Abstract
A pot trial was conducted during the boro (dry) season to evaluate the impact of six traditional organic amendments (OAs) on the growth of SL-8 rice variety in both agricultural and cadmium (Cd) stressed soil at 2% and 4% application rates. Traditional OAs used in the study were cow dung, mustard oil cake (MOC), rice husk, saw dust, tea leaf and vermi compost (VC). Except for cow dung all other OAs were found to remove 99% of Cd from the aqueous solution, while cow dung removed 95%. Rice grain grown in OA-added soil in all application rates contained less Cd than the control. A 2% application rate was found to be more effective in reducing both Cd bioavailability and Cd in grain. OA application in soil significantly influenced soil pH in all cases. Though both bioavailable Cd in soil and grain Cd were reduced by the OA addition, the Cd uptake tendency of SL-8 rice variety markedly increased because of Cd spiking in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a toxic heavy metal, commonly bioavailable as Cd2+ in soil and is non-essential for crops. Cd is added to soil through both natural and anthropogenic processes (Reboredo et al. 2019; Gil et al. 2022). Uptake of Cd from soil solution is the point of entry of this element into the food chain (Moreno-Jimenez et al. 2016; Wei et al. 2019). Suzuki et al. (1988) claimed that Cd-rich food is usually produced in Cd-rich soil. Food from plant sources such as grain and vegetable are the major sources of Cd for humans (WHO 2015; Song et al. 2017). Bangladesh is a country where significant Cd addition to crop land is practiced (Bhattacharyya et al. 2008; Islam et al. 2018). Once incorporated into the soil it is difficult to withdraw the metal and its further degradation occurs (Jiang et al. 2012). A Cd concentration between 0.83 and 4.08 mg/kg (mean 2.17 mg/kg) has been detected in agricultural surface soils of Bangladesh (Mamun et al. 2021). Rice grain accumulates Cd and then transfers it to consumers (Li et al. 2017). This is a major human food safety issue worldwide and especially in countries like Bangladesh where per capita rice consumption is the highest, i.e. 181.3 kg/year (FAO 2020).
Of the various dietary Cd sources rice consumption is the major one for the people of Bangladesh. Long-term rice consumption that contains more than 0.2 mg/kg Cd may irreversibly cause kidney damage, cartilage disease, liver dysfunction, etc. (Zhu et al. 2020). Cd deposition in rice grain is shaped by many factors like rice varieties (Kibria et al. 2022), Cd bioavailability (Li et al. 2017), soil pH (Hou et al. 2021), organic matter (OM) in soil (Filipovic et al. 2018; Zhu et al. 2020), redox potential, microbial activity (Zhu et al. 2020), etc. Hence, new strategies need to be devised urgently to minimize bioavailable Cd in soil so that it does not appear in rice grain.
The application of organic amendments (OA) to reduce solubility and bioavailability of toxic metals has increased worldwide (Song et al. 2021). OA provides plants with essential nutrients, while improving soil’s physical, chemical and biological properties (Saengwilai et al. 2020). OA added to soil immobilize metallic ions primarily by adsorption, ion exchange, surface complexation and precipitation (Hamid et al. 2019). Colloidal properties of OA especially ion exchange capacity and effective surface area enhance adsorption of metals on their surfaces and subsequently reduce metal bioavailability (Hamid et al. 2018). Of all soil properties soil pH noticeably influences Cd bioavailability (Sauve et al. 2000). Organic wastes like farmyard manure, compost, VC, biochar, crop straw and sewage sludge are commonly applied as OA in soil to provide plants with nutrient and immobilize Cd (Liu et al. 2020). Several authors confirm the incorporation of crop straw in soil to affect metal bioavailability to plants (Wang et al. 2015; Yin et al. 2016). Mohamed et al. (2010) stated that the use of rice straw in soil elevates soil pH and ultimately minimizes Cd bioavailability by binding the water soluble bivalent Cd2+ ion. Bioavailable Cd reduction was also reported by using corn and rape straw in an eight year-long field experiment by Rui et al. (2020). Other researchers used cattle manure (Wang et al. 2012), farmyard manure (Grüter et al. 2017), compost (Pardo et al. 2014), animal waste (Sato et al. 2010), etc., as OA to reduce bioavailable Cd in agricultural and polluted soil. VC was also found as an effective agent to curtail bioavailable Cd by both adsorption and forming organometallic complexes (Zhang et al. 2019). The effectiveness of OA to diminish Cd bioavailability depends on many factors such as nature of the OM (Li et al. 2018), stages of decomposition (Putwattana et al. 2015), application rate, application duration (Rui et al. 2020), soil type (Shi et al. 2011), soil pH (Zhu et al. 2020; Jiale et al. 2021), etc. Hamid et al. (2019) reported the availability of amendments as one of the key factors that can minimize Cd bioavailability in soil.
No effective measures have been operated in the soils of Bangladesh so far to minimize Cd availability for plants’ uptake. There is insufficient information on the effectiveness of traditional OA to reduce Cd availability of rice grain grown in the soils of Bangladesh. Moreover, information about the best rate of OA application for agriculture in Cd contaminated soil is not available (Mamun et al. 2021). Meharg et al. (2013) studied rice grain Cd of 12 countries and found that the maximum Cd (99 µg/kg) existed in grains from Bangladesh. The present study is designed to observe the influence of widely used traditional OA on Cd bioavailability in both agricultural and Cd-stressed soil, and its effect on grain Cd concentration in rice. The effect of OA application rate (2% and 4%) was also measured in this study. Most soils in Bangladesh have less than 1.5% and some soils have even less than 1% OM (FRG 2018). The recommended doses (not more than 0.25%) of organic fertilizer for agricultural land is very low (FRG 2018). Consequently, the outcome of the study will provide information on the effectiveness of traditional OA and the best application rate required to reduce Cd in rice grain.
Materials and Methods
Surface soil (0–20 cm depth) was collected from multiple sites to prepare a composite sample from a rice-growing field (22.802° N, 89.533° E). The land has served to cultivate rice for the last 20 years. After air drying the soil was crushed using a wooden hammer so that the sample could pass through a 4 mm sieve for use in pots. For laboratory determination soil was ready to pass through a 2 mm sieve. Properties of the soil are summarized in Table 1. The soil is non-saline and neutral in pH. It was clay textured and the clay content amounted to 58.74%. Lower CEC (45 cmol/kg) means that the clay fraction is composed mostly of less active clay. Cd content of the soil was a bit higher (6.16 mg Cd/kg soil) probably due to the application of agrochemicals for 20 consecutive years during rice cultivation. Cd distribution data reveal that most Cd is present in an OM bound fraction followed by MnO bound > FeO bound > Exchangeable > Carbonate bound. A small amount of Cd (2.44% of total) was found in the bioavailable pool.
Six OA, namely cow dung, MOC, rice husk, saw dust, tea leaf (residue left after tea preparation) and VC were used in the experiment. Collected OAs were air dried in a shed, ground and passed through a 2 mm sieve. Some basic data of the OAs are given in Table 2. Total Cd content of cow dung is high (2.35 mg/kg) whereas the other OAs contain low Cd (0.05 mg/kg). Bioavailable Cd content of the OAs is very low (0.001 mg/kg) and this indicates that most of the Cd exists in organic combination and not subject to exchange. CEC of the materials varied considerably (68–310 cmol/kg).
Six OA used in this study to reduce Cd bioavailability in soil was first tested in a laboratory to assess their Cd2+ removal efficiency from aqueous solution. Standard Cd solution (1000 mg/L) was prepared by dissolving CdCl2.H2O (Sigma-Aldrich, Germany). As soil solution contains less Cd, for example 5 µg/L (Kubier et al. 2019) by diluting approximately 2 mg Cd/L solution was prepared; 1.569 mg Cd/L was later recovered in ICP-OES (Spectro Genesis, Germany). The initial pH was adjusted to 7 for the feed solution. 50 ml solution was put into a centrifuge tube and 1 g (@ 2% w/v) OA added to it. After 2 hours’ shaking at 200 rpm the mixtures were filtered with ash-less filter paper (Whatman no. 42). Cd2+ concentration of the aliquots was determined by ICP-OES.
Each amendment was tested in five replications. Using the following equation the percentage Cd removed from the aqueous solution was calculated (Cheraghi et al. 2015):
Where, Co = Initial Cd2+ concentration (mg/L) and Ce = Cd2+ concentration after adsorption (mg/L).
The study was conducted under open conditions in a net house at Khulna University campus (22.802° N, 89.533° E). Earthen pots (40 cm height and 30 cm diameter) were prepared for taking 5 kg soil. 100 g of each OA were added with 5 kg soil to obtain a 2% rate (40 t OA/ha soil) of amendment, while 200 g was added to get a 4% rate (80 t OA/ha soil) of amendment. Added OA were uniformly mixed with soil. Half of the prepared pots were spiked with Cd (10 mg Cd/kg soil). Standard Cd solution (1000 mg/L) was prepared by dissolving CdCl2.H2O (Sigma-Aldrich, Germany). 25 ml standard Cd solution was diluted up to 2 L by adding water. Half of the soil was poured into the pot and then the prepared Cd solution was added. Finally, the remaining soil was instantly added to ensure homogenous Cd2+ distribution in the soil. Field capacity water was maintained initially (21 days) and then the soil was kept submerged at 3–4 cm (7 days).
The crop was grown under pot conditions in both agricultural soil (without Cd stress) and Cd-stressed soil (10 mg/kg). Two vigorous seedlings that were four weeks old were transplanted into each pot. Saturated water was maintained throughout the experiment till a week before harvest.
Recommended doses of fertilizers (urea 75, TSP 150, muriate of potash 115 and gypsum 150 kg/ha) and pesticides were applied. In total, 78 pots were arranged in a randomized complete block design to reduce experimental errors.
Rice grains were sampled at ripening and washed twice with distilled water. Then these grains were oven dried at 65o C and the chaff of the grain was manually separated. The brown rice samples were preserved for analysis. Rice grains were crushed and ground to homogenize the sample. Soil sampled from experimental pots was air dried, smashed and sieved (2 mm) for Cd analysis.
Hydrometer method (Gilson, USA) served to determine particle size (Gee and Bauder 1986). Soil pH was measured by pH meter (Hanna HI110, USA) using distilled water at a 1:2.5 ratio (Li et al. 2005). EC was measured by EC meter (Hanna HI2315, USA) in 1:5 ratio (Hardie and Doyle 2012). Organic matter analysis was conducted through titration as described by Nelson and Sommers (2001). CEC was determined by using NH4OAc (Lu 2000). Bioavailable soil Cd was determined by utilizing CaCl2 solution (0.01 M) at a ratio of 1:10 after shaking that lasted 2 h (Houba et al. 2000). Grain and soil samples were digested with HNO3-HClO4 acid mixture (2:1 ratio) following the method of Hseu (2004) for total analysis of Cd. ICP-OES (Spectro Genesis, Germany) measured the Cd2+ concentrations. Sequential extraction as described by Tsai et al. (2003) was employed to determine soil Cd fractions.
Bioconcentration factor (BCF) is calculated according to the following equation (Jiale et al. 2021):
Grade-1 water of 0.01 mS/m EC (at 25o C) was used for laboratory analysis. Standard solution purchased from Sigma-Aldrich, Germany (Multi-element IV, SKU- 1,113,550,100) was used to calibrate ICP. Reference rice flour certified material for trace elements (SKU- IRMM804-15G, Sigma-Aldrich, Germany) was used from which 94.5–96.0% Cd was recovered. Reagent blank, replicated samples, triple reading and continuing check verifications (CCVs) were implemented while readings were taken in ICP for each sample batch.
Statistix 10 software was used for data analysis. One-way ANOVA (analysis of variance) at confidence intervals of 95% (P < 0.05) served for the statistical analysis. Where significant, LSD were calculated among the multiple comparisons of means using Tukey multiple range test. When P < 0.05 then the treatment effect was statistically significant.
Results and Discussion
All six OAs were found to be very efficient in removing Cd from aqueous solution at 2% application rate (Fig. 1). Except for cow dung the other five OAs adsorbed more than 99% solution Cd2+ ions. Cow dung removed 95% Cd from the solution. Therefore, OAs are potential agents to adsorb bioavailable Cd from soil solution. Low-cost OAs obtained from animal wastes and agricultural sources are of prime interest for this process (Mathew et al. 2016). Deploying low-cost OAs helps to reduce environmental pollution and minimize production costs. Agricultural use of these OM adds nutrients to soil through decomposition and this also helps to reduce environmental pollution or damage done to the soils. Effective Cd removal from aqueous solution using various biosorbents was reported by several authors (Cheraghi et al. 2015; Tatah et al. 2017; Alalwan et al. 2020; Visa et al. 2020). However, Mathew et al. (2016) argued that the adsorption process depends on several parameters, especially temperature, pH, pressure, adsorbent activation and adsorbent surface area.
Agricultural and Cd-stressed soil (10 mg Cd/kg soil) contained 0.06 and 0.08 mg/kg Cd in control pots (without OA), respectively. The salt CdCl2.H2O added Cd in soil as Cd2+ which increased its bioavailability by about 38%. The effects of OA on Cd bioavailability in agricultural and Cd-stressed soil are presented in Fig. 2a and b. In both cases, the addition of OA noticeably reduced bioavailability of Cd. At a 2% rate of application, cow dung proved to be most effective and reduced Cd bioavailability by about 33% in agricultural soil and about 64% in Cd-stressed soil. MOC also diminished the Cd concentration in bioavailable pool remarkably well. The performance of rice husk, saw dust and tea leaf was very similar. Among the six amendments VC was found to be the least efficient in adsorbing Cd, achieving only 7% in agricultural soil and 43% in Cd-stressed soil compared with the control. Reduction of Cd bioavailability due to OA application was reported in other research (Bolan et al. 2003; Ruttens et al. 2006; Liu et al. 2009; Ok et al. 2011; Park et al. 2011; Wu et al. 2011; Juang et al. 2011). In the case of 4% application, rate the removal of Cd from the soil solution did not increase considerably compared to the 2% application rate. Therefore, up to 2% application of OA apparently reduces soil bioavailable Cd.
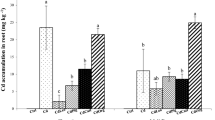
Rice grain Cd content grown under different OA treated soils is presented in Fig. 3a and b. Addition of OA in soil noticeably reduced grain Cd content irrespective of soil and rate of application compared with the control. In agricultural soil, grain Cd content grown in control pots were 0.38 mg/kg and for OA-added pots it was in the 0.15 to 0.30 mg/kg range. Saw dust, tea leaf and VC produced grains with safe amounts of Cd in this soil that made human consumption secure (< 0.2 mg/kg). Cow dung and MOC apparently lowered grain Cd with an increase in the application rate from 2% to 4%. Mamun et al. (2021) recently recommended 1% VC application as the effective dose to reduce Cd uptake from soils in Bangladesh. Cd content in grains grown in the control Cd-stressed soil amounted to 0.45 mg/kg while OA-added soils it was in the 0.27 to 0.40 mg/kg range. 4% OA application rate was not even enough to lower grain Cd below the safe value. In both application rates, rice husk proved to be the most efficient in reducing grain Cd. Other OAs showed significant variation in lowering grain Cd. Many researchers recommended the use of various OAs to reduce Cd toxicity to plants (Rizwan et al. 2016a). The response of plants to Cd concentration considerably varied according to the type of soil and OA application rate (Rizwan et al. 2016b). Liu et al. (2020) stated that reduced Cd uptake by rice is possibly due to the influence of OA on soil properties to reduce Cd bioavailability.
BCF of SL-8 rice variety considerably varied due to the application of OA compared with the control irrespective of soil type and application rate (Fig. 4a and b). In agricultural soil, BCF value ranges from 2.8 to as high as 7.5. In most instances, OA application in both 2% and 4% rates noticeably reduced BCF. Rice husk, saw dust, tea leaf and VC efficiently reduced BCF value at the 2% rate of application whereas cow dung and MOC were efficient at the 4% rate. However, in Cd-stressed soil OA application raised the BCF value higher than that of the control (Fig. 4b). The lowest BCF was found in the control (5.4) and the highest value (10.9) was recorded in rice grains grown in cow dung-treated soils. Again cow dung and MOC emerged as the least effective. Rate of OA application did not show remarkable variation in Cd-stressed soil. Though both bioavailable Cd in soil and grain Cd were reduced by the OA addition the Cd uptake by SL-8 rice variety markedly increased due to Cd spiking in soil. The Cd BCF of rice is significantly higher than wheat and maize (Chen et al. 2021). The authors also reported that soil Cd content, pH, OA and other factors influence Cd BCF of rice.
All OAs greatly influenced the pH of soils (Fig. 5a and b). In agricultural soil, pH variation was in the range of 7.64 to 7.93 in 2% and 7.74 to 7.92 in 4% application rate. In contrast, pH variation in Cd-stressed soil was 7.64 to 7.92 at 2% and 7.64 to 7.83 at 4% OA application. Some amendments increased soil pH whereas others curtailed it. Soil pH lowering was noticeably observed in Cd-stressed soil at 4% application rate. With the increase in OA application rate organic acids are produced through decomposition which reduces soil pH and increases bioavailable Cd in soil (Beesley et al. 2010; Jiang et al. 2012). Moreover, organic acids present in rhizosphere soil can lower the pH value and elevate the bioavailability of heavy metals by activating insoluble forms (Zhi et al. 2020). Therefore, Cd chemistry in soil to a large extent depends on the variation in soil pH. Jiale et al. (2021) reported there was a negative correlation between bioavailable Cd and soil pH. The Authors also stated that the risk of Cd toxicity cannot be neglected specially upon soil acidification which elevates Cd bioavailability and encourages Cd deposition in rice grain. Hence, considering the solubility of Cd with soil pH the application rate of the OA should be judged carefully.
The influence of OA on soil pH also varies with time dynamics after application in soil (Shi et al. 2011). Significant elevation in soil pH due to the addition of crop straw and compost was reported in other studies (Lombi et al. 2003; Zhu et al. 2012; Wang et al. 2015; Sun et al. 2020). Meng et al. (2019) reported that Cd bioavailability may also be effectively reduced by increasing soil pH. The authors showed through linear regression analysis that elevating soil pH bears a positive correlation to immobilizing Cd from soil solution. The elevation in soil pH increases the amount of negative charge on various soil colloidal constituents which enhances Cd sorption to reduce its bioavailability to plants (Naidu et al. 1994; Bolan et al. 2014). The ratio of total soil Cd and soil solution Cd is defined as solid-liquid distribution coefficient (Kd) of Cd (Degryse et al. 2009). The coefficient is mainly soil pH-dependent (Degryse et al. 2009) and Alloway (2013) convincingly showed that each unit pH rise causes an increase in Kd by about a factor of four.
Conclusion
Six traditional OAs used in this study successfully removed more the 95% Cd from aqueous solution. In both agricultural and Cd-stressed soils the addition of OA markedly reduced Cd bioavailability. Addition of OA in soil remarkably reduced grain Cd content irrespective of soil and rate of application compared with the control sample. Saw dust, tea leaf and VC produced grains with safe amounts of Cd for humans to consume (< 0.2 mg/kg). Tea leaf was the most efficient OA for minimizing rice grain Cd in agricultural soil and rice husk appeared to be the most efficient at reducing grain Cd in Cd-stressed soil. 2% (40 t/ha) application of OA apparently reduced Cd bioavailability in soil. BCF of SL-8 rice variety considerably varied due to the application of OA compared with the control irrespective of soil type and application rate. Despite the fact that both bioavailable Cd in soil and grain Cd were reduced by OA addition, the SL-8 rice variety’s uptake of Cd markedly increased as a result of Cd spiking in soil. All OAs clearly had an effect on soil pH which bears close relationship with Cd availability and plant growth. Hence, agrochemicals should be carefully judged before applying them to soil. Lastly, the addition of OA in soil makes the dynamic equilibrium very complicated so more detailed studies are required to evaluate its role in regulating Cd availability in rhizosphere and its subsequent deposition in rice grain.
Data Availability
All data are available.
Code Availability
Not applicable.
References
Alalwan HA, Kadhom MA, Alminshid AH (2020) Removal of heavy metals from wastewater using agricultural byproducts. J Water Supply: Res and Technol—AQUA. https://doi.org/10.2166/aqua.2020.133., 69.2
Alloway BJ (2013) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability, 3rd edition, Springer Science + Business Media, Dordrecht https://doi.org/10.1007/978-94-007-4470-7_2
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287. https://doi.org/10.1016/j.envpol.2010.02.003
Bhattacharyya P, Chakrabarti K, Chakraborty A, Tripathy S, Powell MA (2008) Fractionation and bioavailability of Pb in municipal solid waste compost and pb uptake by rice straw and grain under submerged condition in amended soil. Geosci J 12(1):41–45. https://doi.org/10.1007/s12303-008-0006-9
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils to mobilize or to immobilize? J of Hazardous Materials 266:141–166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Bolan NS, Adriano DC, Duraisamy A, Mani P (2003) Immobilization and phytoavailability of cadmium in variable charge soils. III. Effect of biosolid compost addition. Plant Soil 256:231–241. https://doi.org/10.1023/A:1026288021059
Chen J, Wang J, Wang YW, Yao QX, Su DC (2021) Influencing factors of Cadmium Bioaccumulation factor in crops. Environ Sci 42(4):2031–2039. https://doi.org/10.13227/j.hjkx.202008161
Cheraghi E, Ameri E, Moheb A (2015) Adsorption of cadmium ions from aqueous solutions using sesame as a low-cost biosorbent: kinetics and equilibrium studies. Int J Environ Sci Technol 12:2579–2592. https://doi.org/10.1007/s13762-015-0812-3
Chowdhury S, Mahbub H, Miah TH (2013) Role of SL8H super hybrid rice to achieve food security in Bangladesh: interpretations of survey results. Int J Agric Policy Res 1(3):053–061
FAO (2020) Food outlook, biannual report on global food markets, pp 67 Food and Agricultural Organization of the United Nations. Available from: http://www.fao.org/3/cb1993en/CB1993EN.pdf. Accessed 01 March 2022
Filipović L, Romić M, Romić D, Filipović V, Ondrašek G (2018) Organic matter and salinity modify cadmium soil (phyto)availability. Ecotoxicol and Environ Saf 147:824–831. https://doi.org/10.1016/j.ecoenv.2017.09.041
FRG (Fertilizer recommendation guide) (2018) Bangladesh Agricultural Research Council, Bangladesh www.bare.gov.bd (assessed 15-12-2021)
Gee GW, Bauder JW (1986) Particle-size analysis. In Methods of soil analysis, part 1- physical and mineralogical methods, A. Klute (Eds.), American Society of Agronomy, Inc and Soil Sci Soc of America, Inc, Madison, Wisconsin, 383–411 https://doi.org/10.2136/sssabookser5.1.2ed
Gil JP, LópezZuleta1 S, QuirogaMateus RY, BenavidesErazo J, Chaali N, Bravo D (2022) Cadmium distribution in soils, soil litter and cacao beans: a case study from Colombia. Int J Environ Sci Technol 19:2455–2476. https://doi.org/10.1007/s13762-021-03299-x
Grüter R, Alexandra M, Rainer S, Susan T (2017) Green manure effects on zinc and cadmium accumulation in wheat grains (Triticum aestivum L.) on high and low zinc soils. Plant Soil 422:437–453. https://doi.org/10.1007/s11104-017-3486-4
Hamid Y, Tang L, Wang X, Bilal H, Yaseen M, Yaseen M, Aziz MZ, Yang X (2018) Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci Rep 8:17839. https://doi.org/10.1038/s41598-018-35881-8
Hamid Y, Tang L, Sohail MI, Cao X, Hussain B, Aziz MZ, Usman M, He ZL, andYang X (2019) An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain.Sci Total Environ 660:80?96. https://doi.org/10.1016/j.scitotenv.2018.12.419
Hardie M, Doyle R (2012) Measuring Soil Salinity. Plant Salt Tolerance. Springer Nat 415–425. https://doi.org/10.1007/978-1-61779-986-0_28
Hou Q, Yang Z, Ji J, Yu T, Yuan J (2021) Effects of Soil pH and Mineral Nutrients on Cadmium Uptake by Rice Grain in the Pearl River Delta, China. Bull Environ Contam Toxicol 106:99–108. https://doi.org/10.1007/s00128-020-03057-8
Houba VJG, Temminghoff EJM, Gaikhorst GA, Vark WV (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plantanal 31(910):1299–1396. https://doi.org/10.1080/00103620009370514
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresource Technol 5:53–59. https://doi.org/10.1016/j.biortech.2004.02.008
Islam MA, Romic´ D, Akber MA, Romic´ M (2018) Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environ Geochem Health 40(1):59–85. https://doi.org/10.1007/s10653-017-9907-8
Jiale C, Zheng C, Ruan J, Zhang C, Ge Y (2021) Cadmium bioavailability and accumulation in rice grain are controlled by pH and ca in paddy soils with high geological background of transportation and deposition. Bull Environ Contam Toxicol 106:92–98. https://doi.org/10.1007/s00128-020-03067-6
Jiang H, Li T, Han X, Yang X, He Z (2012) Effects of pH and low molecular weight organic acids on competitive adsorption and desorption of cadmium and lead in paddy soils. Environ Monit Assess 184(10):6325–6335. https://doi.org/10.3109/09637486.2011.636343
Juang KW, Pei-Chi H, Chun-Hui Y (2011) Short-term effects of compost amendment on the fractionation of cadmium in soil and cadmium accumulation in rice plants. Environ Sci Pollut Res 19:1696–1708. https://doi.org/10.1007/s11356-011-0684-0
Kibria KQ, Islam MA, Hoque S, Siddique MAB, Hossain MZ, Islam MA (2022) Variations in cadmium accumulation among amon rice cultivars in Bangladesh and associated human health risks. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18762-6
Kibria KQ, Islam MA, Islam MA, Hossain MZ (2021) Screening of cadmium safe rice cultivar in boro season in Bangladesh. Oral presentation, Book of abstracts, The 8th International Conference on Agriculture 2021 (AGRICO 2021) 19th–20th August 2021
Kubier A, Wilkinb RT, Pichlera T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388. https://doi.org/10.1016/j.apgeochem.2019.104388
Li B, Yang L, Wang CQ, Zheng SQ, Xiao R, Guo Y (2018) Effects of organic-inorganic amendments on the cadmium fraction in soil and its accumulation in rice (Oryza sativa L). Environ Sci Pollut Res 26:13762–13772. https://doi.org/10.1007/s11356-018-2914-1
Li H, Luo N, Li YW, Cai QY, Li HY, Mo CH, Wong MH (2017) Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.01.087
Li Z, Li L, Chen GPJ (2005) Bioavailability of cd in a soil–rice system in China: soil type versus genotype effects. Plant Soil 271:165–173. https://doi.org/10.1007/s11104-004-2296-7
Liu L, Chen H, Cai P, Liang W, Huang Q (2009) Immobilization and phytotoxicity of cd in contaminated soil amended with chicken manure compost. J Hazard Mater 163:563–567. https://doi.org/10.1016/j.jhazmat.2008.04.116
Liu N, Jiang Z, Li X, Liu H, Li N, Wei S (2020) Mitigation of rice Cd accumulation by joint application of organic amendments and Se in high Cd contaminated soils. Chemosphere 241, Article ID 125106. https://doi.org/10.1016/j.chemosphere.2019.125106
Lombi E, Haman RE, McGrath SP, McLaughlin MJ (2003) Lability of cd, Cu, and Zn in polluted soils treated with lime, beringite and red mud, and identification of a non-labile colloidal fraction of metals using isotopic techniques. Environ Sci Technol 37(5):979–984. https://doi.org/10.1021/es026083w
Lu R (2000) Soil and agro-chemical analysis methods. Agricultural Science and Technology Press, Beijing, China, pp 205–266
Mamun SA, Saha S, Ferdush J, Tusher TR, Sharif MA, Alam MF, Balks MR, Parveen Z (2021) Cadmium contamination in agricultural soils of Bangladesh and management by application of organic amendments: evaluation of field assessment and pot experiments. Environ Geochem Health 43:3557–3582. https://doi.org/10.1007/s10653-021-00829-x
Mathew BB, Jaishankar M, Biju VG, Beeregowda KN (2016) Role of bioadsorbents in reducing toxic Metals. J Toxicol Article ID 4369604. https://doi.org/10.1155/2016/4369604
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu Y, Li G, Zhao FJ, McGrath S, Villada A, Sommella P, Mangala CS, Silva D, Brammer H, Dasgupta T, Islam RF (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47:5613–5618. https://doi.org/10.1021/es400521h
Meng L, Huang T, Shi J, Chen J, Zhong F, Wu, Xu J (2019) Decreasing cadmium uptake of rice (Oryza sativa L.) in the cadmium-contaminated paddy field through different cultivars coupling with appropriate soil amendments. J Soils Sediments 19:1788–1798. https://doi.org/10.1007/s11368-018-2186-x
Mohamed I, Bocar A, Ming L, Changxiu G, Peng C, Wei L, Qiaoyun H (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soil Sedim 10:973–982. https://doi.org/10.1007/s11368-010-0199-1
Moreno-Jimenez E, Fernandez JM, Puschenreiter M, Williams PN, Plaza C (2016) Availability and transfer to grain of as, cd, Cu, Ni, Pb and Zn in a barley agri-system: impact of biochar, organic and mineral fertilizers. Agric Ecosyst Environ 219:171–178. https://doi.org/10.1016/j.agee.2015.12.001
Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic strength and pH effects on surface charge and cd sorption characteristics of soils. Eur J Soil Sci 45(4):419–429. https://doi.org/10.1111/j.1365-2389.1994.tb00527.x
Nelson DW, Sommers LE (2001) Total carbon, organic carbon, and organic matter. Methods of Soil Analysis. Part 3- chemical methods. Editor-in-chief D L. sparks SSSA Book Series 5. Soil Science Society of America, Inc., American Society of Agronomy, Inc., Madison, Wisconsin, USA, pp 961–1010
Ok YS, Kim SC, Kim DK, Skousen JG, Lee JS, Cheong YW, Kim SJ, Yang JE (2011) Ameliorants to immobilize cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ Geochem Health 33:23–30. https://doi.org/10.1007/s10653-010-9362-2
Pardo T, Bernal MP, Clemente R (2014) Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: I. Effects on trace elements and nutrients solubility and leaching risk. Chemosphere 107:121–128. https://doi.org/10.1016/j.chemosphere.2014.03.023
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185(2–3):549–574. https://doi.org/10.1016/j.jhazmat.2010.09.082
Putwattana N, Kruatrachue M, Kumsopa A, Pokethitiyook P (2015) Evaluation of organic and inorganic amendments on maize growth and uptake of cd and zn from contaminated paddy soils. Int J Phytoremediation 17(2):165–174. https://doi.org/10.1080/15226514.2013.876962
Reboredo F, Simões M, Jorge C, Mancuso M, Martinez J, Guerra M, Ramalho JC, Pessoa MF, Lidon F (2019) Metal content in edible crops and agricultural soils due to intensive use of fertilizers and pesticides in Terras da Costa de Caparica (Portugal). Environ Sci Pollut Res 26:2512–2522. https://doi.org/10.1007/s11356-018-3625-3
Rizwan M, Ali S, Abbas T, Rehman MZ, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016a) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016b) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23:17859–17879. https://doi.org/10.1007/s11356-016-6436-4
Rui Z, Yi-zhong LU, Yi-bing MA, Ju-mei LI (2020) Effectiveness and longevity of amendments to a cadmium-contaminated soil. J Integr Agric 19(4):1097–1104. https://doi.org/10.1016/S2095-3119(19)62821-2
Ruttens A, Colpaert J, Mench M, Boisson J, Carleer R, Vangronsveld J (2006) Phytostabilization of a metal contaminated sandy soil. II. Influence of compost and/or inorganic metal immobilizing soil amendments on metal leaching. Environ Pollut 144:533–539. https://doi.org/10.1016/j.envpol.2006.01.021
Saengwilai P, Weeradej M, Theerawut P, John P (2020) Immobilization of Cadmium in Contaminated Soil using Organic amendments and its Effects on Rice Growth performance. Expos Health 12:295–306. https://doi.org/10.1007/s12403-019-00312-0
Sato A, Hiroyuki T, Wataru O, Eiji N, Masaharu M (2010) Reduction of cadmium uptake in spinach (Spinacia oleracea L.) by soil amendment with animal waste compost. J Hazard Mater 181:298–304. https://doi.org/10.1016/j.jhazmat.2010.05.011
Sauve S, Hendershot W, Allen HB (2000) Solid-solution partitioning of metals in contaminated soils: dependent on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1130. https://doi.org/10.1021/es9907764
Shi J, Yu X, Zhang M, Lu S, Wu W, Wu J, Xu J (2011) Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil–rice system under intensive farming: a case study of Nanhu, China. J Environ Qual 40:1695–1704. https://doi.org/10.2134/jeq2010.0316
Song L, Xiangyang S, Suyan L, Yuanxin L, Qixue M, Wenjie Z (2021) Effects of amendments on the bioavailability, transformation and accumulation of heavy metals by pakchoi cabbage in a multi-element contaminated soil. R Soc Chem 11:4395–4405. https://doi.org/10.1039/d0ra09358k
Song Y, Wang YBN, Mao WF, Sui HX, Yong L, Yang DJ, Jiang DG, Zhang L, Gong YY (2017) Dietary cadmium exposure assessment among the chinese population. PLoS ONE. 12 Article ID 0177978 https://doi.org/10.1371/journal.pone.0177978
Sun J, Qinya F, Jingwen M, Liqiang C, Guixiang Q, Jinlong Y, Limin W, Kiran H, Basit A, Hui W (2020) Effects of biochar on cadmium (cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ Pollut Bioavailability 32(1):36–46. https://doi.org/10.1080/26395940.2020.1714487
Suzuki S, Koyama H, Hattori T, Kawada T, Rivai IF (1988) Daily intake of cadmium: an ecological review, In: Environ and occ chem hazards, Sumino, S (ed.) Natl. University Singapore and Kobe:205–217
Tatah VS, Otitoju O, Ezeonu CS, Onwurah INE, Ibrahim KLC (2017) Characterization and adsorption isotherm studies of cd (II) and pb (II) ions bioremediation from aqueous solution using unmodified sorghum husk. J Appl Biotechnol Bioeng 2(3):113–120. https://doi.org/10.15406/jabb.2017.02.00034
Tsai LJ, Yu KC, Chen SF, Kung PY, Chang CY, Lin CH (2003) Partitioning variation of heavy metals in contaminated river sediment via bioleaching: effect of sulfur added to total solids ratio. Water Res 37:4623–4630. https://doi.org/10.1016/J.WATRES.2003.07.003
Visa A, Maranescu B, Lupa L, Crisan L, Borota A (2020) New efficient adsorbent materials for the removal of cd(II) from aqueous solutions. Nanomater 10(5):899. https://doi.org/10.3390/nano10050899
Wang FY, Ling W, Zhao YS, You JL, Zhi MS (2012) Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil. J Plant Growth Regul 31:549–559. https://doi.org/10.1007/s00344-012-9265-9
Wang S, Huang DY, Zhu QH, Zhu HH, Liu SL, Luo ZC, Cao XL, Wang JY, Rao ZX, Shen X (2015) Speciation and phytoavailability of cadmium in soil treated with cadmium-contaminated rice straw. Environ Sci Pollut Res 22:2679–2686. https://doi.org/10.1007/s11356-014-3515-2
Wei J, Gao J, Cen K (2019) Levels of eight heavy metals and health risk assessment considering food consumption by China’s residents based on the 5th China total diet study. Sci Total Environ 689:1141–1148. https://doi.org/10.1016/j.scitotenv.2019.06.502
WHO (2015) Evaluation of certain food additives and contaminants, 55th report of the Joint FAO/WHO Expert Committee on Food Additives:891. World Health Organization
Wu F, Lin DY, Su DC (2011) The effect of planting oilseed rape and compost application on heavy metal forms in soil and cd and pb uptake in rice. Agric Sci China 10:267–274. https://doi.org/10.1016/S1671-2927(11)60004-7
Yin B, Zhou L, Yin B, Chen L (2016) Effects of organic amendments on rice (Oryza sativa L.) growth and uptake of heavy metals in contaminated soil. J Soil Sedim 16:537–546. https://doi.org/10.1007/s11368-015-1181-8
Zhang Y, Tian Y, Hu D, Fan J, Shen M, Zeng G (2019) Is vermicompost the possible in situ sorbent? Immobilization of pb, cd and cr in sediment with sludge derived vermicompost, a column study. J Hazard Mater 367:83–90. https://doi.org/10.1016/j.jhazmat.2018.12.085
Zhi Y, Qixing Z, Xue L, Chunlei Z (2020) Mechanism of remediation of Cadmium-Contaminated Soil with Low-Energy Plant Snapdragon. Front Chem. https://doi.org/10.3389/fchem.2020.00222
Zhu QH, Huang DY, Liu SL, Zhou B, Luo ZC, Zhu HH (2012) Flooding enhanced immobilization effect of sepiolite on cadmium in paddy soil. J Soils Sediments 12(2):169–177. https://doi.org/10.1007/s11368-011-0444-2
Zhu Y, Ma J, Chen F, Yu R, Hu G, Zhang S (2020) Remediation of Soil Polluted with Cd in a Postmining Area Using Thiourea-Modified Biochar. Int J Environ Res Public Health 17:7654. https://doi.org/10.3390/ijerph17207654
Acknowledgements
This research was funded by Khulna University Research Cell.
Funding
The Authors received fund from Khulna University Research Cell.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
All sections are relevant to the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kibria, K.Q., Islam, M.A., Hoque, S. et al. Effect of Organic Amendments on Cadmium Bioavailability in Soil and its Accumulation in Rice Grain. Bull Environ Contam Toxicol 110, 74 (2023). https://doi.org/10.1007/s00128-023-03717-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03717-5