of the applied Cr (VI) dose. The maximum concentration (6.97 mg/g fresh matter) is recorded in plants exposed to 100 ppm Cr (VI), with a 10% increase compared to the control.
According to Fig. 4A, the increase in Cr (VI) dose in soil led to an increase in protein content in Vicia faba leaves compared to the control. At the concentration of 25ppm, the recorded protein content was 40.86 µg/mg MF. This value reached 62.89 µg/mg MF for plants exposed to 100 ppm Cr (VI).
The proline content in Vicia faba leaves increases with increasing Cr (VI) (Fig. 4B). The difference between the control and the different Cr (VI) treatments is significant. The maximum proline content is recorded in plants treated with 100 ppm Cr (VI) (with a value of 98.57 µg/mg MF), which is 3 times more proline than in the leaves of control plants.
Chromium levels in both aerial and root parts are presented in Fig. 5. Cr (VI) concentrations in both plant parts increased significantly for all applied Cr (VI) doses (25, 50, 75, and 100 ppm). Bioaccumulation of Cr (VI) is higher in the roots, independent of the Cr (VI) concentrations in the soil.
The translocation factor (TF) represents the fraction of metal transported to the aerial part relative to the amount of metal stored in the roots. Based on the results of the Fig. 6, the FT increases with the concentration of Cr (VI) in the soil. The maximum FT recorded is about 56.44% for the 100 ppm dose, i.e. the Cr (VI) accumulated in the shoots represents 56.4% of the total amount accumulated in the roots. The bioaccumulation factor (BAF) represents the amount of the metal accumulated in the whole plant relative to the concentration of that metal in the soil. According to the calculations, Vicia faba plants have a very low bioaccumulation factor for Cr (VI). This factor does not exceed 0.45 even at the high dose of 100 ppm Cr (VI).
Discussion
The exposure of Vicia faba plants to increasing concentrations of Cr (VI) adversely affects morpho-physiological and biochemical parameters. Damage intensity increases with increasing Cr (VI) concentration applied. The results revealed that the growth parameters of Vicia faba L. plants, namely, the length and the aerial and root biomass significantly decreased after exposure to increasing concentrations of Cr (VI). The toxic effects of Cr (VI) on plant growth and biomass are observed in many plants at the concentrations range of 2 to 500 ppm are observed in many plants (Amna et al. 2015; Bouhadi et al. 2019, 2022, 2023a, b; Shiyab 2019). Our results are consistent with those reported by Afshan et al. (2015), These authors showed that the biomass of roots, stem and leaves of Brassica napus L. exposed to Cr (100 and 500 µM) are significantly lower compared to control plants. The reduction in leaf, stem and root dry biomass is 39%, 44% and 43%, respectively for the Cr (100 µM) treatment, and 70%, 73% and 72% for the Cr (500 µM) treatment. The results of Kamran et al. (2017) showed that root and shoot length of Eruca sativa decreased by 42% and 39%, respectively after Cr exposure. Similarly, fresh and dry biomass decreased by 51% and 43%, respectively. This reduction could be due to ultrastructural alterations in plant organs such as diffusely broken cell wall, ruptured thylakoid membranes, interrupted golgibodies, immature nuclei and chromosomal abberations under the higher concentration of Cr (Gill et al. 2015). Cr (VI) exposure induces membrane lipid peroxidation, leading to loss of membrane integrity and increased electrolyte leakage (Ashraf et al. 2022). Our results also showed that exposure of Vicia faba L. to Cr (VI) has adverse effects on chlorophyll (a and b) and carotenoid content (variation No-significant). This decrease could be attributed to blockage of the electron transport chain or degradation of chloroplasts, protein complexes and photosynthetic machinery. Similar results, are observed in Brassica plants exposed to Cr and Cd (Afshan et al. 2015; Ali et al. 2015a). Exposure to Cr (VI) induces the generation of ROS, which act as alternative electron sinks, blocking electron transport, disrupting photosystems and destroying pigments (Shanker et al. 2005).
Other heavy metals showed adverse effects on pigment content in plants, such as cadmium, lead and nickel in tomato (Zeeshan et al. 2020), lead, cadmium, zinc and nickel in cotton and chromium in wheat and mung bean (Jabeen et al. 2016). Also, it has been reported that Cr (VI) induces a strong reduction in pigment content in other plants such as maize (Mohammed et al. 2021a), radish (Mohammed et al. 2021b), Brassica napus (Gill et al. 2015), mint (Bouhadi et al. 2021a), mung bean (Jabeen et al. 2016) and wheat (Ali et al. 2015b).
Under stress, plants usually develop several coping strategies, such as synthesis of soluble solutes (sugars, proline … etc.) and development of antioxidant defense mechanisms (Singh et al. 2015). According to our results, the presence of Cr (VI) in the soil stimulated the synthesis of soluble sugars, proteins (heavy metal chelating proteins, heat shock proteins, enzymatic and non-enzymatic antioxidant proteins) and proline. This synthesis increased with increasing Cr (VI) concentrations in the soil. These results are consistent with those of Jeddi et al. (2021) who recorded a significant increase in proline and soluble sugar content in henna (Lawsonia inermis L.) plants grown at industrial sites contaminated with heavy metals (Zn, Cu, Pb, and Cd). These plants showed an increase of about 147% and 60% in proline and soluble sugar in leaves, and about 104% and 39% in proline and soluble sugar in roots, respectively, compared to plants grown in control soils. Proline and total soluble sugars as osmoprotective agents that preserve cell turgor, participate in establishing osmotic balance by promoting plant tolerance to different types of stress (Rai 2016). According to Aslam et al. (2017), proline detoxifies free radical-induced oxidative damage and serves as a nitrogen and carbon resource. Proline also protects cells from oxidative damage caused by metals (Siddique et al. 2018). Soluble carbohydrate accumulation also improves membrane permeability, enhances antioxidant defenses, and maintains water balance (Zouari et al. 2016). Accumulation of Cr (VI) in plant cells and tissues adversely affects plant physiology and biochemistry by further significantly inhibiting plant growth (Afshan et al. 2015; Kamran et al. 2017). In this study, the bioaccumulation of Cr (VI) in both parts (shoots and roots) of the Vicia faba plant is strongly proportional to the dose of Cr (VI) supplied. When the dose of this element in the soil increases, its bioaccumulation in both parts also increases. The majority of Cr (VI) absorbed by the plant is stored in the roots, the rest being transported to the shoots. Many researchers have reported that the sequestration of Cr (VI) in the vacuoles of root cells as a protective mechanism could explain the increased accumulation of this element in the roots compared to the aerial parts (Kushwaha et al. 2020). Thus, plants have some inherent tolerance to Cr (VI) toxicity (Shanker et al. 2004). Furthermore, the translocation of Cr from roots to shoots is extremely limited and highly dependent on the chemical form of Cr in the tissue (Shahid et al. 2017). This is because Cr (VI) is converted to Cr (III) in plant tissues, which prefers to adhere to cell walls, thus preventing Cr transport (Kabata-Pendias and Szteke, 2015).
Conclusion
Chromium VI toxicity in Vicia faba plants is concentration-dependent. According to our results, exposure of Vicia faba L. to this metal has a negative influence on morpho-physiological and biochemical parameters; furthermore, increasing Cr (VI) concentration leads to more intense damages. Faced with this stressful situation, the plant synthesizes and accumulates more organic solutes such as soluble sugars and proline as a tolerance strategy. The results showed that the Vicia faba plant has a low bioaccumulation factor, studying this property can be very useful for improving the resistance and tolerance of plant species grown in areas contaminated by heavy metals.
References
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015a) Fulvic acid mediates chromium (cr) tolerance in wheat (Triticum aestivum L.) through lowering of cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015b) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L). Environ Sci Pollut Res 22:10669–10678
Amna, Ali N, Masood S, Mukhtar T, Kamran MA, Rafique M, Chaudhary HJ (2015) Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ Monit Assess 187:1–11
Anjum SA, Ashraf U, Khan I, Tanveer M, Shahid M, Shakoor A, Wang L (2017) Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27:262–273
Ashraf MA, Rasheed R, Hussain I, Iqbal M, Farooq MU, Saleem MH, Ali S (2022) Taurine modulates dynamics of oxidative defense, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environ Sci Pollut Res 29(30):45527–45548
Aslam M, Saeed MS, Sattar S, Sajad S, Sajjad M, Adnan M, Iqbal M, Sharif MT (2017) Specific role of proline against heavy metals toxicity in plants. Int J Pure Appl Biosci 5:27–34
Bergman I, Loxley R (1970) The determination of hydroxyproline in urine hydrolysates. Clin Chim Acta 27(2):347–349
Bouhadi M, Ainane A, M’hammed EL, Talbi M, Cherifi O, El yaacoubi A, Ainane T (2019) Role of the macroalgae Corallina officinalis in alleviating the toxicity of hexavalent chromium on Vicia faba L. J Anal Sci Appl Biotechnol 1(2):1–2
Bouhadi M, Atmani E, Talbi Z, Elkouali M, M., Ainane T (2021a) Evaluation of the toxicity of chromium VI contaminated irrigation water on the mint (mentha spicata) crop. Pharmacologyonline 3:421–427
Bouhadi M, Elkouali MH, Talbi M, Ainane T (2021b) Phytoremediation review: bioavailability of heavy metals and the role of bioaccumulative plants in the remediation of contaminated soils. J Anal Sci Appl Biotechnol 3(1):40–47
Bouhadi M, Elkouali MH, Talbi M, Amegrissi F, Fougrach H (2022) Phytoextraction of chromium VI by Raphanus sativus L. under exogenous application of citric acid. Bot Pacifica 11(2):89–93
Bouhadi M, Daoui O, Hajjouji E, Elkhattabi H, Chtita S, Kouali SE, Talbi M, Fougrach H (2023a) Physiological and molecular modeling investigations of the relationship between sulfate and chromium VI uptake in Vicia faba L. Biocatal Agric Biotechnol 47:102554
Bouhadi M, Daoui O, Hajjouji E, Elkhattabi H, Chtita S, Talbi S, M., Fougrach H (2023b) Study of the competition between pi and cr (VI) for the use of Pi-transporter at Vicia faba L. using molecular modeling. Plant Physiol Biochem 196:695–702
Bouhadi M, Abchir O, Yamari I, Youbi E, Azgaoui AEH, Chtita A, Fougrach S (2024) Genotoxic effects and mitosis aberrations of chromium (VI) on root cells of Vicia faba and its molecular docking analysis. Plant Physiol Biochem 207:108361
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater. P. H. Association, Washington, p 366
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Farooq M, Ali S, Hameed A, Bharwana S, Rizwan M, Ishaque W, Farid M, Mahmood K, Iqbal Z (2016) Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S Afr J Bot 104:61–68
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47(1):39–50
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Jeddi K, Siddique KH, Chaieb M, Hessini K (2021) Physiological and biochemical responses of Lawsonia inermis L. to heavy metal pollution in arid environments. South Afr J Bot 143:7–16
Kabata-Pendias A, Szteke B (2015) Trace elements in abiotic and biotic environments. CRC Press, Boca Raton
Kamran MA, Bibi S, Xu R, Hussain S, Mehmood K, Chaudhary HJ (2017) Phyto-extraction of chromium and influence of plant growth promoting bacteria to enhance plant growth. J Geochem Explor 182:269–274
Khadraji A, Bouhadi M, Ghoulam C (2020) Effect of soil available phosphorus levels on Chickpea (Cicer arietinum L.) - rhizobia symbiotic association. Legume Res 43(6):878–883
Kumar P, Tokas J, Singal HR (2019) Amelioration of chromium VI toxicity in sorghum (Sorghum bicolor L.) using glycine betaine. Sci Rep 9(1):16020
Kumari V, Yadav A, Haq I, Kumar S, Bharagava RN, Singh SK, Raj A (2016) Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J Environ Manag 183:204–211
Kushwaha BK, Ali HM, Siddiqui MH, Singh VP (2020) Nitric oxide-mediated regulation of sub-cellular chromium distribution, ascorbate–glutathione cycle and glutathione biosynthesis in tomato roots under chromium (VI) toxicity. J Biotech 318:68–77
Lichtenthaler HK (1987) [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Methods in Enzymology, vol 148. Elsevier, pp 350–382
Mohammed B et al (2021a) Physiological and physico-chemical study of the effect of chromium VI on the nutritional quality of maize (Zea mays L.). Procedia Comput Sci 191:463–468. https://doi.org/10.1016/J.PROCS.2021.07.058
Mohammed B et al (2021b) Effect of Chromium VI on edible plants and their health risks: case of Radish (Raphanus sativus L.)’, E3S Web of Conferences, 319, p. 01109. https://doi.org/10.1051/e3sconf/202131901109
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350
Rai R, Agrawal M, Agrawal SB (2016) Impact of heavy metals on physiological processes of plants: with special reference to photosynthetic system. Plant responses to xenobiotics. Springer, Singapore, pp 127–140
Reale L, Ferranti F, Mantilacci S, Corboli M, Aversa S, Landucci F, Baldisserotto C, Ferroni L, Pancaldi S, Venanzoni R (2016) Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 145:98–105
Sangwan P, Kumar V, Joshi UN (2014) Effect of chromium (VI) toxicity on enzymes of nitrogen metabolism in clusterbean (Cyamopsis tetragonoloba L.). Enzyme research, 2014
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar C, Pathmanabhan G (2004) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. Cv CO 4) roots. Plant Sci 166:1035–1043
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753
Sharma P, Jha AB, Dubey RS (2019) Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Pessarakli M (ed) Handbook of plant and crop stress. CRC press, Boca Raton, pp 93–136
Shiyab S (2019) Morphophysiological effects of chromium in sour orange (Citrus aurantium L.). HortScience 54:829–834
Shukla M, Baksi B, Mohanty SP, Mahanty B, Mansi A, Rene ER, Behera SK (2022) Remediation of chromium contaminated soil by soil washing using EDTA and N-acetyl-L-cysteine as the chelating agents. Prog Org Coat 165:106704
Siddique A, Kandpal G, Kumar P (2018) Proline accumulation and its defensive role under diverse stress condition in plants: an overview. J Pure Appl Microbiol 12(3):1655–1659
Singh M, Singh VP, Dubey G, Prasad SM (2015) Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol Environ Saf 117:164–173
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48(11):3262–3267
Wilkins DA (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol 80(3):623–633
Zeeshan M, Ahmad W, Hussain F, Ahamd W, Numan M, Shah M, Ahmad I (2020) Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J Clean Prod 255:120318
Zhitkovich A (2011) Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol 24(10):1617–1629
Zouari M, Ahmed CB, Zorrig W, Elloumi N, Rabhi M, Delmail D, Abdallah FB (2016) Exogenous proline mediates alleviation of cadmium stress by promoting photosynthetic activity, water status and antioxidative enzymes activities of young date palm (Phoenix dactylifera L.). Ecotoxicol Environ Saf 128:100–108
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouhadi, M., Lahmidi, A., Am, A. et al. Study of the Toxicity and Translocation of Chromium (VI) in Vicia faba Plant. Bull Environ Contam Toxicol 112, 40 (2024). https://doi.org/10.1007/s00128-024-03864-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-024-03864-3