Abstract
Polychlorinated biphenyls (PCBs) are persistent in the environment, bioaccumulate and biomagnify throughout the food chain, and may have adverse effects on human health and wildlife. PCB indicator (PCB 28, PCB 52, PCB 101, PCB 118, PCB 138, PCB 153, and PCB 180) were monitored in human milk using 68 samples from healthy and primiparous mothers from seven cities in Colombia, and the estimated daily intake (EDI) of infants was calculated. The PCB indicator with the highest concentration was PCB 153 with a value of 7.30 ng g−1 lipids. The maximum EDI was calculated as 0.257 μg kg−1 bw−1 day−1. In general, the PCB levels found in the 68 samples were low and did not represent a risk to breastfed infants. Additionally, these results could strengthen Colombia’s efforts to increase the practice of breastfeeding. Finally, the results establish a general overview of population exposure and can be a scientific tool to improve environmental health policies in the country.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polychlorinated biphenyls (PCBs) are categorized as persistent organic pollutants (POPs). They are persistent in the environment, bioaccumulate and biomagnify throughout the food chain, and have adverse effects on human health and wildlife (Wöhrnschimmel et al. 2016; Pessah et al. 2019; Rigét et al. 2019; Reddy et al. 2019). PCBs have long-range environmental transport and worldwide presence, even in regions where they have never been used (Wania and MacKay 1996; Hao et al. 2021; Ding et al. 2022). PCBs have been detected in environmental matrices such as air, water, soils, and sediments (Avino and Russo 2018; Mwanza et al. 2021). Moreover, PCBs have been detected in animal and human tissues, blood, urine, and breast milk (Van den Berg et al. 2017; Avila et al. 2020; Sousa et al. 2022). PCBs are divided into dioxin-like PCBs (dl-PCBs) and non-dioxin-like PCBs (ndl-PCBs), with dl-PCBs being the more toxic congeners of this family of pollutants (Magulova and Priceputu 2016).
Several biomonitoring studies of humans and wildlife have been performed to provide information on the body burden of PCBs (Avila et al. 2020; Jeanjean et al. 2021; Peng et al. 2021; Polachova et al. 2021; Varakina et al. 2021; Gaum et al. 2021; Eftekhari et al. 2021; Esser et al. 2021; Chierichetti et al. 2021). Among all the matrices for human biomonitoring of PCBs, breast milk is the preferred. It is easily obtainable and collected noninvasively, and its lipid content makes the extraction of PCBs simple in comparison to that of other matrices. Human milk concentrations of PCBs are often similar to values in blood and adipose tissue. The exposure of breastfed infants plays an important role in evaluating the benefit–risk profile of breast milk since the benefits of breast milk in infants and the possible adverse health effects occur simultaneously (Esteban and Castaño 2009; Van den Berg et al. 2017; Brajenović et al. 2018; Pajewska-Szmyt et al. 2019).
Breastfeeding reduces a wide variety of health problems in infants, such as otitis media (Abrahams and Labbok 2011), gastroenteritis (Frank et al. 2019), severe lower respiratory tract infections (Tromp et al. 2017), atopic dermatitis (Lien and Goldman 2011), asthma (young children) (Miliku and Azad 2018), obesity (Yan et al. 2014), possibly type 1 and 2 diabetes (Gunderson et al. 2015), childhood leukemia (Su et al. 2021), sudden infant death syndrome (Vennemann et al. 2009), and necrotizing enterocolitis (Herrmann and Carroll 2014). Prolonged breastfeeding has been associated with a reduction in mortality and morbidity, with many more beneficial health effects in later life (Chen and Rogan 2004; Ip et al. 2007; Van den Berg et al. 2017).
In Colombia, in 2005, the median duration of exclusive breastfeeding was 2.2 months, with a median duration of 14.9 months of total breastfeeding (Díaz et al. 2011). In the country, the duration of the exclusive breastfeeding practice among women is now very short, and the practice of breastfeeding until the sixth month of a child’s life is uncommon (Díaz et al. 2011). In 2015, Colombia reported the prevalence of obesity in childbearing women and in children under 5 years of age as 22.4 and 6.3%, respectively, with a 10.8% prevalence of stunting and a 2.3% prevalence of wasting during the same year (Aldana-Parra et al. 2020). A strong association between higher maternal body mass index and the mother’s infant being overweight was found by Aldana-Parra et al. 2020.
The World Health Organization (WHO) has determined the tolerable daily intake of PCBs to be 20 μg kg−1 bw day−1 (Van den Berg et al. 2006; Darnerud et al. 2006). The French Agency for Food, Environmental and Occupational Health & Safety (ANSES) has established a tolerable daily intake of 10 µg kg−1 bw day−1 as a reference value for the group of six PCB congeners called PCB indicators (28, 52, 101, 138, 153, and 180).
In 2005, the European Food Safety Authority (EFSA) indicated a benchmark dose lower confidence limit (BMDL) in breast milk of 630–710 ng g−1 lipids for total PCBs based on the cognitive results of children exposed prenatally (Singh et al. 2015). Finally, in 2008, the human biomonitoring commission (HBM) defined the reference value for the sum of the most persistent PCBs [1.64 × (∑ PCB 138 + PCB 153 + PCB 180)] as 500 ng g−1 lipids in the breast milk of breastfeeding women from Germany.
Based on the above, this study aimed to monitor PCB indicators in breast milk from Colombian mothers and to evaluate the risk of adverse health effects in infants due to PCB intake. The results establish a general overview of population exposure and can be a scientific tool to improve environmental health policies in the country.
Materials and Methods
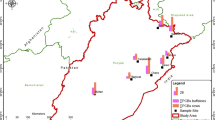
In 2016, 68 samples of breast milk were collected from primiparous and healthy mothers in the Colombian cities of Barranquilla (15), Pasto (9), Medellín (17), Ibagué (8), Cúcuta (10), Santa Marta (3), and Fusagasugá (6). Among these cities, Barranquilla and Santa Marta are situated on the Caribbean coast of Colombia, where the diet of the population is based on food from fisheries mainly by the sea (Cala and Södergren 1999; Duarte-Restrepo et al. 2021). Fusagasugá is a city in the savannah of Bogota that bases its economy on flower growing; the population presents a varied diet that, compared with that on the Caribbean coast, is less rich in food from fishing. Medellín is an industrialized city bordering the coffee belt. Finally, Cúcuta and Pasto are two cities close to the western and southern borders of the country, respectively (FAO 2003; Donato and Haedo 2019; Fuentes lopez et al. 2019). The sampling sites are shown in Fig. 1.
The samples were obtained with the participants’ authorization and without discriminating against race, religious beliefs, or ethnic groups through informed consent approved by the ethics committee of the National Institute of Health (NIH). The samples were 50 mL in volume and were obtained by manual extraction from one of the mother’s breasts into a previously sterilized glass bottle. The samples were stored at − 20°C until they arrived at the laboratory of the Environmental and Occupational Health group of the NIH and were subsequently stored at − 70°C until their analysis. All participants completed a questionnaire about eating habits and sociodemographic information. The questionnaire was developed according to Appendix 4 of the Fourth WHO-coordinated Survey of Human Milk for Persistent Organic Pollutants in cooperation with the United Nations Environment Programme (UNEP).
Standards of PCB indicators 28, 52, 101, 118, 138, 153, and 180 and the internal standard 2,4,5,6-tetrachloro-m-xylene, all with a purity of 99.0%, were purchased from Dr. Ehrenstorfer (Augsburg, Germany). PCB 209 with a purity of 98.8% was used as a surrogate standard for a sample extraction control and was purchased from Ultra Scientific (North Kingstown, United States). The reagents for the extraction and clean-up (formic acid, n-hexane, and potassium oxalate) of the samples were purchased from Merck (Darmstadt, Germany). Stock solutions of each PCB indicator and internal standards were prepared at a concentration of 200 mg L−1; subsequently, three working mix PCB solutions were prepared with isooctane at concentrations of 2000, 200.0, and 20.00 μg L−1. The working solutions were stored at -20 ± 2°C, and their stability for times greater than 4 months was verified. Calibration solutions were prepared by using the appropriate dilutions in a matrix extract from 2.00 to 50.0 μg L−1.
The analytical method was previously validated and is described below. First, 2 mL of a milk sample was extracted with 2 mL of formic acid, 75 mg of potassium oxalate, and 2 mL of n-hexane and was then vortexed for 1.5 min in a 15 mL glass tube and centrifuged at 4500 rpm for 5 min. Next, the organic phase was transferred to another 15 mL glass tube. The extraction process with 2 mL of n-hexane was repeated twice more, and an organic phase volume of approximately 6 mL was obtained. The sample was cleaned-up in a 5 g column of Florisil®. The Florisil®-containing column was conditioned with 25 mL of n-hexane, and samples were eluted with 15 mL of n-hexane. Finally, the organic extract was evaporated to dryness and reconstituted with 20 μL of 2,4,5,6-tetrachloro-m-xylene at 200 μg L−1 and 180 μL of isooctane. The fat content was determined gravimetrically with an independent aliquot of the milk sample and n-hexane.
Samples were analyzed on an HP 6890 N gas chromatograph equipped with a 7683 split/splitless injector connected to an HP-5MS capillary column (30 m, 0.25 mm I.D., 0.25 µm) coupled to a mass selective detector (5975B) manufactured by Agilent Technologies (Palo Alto, CA, USA). The operating conditions of the equipment were as follows: an injection volume of 2 μL, an injector temperature of 280°C, and 60 psi in pulsed splitless mode injection for 0.5 min. The oven had an initial temperature of 100°C for 1.0 min, which was increased at 14°C min−1 to 130°C and then ramped at 8°C min−1 to 300°C, which was maintained for 1 min. The temperature of the transfer line to the mass spectrometer was maintained at 280°C, and the analysis time was 25.4 min with a constant flow of 1.0 mL min−1 of grade 5.0 helium gas. The mass spectrometer was operated in electron impact ionization mode at 70 eV, and the acquisition mode was selected ion monitoring (SIM). The selected acquisition ions for each PCB can be reviewed in (Ávila and Ramírez 2017).
The method trueness was evaluated with recovery tests, and the recovery percentages found to be between 83.0% (PCB 101) and 85.6% (PCB 118). The method detection limits (MDLs) for PCB 28, PCB 52, PCB 101, PCB 118, PCB 138, PCB 153, and PCB 180 were 0.029, 0.035, 0.028, 0.032, 0.025, 0.021, and 0.031 μg L−1, respectively. The method quantification limits (MQLs) for PCB 28, PCB 52, PCB 101, PCB 118, PCB 138, PCB 153, and PCB 180 were 0.086, 0.10, 0.084, 0.10, 0.076, 0.063, and 0.094 μg L−1, respectively. The MDLs for all PCBs were in the range of 0.70 to 1.16 ng g−1 extractible lipids. Linearity was established from 2.00 to 500 μg L−1; however, the working range used for quantifying the samples was 2.00 to 50.0 μg L−1. All samples were spiked with 20 μL of PCB 209 at 200 μg L−1 as a control for the extraction process. The percentage of PCB 209 recovery was between 81.2% and 104.1% in the samples analyzed. Additionally, in each batch of samples (20 samples), one method blank was prepared with an aliquot of cow milk, and the blank was extracted with the same analytical method to verify cross-contamination in the extraction. Furthermore, for each batch of samples, one sample chosen for randomized analysis was spiked twice with analytes to evaluate the recovery of the method during analysis of the samples. The recovery percentages of PCBs in spiked samples were between 84.5% (PCB 28) and 96.3% (PCB 118), and the relative percent difference was < 20% for all PCBs.
The results were organized in an Excel database. When the results were less than MQL but greater than MDL, ½ MQL was input, and when the results were less than MDL, ½ MDL was input in the database to calculate the average, median, 95th percentile, standard deviation, and all other statistical analysis. The estimated daily intake (EDI) values were calculated with Eq. (1), and the results were transformed to logarithms in base 10 to better visualize and interpret the obtained results.
where EDI is the estimated intake dose in μg kg−1 bw−1 day−1, Cmilk is the PCB concentration in human milk in μg g−1 lipids, and Clip is the percentage of lipids in human milk.
Results and Discussion
Summarized results of the PCB indicators (28, 52, 101, 118, 138, 153, and 180) in breast milk are shown in Table 1.
The PCB indicator with the highest concentration was PCB 153, with a value of 7.30 ng g−1 extractable lipids. This indicator was detected in 100% of the breast milk samples analyzed with a median value of < 2.10 ng g−1 extractable lipids, and a 95th percentile value of 5.65 ng g−1 extractable lipids. The higher concentrations of PCBs 138 and 180 were 6.71 and 6.59 ng g−1 extractable lipids, respectively. PCB indicators with a low level of chlorination (PCBs 28, 52) were detected less frequently and in most cases at concentrations less than the MDL. These results are comparable to the results presented by Colombia in the third regional report from Latin America and Caribbean Group (GRULAC) in the global monitoring plan II (GMP II) (GOR-GRULAC 2021). Colombia reported concentrations of PCBs in a national combined human milk sample of 0.42, 0.066, 0.078, 1.35, 2.31, and 1.68 ng g−1 lipids for PCBs 28, 52, 101, 138, 153 and 180, respectively.
The median value of the GRULAC region for the sum of the PCB indicators was 11.00 ng g−1, which is on the same order of magnitude as the results in the present study. In fact, the results shown in Table 1 are slightly less than those of the GRULAC region. Additionally, the results in Table 1 are similar to those of other studies of human milk where PCB 153 was also found at a higher concentration than the other PCB indicators (Paumgartten et al. 2000; Lignell et al. 2009; Çok et al. 2012; Hassine et al. 2012; Müller et al. 2017).
When comparing the results of the present study with similar research in other countries, we found that the levels of PCB indicators in human milk in the 68 samples were either lower or on the same order of magnitude (Rogan et al. 1986; Paumgartten et al. 2000; Rodas-Ortíz et al. 2008; Lignell et al. 2009; Çok et al. 2012; Hassine et al. 2012; Müller et al. 2017; Van den Berg et al. 2017; Brajenović et al. 2018). These findings suggest that the concentrations of the population study mainly correspond to environmental exposure to PCBs and establish a general picture of the levels of PCBs in the country.
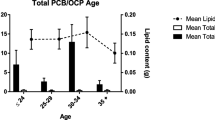
The total amount of PCBs was calculated as the sum of the three most persistent PCBs (138, 153, and 180) multiplied by a factor of 1.64, given that these compounds represent approximately 41% of PCBs present in a human milk sample (Schulz et al. 2012). The maximum total PCB concentration was 33.1 ng g−1 extractable lipids, the median value was < 3.44 ng g−1 extractable lipids, and the 95th percentile value was 23.4 ng g−1 extractable lipids. The levels of total PCB found were less than the BMDL of 630–710 ng g−1 lipids and less than the reference value of 500 ng g−1 lipids, as proposed by the human biomonitoring commission. The results indicate that there is no significant source of exposure to PCBs in the study population (Landrigan et al. 2002; Müller et al. 2017).
The highest levels of PCB indicators were found in the city of Barranquilla (Fig. 2), where five of the 15 samples collected exhibited values between 8.49 and 33.1 ng g−1 extractable lipids of total PCBs. The lowest levels of PCB indicators were found in the cities of Ibagué and Pasto, where all samples exhibited concentrations less than the MQL. The differences in the sample results between cities might be explained in terms of the geographic distribution of the cities (Fig. 1). Barranquilla and Santa Marta are coastal cities with a diet based on the consumption of fishery products and are also a highly industrialized cities (Donato and Haedo 2019; Fuentes lopez et al. 2019), which is in contrast to Ibague and Pasto.
However, the high levels in Barranquilla samples with respect to the other cities cannot be attributed to fish intake or urban industrialization using the data collected in the present study. In fact, these results suggest that new research should be conducted to relate the levels of PCBs between cities considering variables such as feeding, occupation, and educational status, given that the design of this study does not permit determining those relations. Finally, the persistence of PCBs and their ability to travel long distances were confirmed because they were detected throughout the country.
The EDI was calculated using Eq. (1), assuming that the average milk consumption of a 5.0 kg infant is 700 g day−1 (Çok et al. 2012). The results were transformed to base-10 logarithm values for better visualization and interpretation. The results are shown in Fig. 3 and Table 2.
The EDI values were between 0.00316 and 0.257 μg kg−1 bw−1 day−1, the average was 0.0306 μg kg−1 bw−1 day−1, the median was 0.0145 μg kg−1 bw−1 day−1, and the 95th percentile was 0.172 μg kg−1 bw−1 day−1. The calculated EDI values (Table 2) indicate a low intake of PCBs by infants for the 68 samples. The maximum EDI value was less than the tolerable daily intake (TDI) values proposed by the WHO and ANSES of 20 and 10 µg kg−1 bw−1 day−1, respectively.
The PCB levels found in the 68 samples did not represent a risk to breastfed infants, so the results indicate that the breast milk analyzed in this study was safe for consumption by a baby with respect to the PCB concentration. These results could strengthen the efforts of Colombia to increase the practice of breastfeeding.
In general, the results indicate low levels of PCB indicators in the 68 samples of breast milk from Colombia. This finding can be explained by the fact that Colombia and neighboring countries (Ecuador, Brazil, Peru, and Venezuela) were never producers of PCBs and just imported electric transformers with PCBs several decades ago. In fact, Colombia identified 492,835 possible pieces of equipment contaminated with PCBs; only 1.10% (5381) were confirmed to have PCBs, while 43.77% (215,720) were discarded for the presence of PCBs. Additionally, despite the low levels of PCBs found in the 68 samples of human milk from Colombia, conducting new POP monitoring studies is necessary to establish sources of exposure or contamination hot spots to mitigate the exposure and possible adverse effects on the health of the population.
The PCB indicator levels in the 68 samples analyzed from seven cities of Colombia were low with respect to other samples analyzed in the northern hemisphere and are on the same order of magnitude with respect to other samples in the Latin American region. The most frequently detected congeners were PCBs 138, 153, 180, and 118. PCB 118 is categorized as a dioxin-like PCB and thus is considered a more toxic compound in the PCB family. This finding suggests the presence of dioxin-like PCBs in the body burden. Thus, these types of pollutants (dl-PCBs) are important to include in future research. Finally, the PCB intake by infants in the 68 samples analyzed did not represent a risk because the EDI values were very low with respect to the tolerable daily intake suggested by ANSES and the WHO. These results could strengthen the effort by Colombia to promote breastfeeding.
References
Abrahams SW, Labbok MH (2011) Breastfeeding and otitis media: a review of recent evidence. Curr Allergy Asthma Rep 11:508–512
Aldana-Parra F, Vega GO, Fewtrell M (2020) Associations between maternal BMI, breastfeeding practices and infant anthropometric status in Colombia; secondary analysis of ENSIN 2010. BMC Public Health 20:1–15. https://doi.org/10.1186/s12889-020-8310-z
Ávila BS, Ramírez C (2017) Validation of an analytical methodology to determine polychlorinated biphenyls in samples from blood plasma. Biomedica 37:561–570
Avila BS, Ramírez C, Téllez-Avila E, Combariza D (2020) Occupational exposure to polychlorinated biphenyls (PCBs) in workers at companies in the Colombian electricity sector. Int J Environ Health Res. https://doi.org/10.1080/09603123.2020.1806213
Avino P, Russo MV (2018) A comprehensive review of analytical methods for determining persistent organic pollutants in air, soil, water and waste. Curr Org Chem 22:939–953
Brajenović N, Karačonji IB, Jurič A (2018) Levels of polychlorinated biphenyls in human milk samples in European countries. Arch Ind Hygiene Toxicol 69:135–153
Cala P, Södergren A (1999) Occurrence and distribution of organochlorine residues in fish from the magdalena and meta rivers in Colombia. Toxicol Environ Chem 71:185–195. https://doi.org/10.1080/02772249909358791
Chen A, Rogan WJ (2004) Breastfeeding and the risk of postneonatal death in the United States. Pediatrics. https://doi.org/10.1542/peds.113.5.e435
Chierichetti MA, Scenna LB, Ondarza PM, Giorgini M, Di Giácomo E, Miglioranza KSB (2021) Persistent organic pollutants and chlorpyrifos in the cockfish Callorhinchus callorynchus (Holocephali: Callorhynchidae) from Argentine coastal waters: influence of sex and maturity. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.148761
Çok I, Mazmanci B, Mazmanci MA, Turgut C, Henkelmann B, Schramm KW (2012) Analysis of human milk to assess exposure to PAHs, PCBs and organochlorine pesticides in the vicinity Mediterranean city Mersin, Turkey. Environ Int 40:63–69. https://doi.org/10.1016/j.envint.2011.11.012
Darnerud PO, Atuma S, Aune M, Bjerselius R, Glynn A, Grawé KP, Becker W (2006) Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food Chem Toxicol 44:1597–1606
Díaz CE, López R, Herrera I, Arena D, Giraldo C, Gonzáles L (2011) Factors associated with breastfeeding in children less than one year of age in the city of Cartagena. Colombia 42:26–34
Ding Y, Huang H, Wei C, Zhang Y, Wenwen C, Xing X, Qi S (2022) Background levels of OCPs, PCBs, and PAHs in soils from the eastern Pamirs, China, an alpine region influenced by westerly atmospheric transport. J Environ Sci 115:453–464
Donato V, Haedo C (2019). Atlas de la Geografia industrial Colombiana. DANE. Available from: https://atlas.geoecon.info/colombia/. Accessed 24 June 2022
Duarte-Restrepo E, Noguera-Oviedo K, Butryn D, Wallace JS, Aga DS, Jaramillo-Colorado BE (2021) Spatial distribution of pesticides, organochlorine compounds, PBDEs, and metals in surface marine sediments from Cartagena Bay, Colombia. Environ Sci Pollut Res 28:14632–14653. https://doi.org/10.1007/s11356-020-11504-6
Eftekhari S, Aminian O, Esser A, Schettgen T, Kaifie A, Felten M, Kraus T, Moinfar Z (2021) PCB plasma level in different occupational groups in Iran. Toxicol Ind Health 37:458–468
Esser A, Ziegler P, Kaifie A, Kraus T, Schettgen T (2021) Modelling past human internal exposure to lower chlorinated indicator PCBs using proxies—a calculation based on multiple longitudinal PCB analyses. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.147250
Esteban M, Castaño A (2009) Non-invasive matrices in human biomonitoring: a review. Environ Int 35:438–449. https://doi.org/10.1016/j.envint.2008.09.003
FAO (2003) FAO Fisheries & Aquaculture—Perfiles sobre la pesca y la acuicultura por países—La República de Colombia [Internet]. [cited 2020 Jun 17]. Available from: http://www.fao.org/fishery/facp/COL/es
Frank NM, Lynch KF, Uusitalo U, Yang J, Lönnrot M, Virtanen SM, Hyöty H, Norris JM (2019) The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr 19:339. https://doi.org/10.1186/s12887-019-1693-2
Fuentes lopez HJ, Jiménez Reyes LC, Pérez Forero NA (2019) La demografía industrial en Colombia: localización y relocalización de la actividad manufacturera. Cuad Geogr Rev Colomb Geogr 28:43–65
Gaum PM, Kuczynski I, Schettgen T, Putschögl FM, Kraus T, Fimm B, Lang J (2021) Adverse health effects of PCBs on fine motor performance—analysis of a neurophysiological pathway in the HELPcB surveillance program. Neurotoxicology 84:146–154
GOR-GRULAC (2021) Tercer informe de vigilancia regional—America Latina y el Caribe—Plan de vigilancia mundial de los contaminantes orgánicos persistentes - Convenio de Estocolmo sobre COPs. Available from: http://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx. Accessed 24 June 2022
Gunderson EP, Hurston SR, Ning X, Lo JC, Crites Y, Walton D, Dewey KG, Azevedo RA, Young S, Fox G et al (2015) Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med 163:889–898
Hao Y, Li Y, Wania F, Yang R, Wang P, Zhang Q, Jiang G (2021) Atmospheric concentrations and temporal trends of polychlorinated biphenyls and organochlorine pesticides in the Arctic during 2011–2018. Chemosphere 267:128859
Hassine SB, Ameur WB, Gandoura N, Driss MR (2012) Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere 89:369–377. https://doi.org/10.1016/j.chemosphere.2012.05.035
Herrmann K, Carroll K (2014) An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med 9:184
Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J (2007) Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) [Internet]. [cited 2021 Dec 22]:1–186. Available from: http://europepmc.org/books/NBK38337
Jeanjean M, Goix S, Periot M, Douib K, Dron J, Etienne MP, Marchand P, Austruy A, Revenko G, Chamaret P (2021) Environmental and dietary exposures near a major industrial harbour (Fos-sur-Mer, France) identified as a significant pathway for PCBs and PCDD/Fs accumulation in residents’ blood serum. Expo Health 13:447–464. https://doi.org/10.1007/s12403-021-00395-8
Landrigan PJ, Sonawane B, Mattison D, McCally M, Garg A (2002) Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environ Health Perspect. https://doi.org/10.1289/ehp.021100313
Lien TY, Goldman RD (2011) Breastfeeding and maternal diet in atopic dermatitis—PMC. Can Fam Physician 57:1403–1405
Lignell S, Aune M, Darnerud PO, Cnattingius S, Glynn A (2009) Persistent organochlorine and organobromine compounds in mother’s milk from Sweden 1996–2006: compound-specific temporal trends. Environ Res 109:760–767. https://doi.org/10.1016/j.envres.2009.04.011
Magulova K, Priceputu A (2016) Global monitoring plan for persistent organic pollutants (POPs) under the Stockholm Convention: triggering, streamlining and catalyzing global POPs monitoring. Environ Pollut 217:82–84
Miliku K, Azad MB (2018) Breastfeeding and the developmental origins of asthma: current evidence, possible mechanisms, and future research priorities. Nutrients. https://doi.org/10.3390/nu10080995
Müller MHB, Polder A, Brynildsrud OB, Karimi M, Lie E, Manyilizu WB, Mdegela RH, Mokiti F, Murtadha M, Nonga HE et al (2017) Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human breast milk and associated health risks to nursing infants in Northern Tanzania. Environ Res 154:425–434
Mwanza MM, Ndunda EN, Bosire GO, Nyamori VO, Martincigh BS (2021) Advances in sample pretreatment and detection of PCBs in the environment. J Hazard Mater Adv 4:100028
Pajewska-Szmyt M, Sinkiewicz-Darol E, Gadzała-Kopciuch R (2019) The impact of environmental pollution on the quality of mother’s milk. Environ Sci Pollut Res 26:7405–7427. https://doi.org/10.1007/s11356-019-04141-1
Paumgartten FJR, Cruz CM, Chahoud I, Palavinskas R, Mathar W (2000) PCDDs, PCDFs, PCBs, and other organochlorine compounds in human milk from Rio de Janeiro. Brazil Environ Res 83:293–297
Peng FJ, Emond C, Hardy EM, Sauvageot N, Alkerwi A, Lair ML, Appenzeller BMR (2021) Population-based biomonitoring of exposure to persistent and non-persistent organic pollutants in the Grand Duchy of Luxembourg: results from hair analysis. Environ Int. https://doi.org/10.1016/j.envint.2021.106526
Pessah IN, Lein PJ, Seegal RF, Sagiv SK (2019) Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 138:363–387. https://doi.org/10.1007/s00401-019-01978-1
Polachova A, Gramblicka T, Bechynska K, Parizek O, Parizkova D, Dvorakova D, Honkova K, Rossnerova A, Rossner P, Sram RJ et al (2021) Biomonitoring of 89 POPs in blood serum samples of Czech city policemen. Environ Pollut 291:118140. https://doi.org/10.1016/j.envpol.2021.118140
Reddy AVB, Moniruzzaman M, Aminabhavi TM (2019) Polychlorinated biphenyls (PCBs) in the environment: recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem Eng J 358:1186–1207
Rigét F, Bignert A, Braune B, Dam M, Dietz R, Evans M, Green N, Gunnlaugsdóttir H, Hoydal KS, Kucklick J et al (2019) Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota. Sci Total Environ 649:99–110. https://doi.org/10.1016/j.scitotenv.2018.08.268
Rodas-Ortíz JP, Ceja-Moreno V, González-Navarrete RL, Alvarado-Mejía J, Rodríguez-Hernández ME, Gold-Bouchot G (2008) Organochlorine pesticides and polychlorinated biphenyls levels in human milk from Chelem, Yucatán, México. Bull Environ Contam Toxicol 80:255–259
Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tingelstad J, Tully M (1986) Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health 76:172–177
Schulz C, Wilhelm M, Heudorf U, Kolossa-Gehring M (2012) Update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int J Hygiene Environ Health 215:150–158. https://doi.org/10.1016/j.ijheh.2011.06.007
Singh K, Nong A, Feeley M, Man CH (2015) The use of biomonitoring equivalents for interpreting blood concentrations in population studies: a case for polychlorinated biphenyls. AIMS Environ Sci 2:21–41
Sousa S, Maia ML, Delerue-Matos C, Calhau C, Domingues VF (2022) The role of adipose tissue analysis on environmental pollutants biomonitoring in women: the European scenario. Sci Total Environ 806:150922
Su Q, Sun X, Zhu L, Yan Q, Zheng P, Mao Y, Ye D (2021) Breastfeeding and the risk of childhood cancer: a systematic review and dose-response meta-analysis. BMC Med 19:1–23. https://doi.org/10.1186/s12916-021-01950-5
Tromp I, De Jong JK, Raat H, Jaddoe V, Franco O, Hofman A, De Jongste J, Moll H (2017) Breastfeeding and the risk of respiratory tract infections after infancy: the generation R study. PLoS ONE. https://doi.org/10.1371/journal.pone.0172763
Van den Berg M, Feeley M, De Vito M, Hakansson H, Rose M, Tysklind M, Hanberg A, Farland W, Peterson RE, Haws L et al (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93:223–241
Van den Berg M, Kypke K, Kotz A, Tritscher A, Lee SY, Magulova K, Fiedler H, Malisch R (2017) WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Arch Toxicol 91:83–96
Varakina Y, Lahmanov D, Aksenov A, Trofimova A, Korobitsyna R, Belova N, Sobolev N, Kotsur D, Sorokina T, Grjibovski AM et al (2021) Concentrations of persistent organic pollutants in women’s serum in the European Arctic Russia. Toxics. https://doi.org/10.3390/toxics9010006
Vennemann MM, Bajanowski T, Brinkmann B, Jorch G, Yücesan K, Sauerland C, Mitchell EA (2009) Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics 123:e406–e410
Wania F, MacKay D (1996) Peer reviewed: tracking the distribution of persistent organic pollutants. Environ Sci Technol 30:390A-396A
Wöhrnschimmel H, Scheringer M, Bogdal C, Hung H, Salamova A, Venier M, Katsoyiannis A, Hites RA, Hungerbuhler K, Fiedler H (2016) Ten years after entry into force of the Stockholm Convention: what do air monitoring data tell about its effectiveness? Environ Pollut 217:149–158
Yan J, Liu L, Zhu Y, Huang G, Wang pp. (2014) The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. https://doi.org/10.1186/1471-2458-14-1267
Acknowledgements
The authors acknowledge the Colombian milk bank from Barranquilla, Pasto, Medellín, Ibagué, Cúcuta, Santa Marta, and Fusagasugá for support in sample collecting and United Nations Environment Programme –Colombia (UNEP-COL) for financially supporting this project (COL/84851-71268).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avila, B.S., Ramírez, C. & Tellez-Ávila, E. Human Biomonitoring of Polychlorinated Biphenyls (PCBs) in the Breast Milk of Colombian Mothers. Bull Environ Contam Toxicol 109, 526–533 (2022). https://doi.org/10.1007/s00128-022-03577-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-022-03577-5