Abstract
In the present study, water physicochemical and microbiological parameters, as well as bioassays using Allium cepa L. seeds and the fish species Astyanax jacuhiensis were used to assess the water quality of two rivers – Ilha River and Paranhana River –, located in southern Brazil. Water samples were collected at the source and mouth of the rivers and then, laboratory experiments were performed. The results evidenced high levels of aluminum and iron in water samples collected at the four sampling sites. The micronucleus (MN) test in fish showed significant difference in the frequencies of nuclear abnormalities (NA) in the mouth of the Paranhana River in comparison to control group in one sampling period, whereas the A. cepa test evidenced significant spatial differences in cytotoxicity between the source and mouth of both rivers. Therefore, these data evidence the poor water quality of the rivers studied as well as the potential toxicity to the aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The rapid urban development combined with agricultural practices and inadequate systems of collection and treatment of effluents has led to a global concern about the pollution of water resources. Considering the interactions between complex chemical mixtures in effluents, conventional analyses of water physicochemical parameters are not suitable for hazard assessment (Silveira et al. 2017). Thus, studies integrating water quality parameters and bioassays are critical in the water quality evaluation.
Plants are excellent biological systems because they are good bioindicators of toxicity, with high sensitivity to detect cytotoxic and mutagenic agents through different endpoints, including effects in root meristematic cells (Matos et al. 2017). The Allium cepa test is a simple and reliable bioassay for evaluating cytotoxicity and mutagenicity of environmental substances (Leme and Marin-Morales 2009; Gupta et al. 2018). In addition, fishes are also often used as biological indicators of water quality, and biomonitors for the presence of genotoxic aquatic pollutants (Lemos et al. 2007). The micronucleus test in fish is widely used to detect DNA-damaging substances in the water (Bolognesi and Hayashi 2011).
In southern Brazil, the Ilha River and the Paranhana River are the main tributaries of the Sinos River – one of the most polluted rivers in Brazil and that supplies water for more than 1.6 million inhabitants. Although several studies have been conducted in the Sinos River basin (Nunes et al. 2011; Bianchi et al. 2015; Souza et al. 2016; Alves et al. 2018; Peteffi et al. 2019), only a few have focused on the water quality of the tributaries (Fontanella et al. 2008; Rodrigues et al. 2016; Dalzochio et al. 2019). The assessment of the water quality of these rivers is important because they are also under anthropogenic impacts, related mainly to sewage and industrial discharges, agricultural runoffs and solid waste disposal and thus, they may contribute negatively to the water quality of the basin. Hence, the objectives of this study were to: (1) assess physicochemical and microbiological parameters of water samples collected at four sites of these two Sinos River tributaries; (2) evaluate micronucleus (MN) and nuclear abnormalities (NA) frequencies in fish exposed under laboratory conditions to the water samples and; (3) analyze mitotic index (MI), micronucleus (MN) and chromosomal aberrations (CA) frequencies in A. cepa seeds exposed to water samples.
Methods and Materials
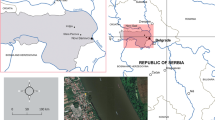
The Sinos River basin is situated northeast of the State of Rio Grande do Sul, southern Brazil. Sampling sites are located in the Ilha River and Paranhana River. Sites 1 and 2 (S1 and S2) are located in the source and mouth of the Ilha River, respectively (29°33′21.16″S; 50°37′45.55W and 29°40′56.42″S; 50°44′22.86″W). These sites are mainly under agricultural impacts, especially S2. Sites 3 and 4 (S3 and S4, source and mouth, respectively) (29°25’29.39”S; 50°46’17.05”W and 29°39’32”S; 50°48’07”W) are located in the Paranhana River, where discharges of industrial and domestic effluents occur.
Approximately 15 L of water samples were collected at each site for water quality parameters analysis and laboratory experiments with fish and A. cepa in August and November/2015 and February and May/2016. The collection, transport and analysis procedures were in accordance with the recommendations of the Standards Methods for the Examination of Water and Wastewater (APHA 2005). The following physicochemical and microbiological parameters were analyzed: biochemical oxygen demand (BOD5), total phosphorous, ammoniacal nitrogen, aluminum, iron, lead, total chromium, nickel, zinc, total and thermotolerant coliforms. The laboratory where water analyses were performed is accredited by the State Environmental Protection Agency (FEPAM), and also followed the APHA recommendations.
For fish experiments, Astyanax jacuhiensis specimens measuring 3–4 cm and weighing 5 g were obtained from a local fish farm. The animals obtained from this fish farm were apparently healthy (with no damage to the skin and fins) and, in previous studies from our research group, they always showed basal levels of cytogenetic damage (Bianchi et al. 2015, 2019). This fish species was chosen because it is native in southern Brazil (Lima et al. 2003) and has successfully been used as a bioindicator organism in previous studies (Bianchi et al. 2015, 2019; Marins et al. 2020). Before each sampling campaign, fish were fed daily and acclimated in glass aquaria containing 5 L of dechlorinated tap water, under constant aeration and natural photoperiod for seven days at 21 ± 1 °C. Then, fish were exposed to water samples collected at each site (n = 10 per group) for five days. A control group was maintained in dechlorinated tap water during the same period. A semi-static procedure was carried out and 50 % of water volume was renewed with the water from each site (stored at 4°C until renewal) at the third day after the exposure had begun. By the end of the exposure period, fish were killed for blood collection. This study was approved by the Ethics Committee for Animal Experimentation of Universidade Feevale (protocol n. 02.13.022).
For the MN test, blood smears were prepared and fixed in absolute alcohol for 10 min and air-dried overnight. Slides were then stained with Giemsa 5% for 10 min and rinsed in tap water. Slides were coded and analyzed under light microscopy according to criteria described by Al-Sabti and Metcalfe (1995). A total of 2000 erythrocytes per fish were counted at 1000× magnification under an oil-immersion objective. Other nuclear anomalies, e.g. invaginations, buds and binucleated cells were assessed as described by Carrasco et al. (1990), and were grouped as nuclear abnormalities (NA) (Seriani et al. 2015; Vieira et al. 2016).
For A. cepa bioassay, seeds were previously germinated in Petri dishes containing filter paper with distilled water at 25°C. Roots measuring approximately 1 cm were exposure to water samples for 24 h. A control group was maintained in distilled water. After exposure, roots were fixed in Carnoy solution overnight and stored in alcohol 70% at 4°C. For slides preparation, roots were hydrolyzed with HCl 1 N at 60°C for 10 min, rinsed with distilled water and stained with acetic orcein 1% for 30 min. A total of 10 slides containing two roots were prepared for each group. The microscopic analysis included cytotoxicity evaluation, where the number of cells under division was recorded (analysis of 1000 cells) – corresponding to the mitotic index (MI), and the genotoxicity evaluation, where the frequency of micronucleated cells (analysis of 1000 interphase cells) and CA (analysis of 100 anaphase-telophase cells) were estimated. CA included chromosome losses, bridges and chromosome laggards (Silveira et al. 2017; Liman et al. 2020).
Statistical analysis was performed using the Kruskal-Wallis test, followed by the Dunn’s multiple comparison test, when appropriated. All analyses were performed using the Statistical Package for the Social Sciences – SPSS 22 considering a significance level of p ≤ 0.05.
Results and Discussion
Water physicochemical and microbiological analysis revealed mean values of aluminum and iron above the limits established by the Brazilian legislation at all sampling sites. Mean values of thermotolerant coliforms also exceeded the limits at all sites, except for S1, and nickel was found above the limits only at S2 (Table 1). Ammoniacal nitrogen, copper, total chromium and lead were not detected in the samples. Previous studies conducted on the basin also detected concentrations of iron, aluminum and thermotolerant coliforms above the limits established by the Brazilian legislation (Dalla Vecchia et al. 2015; Souza et al. 2016; Bianchi et al. 2019). These studies reported higher levels of such parameters at sites located in the middle and lower section of the basin, where anthropogenic activities are more pronounced. The high levels of metals, such as aluminum, iron and nickel, could be related to the lack of basic sanitation, discharges of industrial effluents and disposal of solid waste near the rivers, as well as to the soil composition of the region (Jorgensen et al. 2012; Oliveira and Henkes 2013). In general, higher values of thermotolerant coliforms were detected at the mouth of rivers (S2 and S4), suggesting contamination by sewage discharge and/or fecal material of swine and cattle farms (Blume et al. 2010). These results highlight the lack of basic sanitation in the tributaries, which can negatively affect the basin.
Plant and fish bioassays are tools that can demonstrate potential toxic effects reflecting interactions among mixtures of contaminants present in water resources. Considering the genotoxic effects in fish, frequencies of MN were low and varied from 0 to 0.27‰. Statistically significant differences were observed only in Nov/15, when higher MN frequencies were found in S4 in comparison to control group (p = 0.03). Frequencies of NA varied from 0.83 to 3.77‰ and there were no statistical differences among groups (Table 2). Microscopic findings are demonstrated in Fig. 1. Low frequencies of MN and NA were also found in other studies which aimed to assess the genotoxic potential of the Sinos River using A. jacuhiensis. Bianchi et al. (2015) found mean frequencies of MN below 0.25‰ and of NA below 1 ‰ in fish exposed for 96 h to water samples collected at different sites of the basin. Frequencies of MN ranging from 0 to 0.4 ‰ and NA ranging from 0.83 to 5,44‰ were also found in A. jacuhiensis exposed for 72 h to water samples collected at five sites of the basin (Bianchi et al. 2019). A. jacuhiensis is a native species, widely distributed in aquatic environments in Brazil and is tolerant to oscillations of water physicochemical parameters (Bemvenuti and Moresco, 2005), thus it has been successfully used in ecotoxicological studies (Bianchi et al. 2015; Marins et al. 2020), as well as in the present study. Nonetheless, the increased frequency of MN at S4 could the related to contamination by urban effluents as a consequence of the presence of industries and higher population density in the area in comparison to S3.
In the A. cepa bioassay, MI varied from 5.98 to 10.86. It was possible to observe a significant decrease of MI in the mouth of the rivers (S2 and S4) in comparison to the sources (S1 and S3) and control group in all sampling periods (p < 0.001), except for Aug/15. However, a significant decrease of MI was found in the mouth of both rivers in comparison to control group in Aug/15 (p < 0.001). Dourado et al. (2017) and Nunes et al. (2007) have also evidenced inhibition of MI in roots exposed to water samples from contaminated areas. In this context, metals could induce disturbances in cell cycle or dysfunction of chromatin by interaction of metal and DNA (Glinska et al. 2007). Morever, similar findings were observed by Rodrigues et al. (2016) who observed cytotoxic effects in A. cepa roots exposed to water samples collected in the Ilha River.
Frequencies of MN varied from 0.4 to 1.3, whereas frequencies of CA varied from 0 to 0.80. However, there were no significant differences among groups (Table 3). Similarly, Nunes et al. (2007) also observed only cytotoxic effects in A. cepa roots exposed to water samples from the Sinos River. Thus, although metals and other substances could have induced cytotoxic effects in the present study, they did not cause mutagenic and genotoxic damage in A. cepa roots cells exposed to water samples. Microscopic findings are demonstrated in Fig. 2.
In this study, the analysis of water quality parameters evidenced the poor water quality of the tributaries of the Sinos River, especially considering the levels of iron, aluminum and thermotolerant coliforms. In addition, a spatial variation in the cytogenotoxic potential of pollutants was observed using the fish and plant bioassays. However, it is important to highlight that only two sampling sites were selected for each tributary and that, in order to better assess the status of the water quality, more sampling sites should be further evaluated. The most relevant results were the toxicity on mitotic activity of A. cepa root meristem induced by water samples of the four sampling sites. The cytotoxicity observed in the mouth of both rivers raises environmental concerns. Thus, the use of different approaches to assess the water quality is important for evaluating the environmental risks posed by contaminants.
References
Alves DD, Riegel RP, Quevedo DM, Osório DMM, Costa GM, Nascimento CA, Telöken F (2018) Seasonal assessment and apportionment of surface water pollution using multivariate statistical methods: Sinos River, southern Brazil. Environ Monit Assess 190(7):384. https://doi.org/10.1007/s10661-018-6759-3
Al-Sabti K, Metcalfe C (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res 343:121–135. https://doi.org/10.1016/0165-1218(95)90078-0
APHA - American Public Health Association (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington, D.C., p 1220
Bemvenuti MA, Moresco A (2005) Peixes: áreas de banhados e lagoas costeiras do extreme sul do Brasil. Editora ABRH, Porto Alegre, p 63
Bianchi E, Dalzochio T, Simões LAR, Rodrigues GZP, da Silva CEM, Gehlen G, Nascimento CA, Spilki FR, Ziulkoski AL, da Silva LB (2019) Water quality monitoring of the Sinos River Basin, Southern Brazil, using physicochemical and microbiological analysis and biomarkers in laboratory-exposed fish. Ecohydrol Hydrobiol 19(3):328–338. https://doi.org/10.1016/j.ecohyd.2019.05.002
Bianchi E, Goldoni A, Trintinaglia L, Lessing G, Silva CEM, Nascimento CA, Ziulkoski AL, Spilki FR, Silva LB (2015) Evaluation of genotoxicity and cytotoxicity of water samples from the Sinos River Basin, southern Brazil. Braz J Biol 75(2):68–74. https://doi.org/10.1590/1519-6984.1913. )
Blume KK, Macedo JC, Meneguzzi A, Silva LB, Quevedo DM, Rodrigues MAR (2010) Water quality assessment of the Sinos River, Southern Brazil. Braz J Biol 70(4):1185–1193. https://doi.org/10.1590/S1519-69842010000600008
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can J Fish Aquatic Sci 47(11):2123–2136. https://doi.org/10.1139/f90-237
Dalla Vecchia A, Rigotto C, Staggemeier R, Soliman MC, De Souza FG, Henzel A, Heinzelmann LS, Santos EL, Nascimento CA, Quevedo DM, Fleck JD, Heinzelmann LS, Almeida SEM, Spilki FR (2015) Surface water quality in the Sinos River basin, in Southern Brazil: tracking microbiological contamination and correlation with physicochemical parameters. Environ Sci Pollut Res 22(13):9899–9911. https://doi.org/10.1007/s11356-015-4175-6
Dalzochio T, Souza MS, Simões LAR, Silva GJH, Rodrigues GPZ, Andriguetti NB, Silva LB, Gehlen G (2019) Impact of anthropogenic activities on water quality of the Paranhana River, Southern Brazil. Braz Arch Biol Technol 62:e19180523. https://doi.org/10.1590/1678-4324-2019180523. )
Dourado PLR, Rocha MP, Roveda LM, Junior JLR, Cândido LS, Cardoso CAL, Morales MAM, Oliveira KMP, Grisolia AB (2017) Genotoxic and mutagenic effects of polluted surface water in the midwestern region of Brazil using animal and plant bioassays. Gen Mol Biol 40(1):123–133. https://doi.org/10.1590/1678-4685-gmb-2015-0223. )
Fontanella AC, Coutinho A, Perry C, Rheinheimer C, Schneck F, Lob G, Mattei G, Silva J, Mahfus J, Tallini K, Amaral KF, Vasconcelos M, Bergmann M, Langone P, Pereira R, Silva RRV, Ávila T, Soldatelli V, Hartz SM, Rodrigues GG, Guerra T (2008) Diagnóstico ambiental da bacia hidrográfica do Rio da Ilha, Taquara, Rio Grande do Sul, Brasil. Rev Bras Bioci 7(1):23–41
Glinska S, Bartczak M, Oleksiak S, Wolska A, Gabara B, Posmyk M, Janas K (2007) Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol Environ Safe 68(3):343–350. https://doi.org/10.1016/j.ecoenv.2007.02.004
Gupta K, Mishra K, Srivastava S, Kumar A (2018) Cytotoxic assessment of chromium and arsenic using chomosomal behavior of root meristema in Allium cepa L. Bull Environ Contam Toxicol 100:803–808. https://doi.org/10.1007/s00128-018-2344-2
Jorgensen S, Tundisi JG, Tundisi TM (2012) Handbook of inland aquatic ecosystem management. CRC Press, Boca Raton, p 427
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81. https://doi.org/10.1016/j.mrrev.2009.06.002
Lemos CT, Rodel PM, Terra NR, Oliveira CD, Erdtmann B (2007) River water genotoxicity evaluation using micronucleus assay in fish erythrocytes. Ecotoxicol Environ Safe 66:391–401. https://doi.org/10.1016/j.ecoenv.2006.01.004
Lima FCT, Malabarba LR, Buckup PA, Silva JFP, Vari RP, Harold A, Benine R, Oyakawa OT, Pavanelli CS, Menezes NA, Lucena CAS, Malabarba MCSL, Lucena ZMS, Reis RE, Langeani F, Moreira C (2003) Genera incertae sedis in characidae. In: Reis RE, Kullander SO, Ferraris CJ Jr (eds) Checklist of the freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre, pp 106–168
Liman R, Acikbas Y, Cigerci IH, Ali MM, Kars MD (2020) Cytotocic and genotoxic assessment of silicon dioxide nanoparticles by Allium and comet tests. Bull Environ Contam Toxicol 104:215–221. https://doi.org/10.1007/s00128-020-02783-3
Marcon AE, Ferreira DM, Moura MFV, Campos TFC, Amaral VS, Agnez-Lima LF, Medeiros SBR (2010) Genotoxic analysis in aquatic environment under influence of cyanobacteria, metal and radioactivity. Chemosphere 81:773–780. https://doi.org/10.1016/j.chemosphere.2010.07.006
Marins AT, Severo ES, Leitemperger JW, Cerezer C, Muller TE, Costa MD, Weimer GH, Bandeira NMG, Prestes OD, Zanella R, Loro VL (2020) Assessment of river water quality in an agricultural region of Brazil using biomarkers in a native neotropical fish, Astyanax spp. (Characidae). Bull Environ Contam Toxicol 104(5):575–581. https://doi.org/10.1007/s00128-020-02821-0
Matos LA, Cunha ACS, Sousa AA, Maranhão JPR, Santos NRS, Gonçalves MMC, Dantas SMMM, Sousa JMC, Peron AP, Silva FCC, Alencar MVOB, Islam T, Aguiar RPS, Melo-Cavalcante BCC, Junior HFJ (2017) The influence of heavy metals on toxicogenic damage in a Brazilian tropical river. Chemosphere 185:852–859. https://doi.org/10.1016/j.chemosphere.2017.07.103
Nunes EA, Lemos CT, Gavronski L, Moreira TN, Oliveira NCD, Silva J (2011) Genotoxic assessment on river water uing different biological systems. Chemosphere 84:47–53. https://doi.org/10.1016/j.chemosphere.2011.02.085
Oliveira LA, Henkes JA (2013) Poluição da água: poluição industrial no rio Sinos-RS. Estudo de caso. Gest Sust Ambient 2:186–211. https://doi.org/10.19177/rgsa.v2e12013186-221
Peteffi GP, Fleck JD, Kael IM, Rosa DC, Antunes MV, Linden R (2019) Ecotoxicological risk assessment due to the presence of bisphenol A and caffeine in surface waters in the Sinos River Basin - Rio Grande do Sul - Brazil. Braz J Biol 79(4):712. https://doi.org/10.1590/1519-6984.189752
Rodrigues GZP, Dalzochio T, Gehlen G (2016) Uso do bioensaio com Allium cepa L. e análises físico-químicas e microbiológicas para avaliação da qualidade do Rio da Ilha, RS, Brasil. Acta Toxicol Argent 24(2):97–104
Souza MS, Rodrigues GZP, Dalzochio T, Goldoni A, Simões LAR, Gehlen G, Silva LB (2016) Avaliação da qualidade da água do Rio dos Sinos (Brasil) por meio do teste de micronúcleos em Cyprinus carpio e de análises físico-químicas e microbiológicas. Acta Toxicol Argent 24(3):193–199
Seriani R, Abessa DMS, Moreira LB, Cabrera JPG, Sanches JQ, Silva CLS, Amorin FA, Rivero DHRF, Fitorra LS, Carvalho-Oliveira R, Macchione M, Ranzani-Paiva MJT (2015) In vitro mucus transportability, cytogenotoxicity, and hematological changes as non-destructive physiological biomarkers in fish chronically exposed to metals. Ecotoxicol Environ Saf 112:162–168. https://doi.org/10.1016/j.ecoenv.2014.11.003
Silveira MAD, Ribeiro DL, Vieira GM, Demarco NR, d’Arce LPG (2017) Direct and indirect anthropogenic contamination in water sources: evaluation of chromosomal stability and cytotoxicity using the Allium cepa test. Bull Environ Contam Toxicol 100:216–220. https://doi.org/10.1007/s00128-017-2232-1
Vieira CED, Costa PG, Lunardelli B, Oliveira LF, Cabrera LC, Risso WE, Primel EG, Meletti PC, Fillmann G, Martinez CBR (2016) Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in Southern Brazil. Sci Total Environ 542:44–56. https://doi.org/10.1016/j.scitotenv.2015.10.071
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul—FAPERGS (scholarships), Universidade Feevale, and Conselho Nacional de Desenvolvimento Científico e Tecnólogico (CNPq) (grant number 459718/2014-2). Luciano Basso da Silva is a CNPq researcher (308244/2015-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dalzochio, T., Zwetsch, B.G., Simões, L.A.R. et al. Combination of Water Quality Parameters and Bioassays for the Assessment of Two Rivers, Southern Brazil. Bull Environ Contam Toxicol 108, 678–684 (2022). https://doi.org/10.1007/s00128-021-03408-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03408-z