Abstract
Serpentine soils containing high levels of nickel and other metals are particularly preferred by some plants that accumulate nickel in their bodies. In this study, the Ni, Co, and Cr accumulation capacities of A. murale grown in Guleman’s serpentine soils were measured. In this respect, 12 A. murale and their soils were collected from the mining site and surroundings. Afterwards, the collected samples were measured in order to evaluate the translocation and accumulation amounts of Ni, Cr, and Co. For that, soil and plant samples were analyzed using inductively coupled plasma mass spectroscopy (ICP-MS). The mean Ni concentrations in the soil, roots, and shoots of A. murale were measured as 2475, 7384, and 7694 mg/kg, respectively. The mean Cr concentrations in the soil, roots, and shoots of A. murale were measured as 742, 33, and 8.4 mg/kg while the mean Co concentrations of A. murale in the soil, roots, and shoots were 166, 10.2, and 23.5 mg/kg, respectively. Then, ECR and ECS values were calculated for Ni, Co, and Cr. The results indicated that A. murale grown in Guleman’s serpentine soils may be helpful for the rehabilitation studies of mining soils contaminated by Ni and can be utilized for phytoextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultramafic soils, which contain high levels of metals such as Ni, Cr, and Co, but low levels of plant nutrients like P, K, and Ca, are formed by the weathering of ultramafic rocks. These rocks contain ferromagnesian silicate minerals and less than 45% silica (Alexander 2009; Alfaro et al. 2015; Galey et al. 2017; Nascimento et al. 2022; Gundogar and Sasmaz 2022; Sasmaz et al. 2023). The ultramafic rocks are composed of periotite, dunites, and serpentinite. Serpentine soils have high concentrations of heavy metals such as Mn, Ni, Co, Cr, and Zn. While some levels of these metals are essential for plant growth at trace levels, high levels can cause toxicity by interfering with cellular functions (Reeves et al. 1999; Wu and Hendershot 2010; Ho et al. 2013). Various methods have been used to rehabilitate heavy metal-contaminated sites. Traditional methods such as soil washing, excavation, and solidification are costly and only provide isolation of the polluted sites (Gavrilescu 2022). Various methods have been used to rehabilitate heavy metal-contaminated sites. Traditional methods such as soil washing, excavation, and solidification are costly and only provide isolation of the polluted sites (Gavrilescu 2022). Therefore, nature-based studies that use plants tolerant to heavy metals in order to preserve human health and the environment have been increasing since the end of the 1990s (Sharma et al., 2021).
Phytoremediation is a nature-based technology that provides the remediation of land and long-term management of soils that have been damaged or contaminated with trace metals by using plants (Baker et al., 1989,1993; Freitas et al. 2004). In this method, plants that can accumulate and store metals in their bodies from the soil are used, thus removing the metals from the soil and remediating it. Plants with more than 1,000 mg/kg dry weight of Ni, Co, Cu, Cr, Pb, or 10,000 mg/kg dry weight of Zn or Mn in their tissue are known as hyperaccumulators, which store trace elements in their above-ground organs (Baker and Brooks 1989; Bani et al. 2007). These species can accumulate trace elements up to 100 times in plant dry matter compared to other species living on the same soil available concentrations. These plant species that have the potential to accumulate unusually high metal concentrations in their harvestable biomass are able to survive on serpentine soils (Brady et al. 2005). Therefore, by applying hyperaccumulator plants that contain heavy metals that can be harmful to other plants, heavy metals are removed from the soil, making it more suitable for plants that are less sensitive to metals.
A hyperaccumulator plant can thrive in soils with extremely high heavy metal concentrations. These plants can absorb heavy metals and store them in different regions of their bodies through their roots. The roots of hyperaccumulator plants are heavier than soil when compared to non-hyperaccumulator species. The amount in question is so large that it is toxic to many similar species that have not adapted to living in soils with high heavy metal content. Hyperaccumulator plants uptake metals at a very high rate in their bodies, deliver them to the stem at a higher rate, and have high levels of metal in their stems and leaves that they store in large quantities (Rascio et al., 2011). A variety of serpentine plants, particularly the leaves, can collect extremely high levels of Ni in their above-ground portions, and at least 400 Ni hyperaccumulators are known (Ghaderian, 2007). Plants growing on serpentine soils have slightly higher Ni contents in their leaves, usually around 10–100 mg/kg, than plants growing on conventional soils (0.2-5 mg/kg) (Reeves 1992; Ghaderian, 2007). A. murale is one of the best-known hyperaccumulators, capable of colonizing serpentine soils and accumulating above 2% (w/w) nickel (Reeves and Baker 2000).
Nickel is a physiologically significant and essential minor element for plant development, but excessive amounts can cause toxicity (Fargasova 2008). The resistance of different plant species to nickel varies, with some plants being introduced as Ni hyperaccumulators, while others are introduced as non-accumulators due to their high sensitivity to Ni (Freeman et al. 2004). Maximum nickel concentrations in plants have been estimated to be in the range of 0.1 to 5 mg/kg on a dry-weight basis (Kabata-Pendias 2011). Maximum permitted Ni levels in agricultural soils have been stated as ranging from 20 to 100 mg/kg, depending on the country (Jones 1997). Excess nickel, like many other heavy metals, is toxic to plants, and the deadly threshold level of Ni in the soil is unknown (Seregin and Kozhevnikova 2006). Nickel can produce toxicity in serpentine soils because of its high solubility in the soil solution.
In humans, the hexavalent chromium has the potential to cause cancer (WHO 1988). As a result, in the United States, the recommended daily allowance (RDA) for Cr in adults is 50–200 micrograms per day (Noel et al. 2003). Cr levels in plant species greater than 5.0 mg/kg are deemed excessive or dangerous on a dry-weight basis (Kabata-Pendias 2011). According to several countries, the maximum permitted values of Cr in agricultural soils are 50–100 mg/kg (Jones 1997). On a dry-weight basis, Co concentrations in uncontaminated areas range from 0.03 to 1.0 mg/kg for plants and 2.0 to 27.0 mg/kg for soil (Kabata-Pendias 2011). Co concentrations in agricultural soils should be between 20 and 50 mg/kg (Jones 1997). When present in trace amounts, chromium (Cr), nickel (Ni), and cobalt (Co) are considered required elements; however, when present in excessive proportions, the same metals pose a threat to soil, surface water, and groundwater (Kabata-Pendias 2011). Therefore, these metals that have excessive proportions should be removed from these environments by using various techniques. The aim of the present work is to investigate the Ni, Co, and Co uptake amount in the root and shoots of A. murale grown in Guleman serpentine soil.
Materials and methods
The Study area
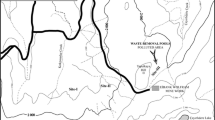
This research was carried out in Elazig, Turkey, in the Kef region of the Guleman mining area, where mining operations have been applied since 1936 (Fig. 1). The Guleman region, one of Turkey’s most important chrome ore-producing districts, is separated into various mining sectors based on the nature of the deposits, lithological properties, geographical location, and structural position. The ultramafic rocks (dunites, peridotites, and pyroxenites) that outcrop around Guleman are linked to this deposit (Engin et al. 1983). These rocks also contain alot of Cr, Ni, and Co. In the study area, chrome ore is extracted using open pit operations or galleries.
The soil and Plant Samples
The soil and plant samples were collected from 12 different locations throughout the mining zone (Fig. 1). The A. murale has deep root systems that can survive for 1–2 years in the wild and can grow in different climatic conditions. These plants were collected from random places on the Guleman mine site and the serpentine soils in its vicinity. Plant root, plant shoot and soil samples were collected separately from each point in the Bahro (9 AL samples) and Dereboyu (3 ALK samples) mining areas in the study area. Then, 12 plant roots, 12 plant shoots and 12 soil samples were analyzed separately for Ni, Cr and Co. The soil samples were taken from the root-feeding places of A. murale at depths between 0.10 and 0.40 m.
Sample Preparation
Firstly, the collected soil samples were pulverized using hand mortars after being cooked at 100 °C for 4 h. Then, these samples were digested in a combination of HNO3 and HCl for one hour at 95 °C (Merck, Darmstadt, Germany); HCl; H2O (1:1:1, v/v; 6mL per 1.0 g of soil). After all the soil samples were dissolved in the mixture, ICP-MS was used to measure the digestion of Ni, Cr, and Co by the soil.
On the other hand, the root and shoot samples of the plant were separated and thoroughly washed with tap water. Then, these samples were dried in an oven at 60 °C for 24 h. After that, the dried plant samples were ashed for 24 h at 300 °C and these ashed samples were digested for one hour in HNO3, followed by one hour at 95 °C in a mixture of HNO3 (Merck, Darmstadt, Germany); HCl; H2O (1:1:1, v/v; 6 mL per 1.0 g of the ashed sample). As with the soil samples, ICP-MS was used to measure the digestion of Ni, Cr, and Co by the plants.
ICP-MS (Inductively Coupled Plasmass Spectroscopy)
The ICP-MS working conditions were provided by Sasmaz and Yaman (2008). In this study, the ICP-MS (Perkin-Elmer ELAN 9000) technology was used to determine the uptake of Ni, Cr, and Co by soil and plant samples. As a result of that analysis, the calculation of ECR and ECS values of plants and soil samples has been done. Detection limits of ICP-MS for Ni, Co and Cr are 0.1, 0.01 and 0.1 mg/kg, respectively.
Enrichment Coefficients of Roots (ECR)
The ECR value is used to determine metal buildup in the plant’s upper section or element transfers from the soil to the plant root (Chen et al. 2005; (Sasmaz et al. 2015a, 2016). In this respect, the ECR values are calculated by dividing the metal concentration in the soil by the metal concentration in the root of the plant (ECR: Plant root concentration/Soil concentration).
Enrichment Coefficient for Shoots (ECS)
The ECS which represents the accumulation capability in phytoremediation studies is a calculation of dividing the metal value in the soil by the metal value in the plant shoot (ECS: Plant shoot concentration/Soil concentration) (Zhao et al. 2002; (Sasmaz et al. 2015a). Also, the ECS identifies how well a plant can absorb and retain energy (Wei et al. 2002; (Sasmaz et al. 2015a, b, 2016).
Translocation Factors (TLF)
The TLF is used to determine the metal ratio of transport from the plant roots to the shoot. In hyperaccumulator plants, the translocation factor is greater than one. That number indicates the capacity of metals to move (rather than accumulate) from the root to the shoot, which is critical in phytoremediation research (Sasmaz and Yaman 2006; Yıldırım and Sasmaz 2016).
Quality Assurance
Quality Assurance through the process of external auditing by recognized organizations; all facilities maintain ISO registrations and accreditations. These accreditations and registrations meet the requirements of the ISO standards and provide independent verification that the management systems have been implemented. All BVM facilities are registered to ISO 9001 and they are pending to the Bureau Veritas corporate registration. Additionally, a number of analytical hubs have received ISO/IEC 17,025 accreditation for specific laboratory procedures.
Results
Ni Concentrations in soil, Roots and Shoots of Alyssum murale
The mean concentrations of Ni in roots and shoots of A. murale samples and their soil samples and the calculated values ECR, ECS and TLF are shown in Fig. 2.
The levels of Ni in soil samples were found to range from 1018 mg/kg to 3025 mg/kg according to the results (Fig. 2). The levels of Ni in root samples varied between 227 and 13,808 mg/kg, while in shoot samples, this value was between 505 and 16,054 mg/kg. The concentrations of Ni in the soil samples were higher only in two samples (ALK-1 and ALK-2) in comparison to the root and shoot values. In contrast, the shoot concentrations of the other 10 samples were higher than the soil and root values of these samples.
In comparison to soil and shoot values, apart from the ALK-1 and ALK-2 samples, soil values were several times lower than shoot values, indicating that the concentration of Ni in the soil was transferred to the roots of the samples. However, these Niconcentrations were not retained in the roots, but were transferred to the shoots and accumulated there.
The highest accumulation of Ni in shoot samples was measured by the AL-6 sample, while the lowest amount was accumulated by the ALK-1 sample. Additionally, the highest transferred Ni concentrations from soil to the roots were measured in the AL-5 sample and the lowest in the ALK-1 sample, as was seen in the shoot values.
Previous research by Ghaderian et al. (2007) indicated that total Ni contents in serpentine soils typically vary from 500 to 8000 mg/kg. Additionally, normal plants and agricultural species experience substantial phytotoxicity at levels lower than 100 mg/kg, as reported by Reeves (1992) and Chaney et al. (2008). According to these previous research results, it was determined that the concentrations of collected A. murale samples were much higher in comparison to the normal plant values and agricultural species (mean 2474.46 mg/kg). However, as indicated in Gharderian’s study, the Ni concentrations of these samples are within the range of serpentine soils (500–8000 mg/kg).
Cr Concentrations in soil, Roots and Shoots of Alyssum murale
The concentrations of Cr in roots and shoots of A. murale samples, soil samples, and the calculated values ECR, ECS, and TLF are shown in Fig. 3. The concentrations of Cr in the soil samples were found to range from 548.7 to 1079.9 mg/kg, with a mean of 742.2 mg/kg. The concentrations of Cr in the roots were measured to be between 6.3 and 113.2 mg/kg, while the range for shoot values was 4.6–25.2 mg/kg (as shown in Fig. 3).
As illustrated in Fig. 3, the concentrations of Cr in the soil samples are significantly higher than the concentrations found in the roots. Additionally, none of the Cr concentrations in the shoot values of the samples were found to be higher than the soil concentrations of the respective samples. This also indicates that A. murale does not accumulate Cr in its organs. The results also suggest that the accumulation capacity of A. murale is not sufficient for storing Cr in its organs.
Co Concentrations in soil, Roots and Shoots of Alyssum murale
Co concentrations in roots and shoots of A. murale samples, soil samples, and the calculated values ECR, ECS, and TLF are shown in Fig. 4. The concentrations of Co in the soil samples were measured to be between 85 and 204 mg/kg (mean: 166 mg/kg). Additionally, Co concentrations in the samples were 3.25–29.1 mg/kg and 4.22–52.1 mg/kg in root and shoot values, respectively (as shown in Fig. 4).
As seen in Fig. 4, the Co concentrations in soil samples are notably higher when compared to the root and shoot values for all samples. Only in the AL-5 sample, the shoot and root concentrations were similar to each other. However, both concentrations in the plant were several times lower than the soil concentrations, as seen in Fig. 4. The Cr concentrations in soil were also higher than the shoot and root values, with the roots of ALK-1 and ALK-2 showing higher uptake than the shoots, while the Cr values in shoots were higher for the rest of the samples. In addition, the concentrations of Co in the soil and plants were relatively lower than Ni and Cr.
Freitas et al. (2004) conducted an experiment in a serpentinized terrain in the north–east of Portugal and found that plant tissues had significant levels of Ni, Cr, and Co: Ni 38,105, Cr 129.3, and Co 145.1 mg/kg. As a result, our findings are consistent with earlier findings from other studies. Table 1 represents the comparison of correlation coefficients between Ni, Cr, and Co and some other metals in the Guleman serpentine soils. The value of the metals that are closer to 1 indicate a stronger correlation, as shown in Table 1. According to the table, Ni shows positive correlations with Fe, Co and Mg. This correlation result suggests that both Ni and other correlated metals are enriched together within serpentine soils. On the other hand, there is a correlation between Co and the metals Cu, Pb, Ag, Mn, Fe, Cd, As, Sb, U, Th, P, Ba, Tl and Se. Cr shows a positive correlation with U, Ca, P, Ba and Fe in serpentine soils. In addition, Ni shows significant negative correlations with only Na and Ca within serpentine soils (Table 1).
ECR, ECS and TLF Values
According to previous studies in the literature, the metal concentration transferred to the root of the samples from the soil is represented by the ECR value. Similarly, the metal concentration transferred to the shoot of the samples from the soil is represented by the ECS value. The ECR, ECS, and TLF values show that A. murale is not an appropriate plant for Co accumulation as none of the values for each sample are close to 1. Additionally, because the ECR and ECS values are lower than 1, it can be stated that A. murale samples cannot be used for remediation of Cr from serpentine soils.
Discussion
Ni concentrations in the soil in various studies on Alyssum are as follows: 1070–3240 mg/kg in Albania, 1160–2660 mg/kg in Greece, 2333–3278 mg/kg in Bulgaria (Bani et al. 2010, 2013), 443 mg/kg in Serbia (Tumi et al. 2012), 637–2232 mg/kg in Bosnia and Herzegovina (Stamenkoviç et al., 2017) and 102–2348 mg/kg in the north-east of Portugal (Freitas et al. 2004). The highest Ni concentration was found in Brockman soil in Maryland and Oregon, with a concentration of 3590 mg/kg. This was followed by the medium Ni soil in Maryland and Oregon with a concentration of 930 mg/kg (Abou-Shanab et al. 2006), 3600 mg/kg in Albania (Broadhurst and Chaney 2016), 1,197–337 mg/kg in Lesbos Island-Greece (Kazakou et al. 2010), 1543–2570 mg/kg in Kosovo (Salihaj et al. 2016), 4600–5900 mg/kg in southwest Oregon (Centofanti et al. 2012), 650–760 mg/kg in the west (Harsin) of Iran and 600–830 mg/kg in the northwest (Khoy) of Iran (Ghaderian et al. 2007), 1070–3240 mg/kg in Albanian and Greek serpentine soils (Bani et al. 2009), with a mean of 1574 mg/kg in Tras-os-Montes (NE Portugal) (Lazaro et al. 2006), 2404–2833 mg/kg in Kızıldağ (Derebucak, Konya-Turkey) (Reeves et al. 2009), 2724–3028 mg/kg in NE Portugal, 2199 mg/kg in NW Spain (Becerra-Castro et al. 2009), 1463–2272 mg/kg in Serbia (Tomovic et al. 2013), 1530 mg/kg in Kızıldağ (Konya-Turkey) (Aksoy et al. 2015), 819–3579 mg/kg in Albania (Shallari et al. 1998), 471–1028 mg/kg in Urals-Russia (Teptina et al., 2015), 230–4538 mg/kg in Fındıkpınarı-Erdemli /Mersin (Özdemir and Demir 2010), 772–2387 mg/kg in Yahyalı (Kayseri-Turkey) (Çelik et al. 2018), 2522–3800 mg/kg in Kızıldağ national park (Isparta) (Saglam 2017), 4450 to 8960 mg/kg in Port Colborne, Ontario (Dehghani et al. 2021).
The levels of Cr in the samples were detected to be between 548.7 and 1079.9 mg/kg (mean: 742.2 mg/kg). Surface soil Cr content varies by country, with values as high as 43 mg/kg in Serbia (Tumi et al. 2012), 153–1748 mg/kg in Bosnia and Herzegovina (Stamenkovic et al. 2017), 200–6822 mg/kg in Portugal (Freitas et al. 2004), 1,118–263 mg/kg in Lesbos Island, Greece (Kazakou et al. 2010), 218–1206 mg/kg in Kosovo (Salihaj et al. 2016), 1600–3100 mg/kg in southwest Oregon (Centofanti et al. 2012), 677–3250 mg/kg in Albanian and Greek serpentine soils (Bani et al. 2009), mean 4384 mg/kg in Tras-os-Montes (NE Portugal) (Lazaro et al. 2006), 404–561 mg/kg in Kızıldağ (Derebucak, Konya-Turkey) (Reeves et al. 2009), 909–2997 mg/kg in NE Portugal, 1066 mg/kg in NW Spain (Becerra-Castro et al. 2009), 258 mg/kg in Kızıldag (Konya-Turkey) (Aksoy et al. 2015), 365–3865 mg/kg in Albania (Shallari et al. 1998), 349–868 mg/kg in Serbia (Tomovic et al. 2013), 122–474 mg/kg in Urals-Russia (Teptina et al., 2015), 214–347 mg/kg in Yahyalı (Kayseri-Turkey) (Çelik et al. 2018), 171–354 mg/kg in Kızıldağ national park (Isparta) (Saglam 2017).
The Co content of soil samples from different countries ranges from 90 to 228 mg/kg in Maryland and Oregon (Abou-Shanab et al. 2006), 56-174.9 mg/kg in Portugal (Freitas et al. 2004), 82 mg/kg in Serbia (Tumi et al. 2012), 76–296 mg/kg in Bosnia and Herzegovina (Stamenković et al., 2017), 13.34–76.2 mg/kg in Lesbos Island (Greece) (Kazakou et al. 2010), 49.5–95.8 mg/kg in Kosovo (Salihaj et al. 2016), 200–300 mg/kg in southwest Oregon (Centofanti et al. 2012), 93–267 mg/kg in Albanian and Greek serpentine soils (Bani et al. 2009), mean 180 mg/kg in Trasos-Montes (NE Portugal) (Lazaro et al. 2006), 123–177 mg/kg in Kızıldağ (Derebucak, Konya-Turkey) (Reeves et al. 2009),148–176 mg/kg in NE Portugal, 149 mg/kg in NW Spain (Becerra-Castro et al. 2009), 78 mg/kg in Kızıldağ (Konya-Turkey)(Aksoy et al. 2015), 130–476 mg/kg in Albania (Shallari et al., 1997), 104–176 mg/kg in Serbia (Tomovic et al. 2013), 37–87 mg/kg in Urals-Russia (Teptina et al., 2015), 14.7–97.4 mg/kg in Kizildag national park-Isparta (Saglam 2017).
The Ni content of plants in different countries varies, with values of 2.93 and 6.79 mg/kg in the roots and shoots, respectively, in Serbia (Tumi et al. 2012), 983–5885 and 1336.8-7409.5 mg/kg in the roots and shoots, respectively, in Bosnia and Herzegovina (Stamenkoviç et al., 2017), 343-13765 mg/kg in the shoots in Thessaloniki, Greece (Whiting et al. 2003), 816–1014 and 1413–1736 mg/kg in the roots and shoots, respectively, in the North Caucasus (Drozdova et al. 2017), 4729-13,676 mg/kg in the shoots, and 8.8–90 mg/kg in the roots and shoots, respectively, in Albanian and Greek serpentine soils (Bani et al. 2009), 90-2340 and 150–4500 mg/kg in the roots and shoots, respectively, in Tras-os-Montes (NE Portugal) (Lazaro et al. 2006), 758–1500 and 5866–9300 mg/kg in the roots and shoots, respectively, in Albania (Shallari et al., 1997 and Bani et al. 2013), 9.4–657 and 11.6–676 mg/kg in the roots and shoots, respectively, in Serbia (Tomovic et al. 2013), 10–69 mg/kg in the underground and 8–12 mg/kg in the aboveground parts of plants, 5.8–6.8 mg/kg in the underground and 4-5.4 mg/kg in the aboveground parts of plants in Yahyalı (Kayseri-Turkey) (Çelik et al. 2018), 3209, 1.1, and 8.91 mg/kg in the aboveground parts of plants in Kızıldağ (Isparta) (Saglam 2017), and 5570, 3938, 4.09, 1.8, 1.72, and 0.81 mg/kg in the roots and shoots, respectively, in Kızıldağ (Konya-Turkey) (Aksoy et al. 2015), 431 to 598 mg/kg in Port Colborne, Ontario (Dehghani et al. 2021; Freitas et al. 2004) conducted an experiment in a serpentinized terrain in the north–east of Portugal and found that plant tissues had significant levels of Ni, Cr, and Co: 38,105, 129, and 145 mg/kg, respectively.
Conclusions
The soil samples taken to evaluate metal concentrations have a typical ultramafic composition and were found to have high concentrations of Ni, Cr, and Co. According to the findings of this study, the average metal concentrations in soil samples were higher than in the plant samples. As a result of the measurements and calculations, it has been determined that A. murale cannot be chosen as an accumulator plant to uptake Co and Cr from the serpentine soil under these conditions. However, it has been shown that A. murale may be used for Ni accumulation in serpentine soils. The present study indicates that A. murale grown in Guleman’s serpentine soils may be helpful for the rehabilitation of mining soils contaminated by Ni and can be utilized for phytoextraction.
References
Abou-Shanab RAI, Angle JS, Chaney RL (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high ni soils. Soil Biol Biochem 38:2882–2889
Aksoy A, Leblebici Z, Prasad MNV (2015) Metal-accumulating plants from serpentine habitats of Kizildag, Konya province. Turkey. Aust J Bot 63:372–378
Alexander EB (2009) Soil and vegetation differences from peridotite to serpentinite. Northeast Nat 16:178–192
Alfaro MR, Montero A, Ugarte OM, Nascimento CWA, Accioly AMA, Biondi CM, Silva YJAB (2015) Background concentrations and reference values for heavy metals in soils of Cuba. Environ Monit Assess 187:4198
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metalic elements-A review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker AJM, Proctor J, Reeves RD (1993) The vegetation of Ultramafic (Serpentine) Soils. Intercept Ltd, Andover, UK, p 509
Bani A, Echevarria G, Sulçe S, Morel JL, Mullai A (2007) In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil 293:79–89
Bani A, Echevarria G, Mullaj A, Reeves RD, Morel JL, Sulçe S (2009) Ni hyperaccumulation by Brassicaceae in serpentine soils of Albania and NW Greece. Northeastern Naturalist 16:385–404
Bani A, Pavlova D, Echevarria G, Mullaj A, Reeves RD, Morel JL, Sulçe S (2010) Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans. Bot Serb 34(1):3–14
Bani A, Imeri A, Echevarria G, Pavlova D, Reeves RD, Morel JL, Sulçe S (2013) Nickel hyperaccumulation in the serpentine flora of Albania. Fresen Environ Bull 22:1792–1801
Becerra-Castro C, Monterroso C, Garcia-Leston M, Prieto-Fernandez A, Acea MJ, Kidd PS (2009) Rhizosphere microbial densities and trace metal tolerance of the nickel hyperaccumulator Alyssum serpyllifolium subsp. lusitanicum. Int J Phytoremed 11:525–541
Brady KU, Kruckeberg AR, Bradshaw HD (2005) Evolutionary ecology of plant adaptatin to serpentine soils. Annu Rev Ecol Evol Syst 36:243–266
Broadhurst CL, Chaney RL (2016) Growth and metal accumulation of an Alyssum murale nickel hyperaccumulator ecotype co-cropped with Alyssum montanum and perennial ryegrass in serpentine soil. Front Plant Sci 7:451
Çelik J, Aksoy A, Leblebici Z (2018) Metal Hyperaccumulating Brassicaceae from the ultramafic area of Yahyalı in Kayseri province, Turkey.Ecol Res33
Centofanti T, Siebecker MG, Chaney RL, Davis AP, Sparks DL (2012) Hyperaccumulation of nickel by Alyssum corsicum is related to solubility of Ni mineral species. Plant Soil 359:71–83
Chaney RL, Chen KY, Li YM, Angle JS, Baker AJM (2008) Effects of calcium on nickel tolerance and accumulation in Alyssum species and cabbage grown in nutrient solution. Plant Soil 311:131–140
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Dehghani S, Zupfer KR, Vasiluk L, Dutton M, Bellantino-Perco M, Hale BA (2021) Modeling phytoremediation of aged soil ni from anthropogenic deposition using Alyssum murale. Chemosphere 267:128861
Drozdova IV, Alekseeva-Popova NV, Kalimova IB, Belyaeva AI, Smirnova NA (2017) The accumulating ability and nickel tolerance of Brassicaceae species of the North Caucasus in connection with the problem of phytoremediation. J Geochem Expl 182:235–241
Engin T, Balcı M, Simer Y, Ozkan YZ (1983) General geological setting and the structural features of the Guleman peridotite unit and the chromite deposits. Bull Min Res Exp Ins Turkey 95:34–56
Fargasova A (2008) Phytotoxicity of Chromium and Nickel. Ecol Chem Eng 15:335–348
Freeman JL, Persans MW, Nieman K, Albrech C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16:2176–2191
Freitas H, Prasad MNV, Pratas J (2004) Analysis of serpentinophytes from north–east of Portugal for trace metal accumulation-relevance to the management of mine environment. Chemosphere 54:1625–1642
Galey ML, van der Ent A, Iqbal MCM, Rajakaruna N (2017) Ultramafic geoecology of South and Southeast Asia. Bot Stud 58:18
Gavrilescu M (2022) Enhancing phytoremediation of soils polluted with heavy metals. Curr Opin Biotech 74:21–31
Ghaderian SM, Mohtadi A, Rahiminejad MR, Baker AJM (2007) Nickel and other metal uptake and accumulation by species of Alyssum (Brassicaceae) from the ultramafics of Iran. Environ Pollut 145:293–298
Gundogar DY, Sasmaz A (2022) Geochemical Approach to Determine the Possible Precipitation Parameters of the Coniacian–Santonian Mazidagi Phosphates, Mardin, Turkey. Minerals, 12, 1544
Ho CP, Hseu ZY, Chen NC, Tsai CC (2013) Evaluating heavy metal concentration of plants on a serpentine site for phytoremediation applications. Environ Earth Scie 70(1):191–199
Jones JB (1997) The handbook of Trace Elements. CRC Press, Boca Raton, Florida
Kabata-Pendias A (2011) Trace Elements in soils and plants. CRC Press, Boca Raton
Kazakou E, Adamidis GC, Baker AJM, Reeves RD, Godino M, Dimitrakopoulos PG (2010) Species adaptation in serpentine soils in Lesbos Island (Greece): metal hyperaccumulation and tolerance. Plant& Soil 332:369–385
Lazaro JD, Kidd PS, Martyneza CM (2006) A phytogeochemical study of the Tra´s-os-montes region (NE Portugal): possible species for plant-based soil remediation technologies. Sci Tot Environ 354:265–277
Nascimento CWA, Lima LHV, Silva JAB, Biondi CM (2022) Ultramafic soils and nickel phytomining opportunities: a review. 46:1–17
Noel L, Leblanc JC, Guerin T (2003) Determination of several elements in duplicate meals from catering establishments using closed vessel microwave digestion with inductively coupled plasma mass spectrometry detection: estimation of daily dietary intake. Food Addit Contam 20(1):44–56
Özdemir Z, Demir E (2010) Fındıkpınarı-Erdemli Mersin bölgesinde nikel akümülatörü bir bitki Alyssum murale Waldst & Kit. Jeoloji Mühendisliği Dergisi 34(1):57–70
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Reeves RD (1992) The hyperaccumulation of nickel by serpentine plants. In:Baker, A.J.M., Proctor, J., Reeves, R.D. (Eds.), The Vegetation of Ultramafic (Serpentine) Soils. Intercept Ltd, Andover, UK 253–277
Reeves RD, Baker AJM (2000) Metal accumulating plants. In: Raskin, I., Ensley, B.D. (Eds.), Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment. JohnWiley&Sons Inc., NY, USA 193–339
Reeves RD, Baker AJM, Borhidi A, Berazan R (1999) Nickel hyperaccumulation in the serpentine flora of Cuba. Ann Bot 83:29–38
Reeves RD, Adiguzel N, Baker AJM (2009) Nickel Hyperaccumulation in Bornmuellera kiyakii Aytac & Aksoy and Associated plants of the Brassicaceae from Kızıldağ (Derebucak, Konya-Turkey). Turkish J Bot 33:33–40
Saglam C (2017) Heavy metal concentrations in serpentine soils and plants from Kizildag national park (Isparta) in Turkey. Fresenius Environ Bull 26(6):3995–4003
Salihaj M, Bani A, Echevarria G (2016) Heavy metals uptake by hyperaccumulating flora in some serpentine soils of Kosovo. Global NEST J 18:214–222
Sasmaz A, Yaman M (2006) Distribution of chromium, nickel, and cobalt in different parts of plant species and soil in mining area of Keban, Turkey. Commun Soil Scie Plant Anal 37:1845–1857
Sasmaz A, Yaman M (2008) Determination of uranium and thorium in soil and plant parts around abandoned Pb-Zn-Cu mining area. Commun Soil Sci Plant Anal 39:2568–2583
Sasmaz M, Akgul B, Sasmaz A (2015a) Distribution and accumulation of selenium in wild plants growing naturally in the Gumuskoy (Kutahya) mining area Turkey. Bull Environ Contam Toxicol 94(5):598–603
Sasmaz M, Arslan Topal EI, Obek E, Sasmaz A (2015b) The potential of Lemna gibba and Lemna minor to remove Cu, Pb, Zn, and as in gallery water in a mining area in Keban, Turkey. J Environ Manag 163:246–253
Sasmaz M, Akgül B, Yıldırım D, Sasmaz A (2016) Mercury uptake and phytotoxicity in terrestrial plants grown naturally in the Gumuskoy (Kutahya) mining area, Turkey. Int J Phyt 18(1):69–76
Sasmaz A, Kilic AD, Akgul B, Sasmaz B (2023) A spectral approach on mineralogy and geochemistry of garnet skarns in Arc-Type granitoids. Spectrochim Acta Part A Mol Biomol Spectrosc 286:122037
Seregin IV, Kozhevnikova AD (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277
Shallari S, Schwartz C, Hasko A, Morel JL (1998) Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci Total Environ 19(209):133–142
Sharma P, Kumar S (2021) Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: recent advances. Bioresour Technol 339:125589
Stamenkovic UM, Andrejic G, Mihailovic N, Sinzar-Sekulic J (2017) Hyperaccumulation of Ni by alyssum murale waldst. & Kit. From ultramafics in Bosnia and Herzegovina. App Ecol Environ Res 15(3):359–372
Teptina AY, Paukov AG (2015) Nickel accumulation by species of Alyssum and Noccaea (Brassicaceae) from ultramafic soils in the urals. Russia Aust J Bot 63:78–84
Tomovic GM, Mihailovic ML, Tumi AF, Gajic BA, Misljenovic TD, Niketic MS (2013) Trace metals in soils and several Brassicaceae plant species from serpentine sites of Serbia. Arch Environ Protec 39:29–49
Tumi AF, Mihailovic N, Gajic BA, Niketic M, Tomovic G (2012) Comparative study of hyperaccumulation of nickel by Alyssum murale s.l. populations from the ultramafics of Serbia. Pol J Environ Stud 21(6):1855–1866
Wei CY, Chen TB, Huang ZC (2002) Cretan bake (Pteris cretica): an arsenic-accumulating plant. Acta Ecol Sin 22:777–782
Whiting SN, Neumann M, Baker AJM (2003) Nickel and zinc hyperaccumulation by Alyssum murale and Thlaspi caerulescens (Brassicaceae) do not enhance survival and whole-plant growth under drought stress. Plant Cell Environ 26:351–360
WHO (1988) World Health Organization. Geneva, Switzerland. Environmental Health Criteria 61: Chromium
Wu Y, Hendershot WH (2010) The effect of calcium and pH on nickel accumulation in and rhizotoxicity to pea (Pisum sativum) root–empirical relationships and modelling. Environ Pollut 158:1850–1856
Yıldırım D, Sasmaz A (2016) Phytoremediation of as, Ag, and pb in contaminated soils using terrestrial plants grown on Gumuskoy mining area (Kutahya Turkey). J Geochem Expl 182:228–234
Zhao FJ, Dunham SJ, McGrath SP (2002) Arsenic hyperaccumulation by different fern species. New Phytol 156:27–31
Acknowledgements
This work was financially supported by Firat University’s FUBAP Unit (MF.20.16).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konakci, N., Kislioglu, M.S. & Sasmaz, A. Ni, Cr and Co Phytoremediations by Alyssum murale Grown in the Serpentine Soils Around Guleman Cr Deposits, Elazig Turkey. Bull Environ Contam Toxicol 110, 97 (2023). https://doi.org/10.1007/s00128-023-03736-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03736-2