Abstract
In the present study, sea urchin Sterechinus neumayeri tissues were used for the passive biomonitoring of toxic and trace elements at the Comandante Ferraz Station, Antarctica and compared to a pristine region (Botany). As, Ba, Br, Ca, Co, Cr, Fe, K, Na, Rb, Sc, Se and Zn concentrations were determined by instrumental neutron activation analysis (INAA), while toxic metals (Cd, Hg, Ni and Pb), and Cu were determined by atomic absorption spectrometry (GF-AAS). The findings were compared to other organisms commonly applied for biomonitoring purposes and to the sediment concentrations of each sampling region. Urchins from the Ferraz Station area presented higher Br, Co, Cr, Cs, K, Se and Zn levels than the pristine location. The results obtained herein suggest S. neumayeri can be applied to the biomonitoring of Cr and Zn. The present study also contributes to knowledge of the mineral composition of the sea urchin S. neumayeri.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Antarctic region used to be considered preserved from human activities, especially concerning soil and water contamination. However, from the late 90 s, it was found that this region is also vulnerable to human actions, both locally, due to the presence of scientific stations, which perform garbage incineration, fuel storage and use and sewage discharges, and globally, by the global warming phenomenon and the transport of pollutants by winds and sea currents (Majer et al. 2014). Because of this, increased levels of Hg, Pb, organochlorines and plastics have been reported in this region (Santos et al. 2005). As a result, benthic Antarctic organisms located near research stations are more susceptible to contamination by trace and potentially toxic elements, due to low fertility and growth rates (King and Riddle 2001; Santos et al. 2005).
The sea urchin Sterechinus neumayeri (Meissner 1900) is the most abundant species in shallow Antarctic waters, extending from the coast to about 400 m in water depth (Brey and Gutt 1991). These organisms are omnivorous, feeding on the substrate surface, including sediment, although they may seasonally become scavengers, especially in the face of food shortages and low ocean temperatures (McClintock 1994). This species lack of mobility and feeding mode, are considered premises for assessing local marine conditions, especially chemical element bioaccumulation. Therefore, Sterechinus neumayeri may meets the conditions to be applied as a biomonitor, similar to other frequently applied organisms, such as bivalves, oysters, crabs and macroalgae (Alves et al. 2017a). This sea urchin has, in fact, been used as a model organism in the reproductive biology, embryology, ecology and bioaccumulation fields (Grotti et al. 2008; Majer et al. 2014) and toxicology (King and Riddle 2001).
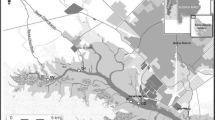
The King George Island region (Fig. 1) is located at the end of the Antarctic peninsula. This island contains the highest density of research stations in the entire Antarctica peninsula, which may be related to the increasing environmental impacts observed in this region (Amaro et al. 2015). A region known as SSSI 8, “Site of Special Scientific Interest”, was created on the west coast of the island, where tourism is prohibited, and many environmental assessments are conducted. King George Island contains the Admiralty Bay, where the Brazilian environmental Antarctic Comandante Ferraz Station is located (Santos et al. 2005). This station was partially destroyed by a fire in February 2012, which consumed about 70% of installations. Some studies have pointed out possible sediment and biota contamination by anthropic activities in this region, especially after the 2012 fire, where high levels of Cu, Pb and Zn were found in surrounding soils (Majer et al. 2014).
Sampling sites of S. neumayeri urchins in Antarctica (adapted from Ribeiro et al. 2011)
The present study analyzed soft Sterechinus neumayeri tissues from the Antarctic Comandante Ferraz Station and Botany regions, by Instrumental neutron activation analysis (INAA), for micronutrients, and Atomic Absorption Spectrometry (AAS) for toxicological elements (Cd, Hg, Ni and Pb) and Cu, in order to contribute with valuable data concerning the nutritional and toxic constituents of this species. In addition, we intended to confirm whether this sea urchin can be used as a test organism for environmental biomonitoring, as scarce data in the literature on the use of these species as biomonitors are available. A passive biomonitoring was performed, which consists in collecting the organisms directly from their environment and processing them according to scientific interest (Market et al. 1999).
Material and Methods
The Sterechinus neumayeri samples were obtained during the summer of 2014/2015, with 10 organisms collected close to the Comandante Ferraz Station effluent disposal outlet (62.05224–62.05035 S to 58.23381–58.23370 W), a region likely to be influenced by human activities (impacted site). Another 10 organisms were also collected from a pristine region, Botany Point (Fig. 1) (62.05400–62.05556 S to 58.18127–58.18612 W). Sampling was performed by dragging and transferring the specimens to nylon canvas bags and later to 30 L gallons of water with intermittent aeration for transport to the laboratory.
Subsequently, biometry was performed and each individual was dissected, the soft tissues (digestive tract and gonads) were removed and later homogenized in order to obtain a tissue pool. The tissues were stored in tubes and frozen at − 20℃. For the chemical analyses, the samples were dried in a ventilated oven at 40℃, for 7 days, homogenized and sieved through a 0.250 mm (60 mesh) nylon sieve and adequately properly.
Concerning the INAA technique, up to 0.15 g of each sample was weighed. Due to the small amount of sample available, only one sample from each organism was analyzed. Next, synthetic mono and multi-elementary standards were prepared, which included the following elements: As, Ba, Br, Ca, Co, Cr, Cs, Fe, K, Na, Rb, Sb, Sc, Se, Th, U and Zn. The standards were prepared by diluting of certified standard solutions (SPEX CERTIPREP, USA). About 0.15 g of certified reference materials (CRM) Mussel Tissue – NIST SRM 2976 and Oyster Tissue – NIST SRM 1566b and Peach Leaves – NIST SRM 1547 were weighed and prepared in the same way as sea urchin tissues. One replicate of each CRM was prepared for each irradiation. The dried sea urchin samples, certified reference materials and synthetic standards were inserted into an aluminum casing for irradiation of a daily 6 to 7-h cycle under a flow of thermal neutrons from 1 to 5 × 1012 n cm−2 s−1 in the IEA-R1 nuclear research reactor at the IPEN-CNEN/SP. The samples and standards were counted on a gamma ray spectrometer consisting of a hyper pure germanium semiconductor crystal detector (HPGe) associated to a CANBERRA electronic data acquisition system, with a resolution of 1.21 and 2.23 keV for the 57Co and 60Co photopeaks, respectively. Details of the analytical procedure have been previously described in Alves et al (2017a).
For the AAS technique, approximately 200 mg of each sea urchin tissue and from the certified reference materials Oyster Tissue (SRM 1566b), Mussel Tissue SRM 2976 and Fish Tissue (IAEA 2010) were weighed, packaged in MARS-ONE TOUCH ™ Teflon® tubes. Subsequently, 10 mL of concentrated HNO3 (Merck™) were added to the samples and the mixtures were digested at room temperature overnight. Two analytical blanks containing only digested HNO3 were also prepared. The following day, the samples, reference materials and analytical blanks were submitted to the “animal tissue” program in a MARS 6 microwave oven (CEM Corporation) for acid digestion. After cooling, the tubes were opened and the contents were transferred to Falcon™ tubes, made up to 50 mL with Milli-Q water and stored 5℃, for further analysis by CV-AAS for Hg and GF-AAS for Cd, Cu, Ni and Pb. For the CV-AAS analysis, a PERKIN ELMER ™ Flow Injection Mercury System 100 was used, with SnCl 2.1% (m/v) as the reducing agent at a flow rate of 6.4 mL/min and 3% HCl (v/v) (carrier solution), at a flow rate of 10.4 mL/min. A hollow Hg cathode lamp at 253.7 nm was used and absorptions were measured using the Winlab 32 software from the same manufacturer. An AAnalyst 800 PERKIN ELMER spectrometer was used for the GF-AAS analyses. Standard solutions, sea urchin samples, CRMs, chemical modifiers, an HNO3 diluent solution and analytical blanks were placed in injection sample containers in the AAS oven. An NH4H2PO4 at 0.5% (m/v) and Mg(NO3)2 at 0.03% (m/v) mixture was used as the chemical modifier for the GF-AAS Cd, Cu and Pb analyses, while a Pd + Mg(NO3)2 at 0.03% (m/v) mixture was used for Ni determinations (Skoog et al. 2014). The following wavelengths (nm) were used: Cd-228.8; Cu -324.8; Ni-232.0 and Pb-283.3.
Z-Score criteria were adopted for precision and accuracy verifications of the INAA analytical technique (Bode 1996; INMETRO 2017). Details of these criteria and how they were calculated have been previously described (Alves et al. 2017a). Measurement uncertainties were calculated for each Z-Score-validated element. The INMETRO (2017) analytical method validation procedure was followed for the AAS technique, expressed as trend/recovery in % and in the expanded uncertainties for each element.
To statistically evaluate concentrations variations, the Shapiro–Wilk test was performed concerning data normality and the Levene test was applied for homoscedasticity assessments (p < 0.05, 95% CI). The means were tested by the t-Student test for independent samples (by variables) where p < 0.05 at 95% CI, in order to indicate concentration differences between the sampling sites, while the Mann–Whitney test was applied for nonparametric data. The Grubbs test (95% CI) was performed to detect outliers. A linear correlation matrix with linear r (Pearson) and p value < 0.05 for significance was applied, and an exploratory PCA was applied to integrate concentration data. The Excel® and Past3 software programs were used for all analysis.
Results and Discussion
If |Z|< 3, the individual result of the control sample (certified reference material) lies within the 99% confidence interval of the target value. Z-score values for As, Ba, Br, Ca, Cr, Cs, Fe, K, Na, Rb, Sc, Se and Zn were all in this interval of (|Z|< 3), indicating good INAA precision and accuracy.
Table 1 displays the results for the certified reference material analyses as detected by the AAS technique, the CV for AAS-determined Hg and for GF- AAS-determined Cd, Cu, Ni and Pb. Recovery values (%) in the order of magnitude ranging from 100 to 10,000 ng g−1 must lie between 80 to 110% (INMETRO 2017). At least one CRM met the validation conditions for the analyzed elements (Table 1).
Most elements from both regions displayed normal distribution by the Shapiro–Wilk test, while K concentrations in both regions, Cu in the Botany region and Ni in the Ferraz Station region exhibited p values < 0.05, characterizing non-normal distributions. Thus, K, Cu and Ni were subjected to the non-parametric Mann–Whitney test, to verify differences in the medians for the Botany and Ferraz Station areas.
The Sterechinus neumayeri tissue element determination results by both the AAS techniques and the INAA technique are presented in Table 2, expressed as means and standard deviation with 95% significance on a dry basis at both sampling sites. The limits of quantification (LoQ), relative standard deviation (RSD), number of individuals (n), t test p values for K, Cu and Ni and Mann–Whitney p values are also presented.
As, Ba, Cd, Cu, Fe, K, Na, Ni, Pb, Rb and Sc were not significantly different between the Botany (non-impacted) and Ferraz Station (possibly impacted) areas, according to a t-test at a. 95% CI (p < 0.05), and according to the Mann–Whitney non-parametric test, under the same conditions.
On the other hand, Br (220 ± 20 mg kg−1 and 300 ± 60 mg kg−1); Co (260 ± 50 ng g−1 and 330 ± 40 ng g−1); Cr (0.5 ± 0.1 mg kg−1 and 0.8 ± 0.2 mg kg−1); Zn (80 ± 10 mg kg−1 and 120 ± 30 mg kg−1); Cs (32 ± 7 ng g−1 and 60 ± 20 ng g−1) and Se (2.1 ± 0.3 mg kg−1 and 2.9 ± 0.5 mg kg−1) at the Botany and Ferraz Station regions, respectively, were significantly different by the t-test, at a 95% CI (p < 0.05). These elements were higher at the Comandante Ferraz Station region, which is possibly impacted. Ca and Hg were below the limit of quantification and were, therefore, excluded from this study.
For the elements analyzed by the INAA technique, the observed relative standard deviation (RSD) suggests a natural variability of these elements in sea urchins due to their metabolism (Marcovecchio 2004). Regarding the AAS results, Cd, Cu, Ni and Pb also exhibited high RSD values, which also suggests natural variability of these element between organisms.
No statistically significant differences between areas studied (t test, p < 0.05, CI 95%) were observed for Ba (9 ± 2 and 9 ± 3 mg kg−1); Fe (200 ± 100 and 200 ± 50 mg kg−1); Na (14,000 ± 2000 and 15,000 ± 2000 mg kg−1); Rb (6.4 ± 0.6 and 6 ± 1 mg kg−1) and Sc (8 ± 1 and 8 ± 4 ng g−1), at Botany and Ferraz Station, respectively. Comparing these results to other organisms (Catharino 2008), the sea urchin species evaluated herein is rich in Na, but this element is not of interest in environmental biomonitoring, due to its association with salinity. Ba concentrations in sediments at Admiralty Bay range at about 363 mg kg−1 (Fávaro et al., 2012). Therefore, the detected Ba contents in the sea urchins are probably a reflection of the availability of this element in sediments ingested during the feeding process. Fe, Rb and Sc exhibit average concentrations of 5200, 56 mg kg−1 and 23.8 µg kg−1, respectively, in sediments (Fávaro et al. 2012). Thus, it is very likely that the content of these elements in S. neumayeri is also due to the availability of these elements in the sediment, as the concentration of these elements in sediments is much higher than in the evaluated organisms.
For a better understanding of the correlation between the chemical elements detected in sea urchins and their role concerning environmental issues, especially regarding marine pollution and biomonitoring, a Correlation matrix (Table 3) for urchins in Ferraz Station and exploratory Principal Component Analysis (PCA) was applied for both regions (Fig. 2).
For the Botany region, a linear correlation matrix was performed which exhibited that Rb had a positive correlation with As (p = 0.02, r = 0.73) and Br (p = 0.01, r = 0.79) and the element Zn has positive linear correlation with K (p = 0.01, r = 0.79). Other elements had no significant linear correlation.
Data from linear correlation matrix for the Ferraz Station is shown in (Table 3). Only data that with Pearson’s r values >|0.7| and p < 0.05 are shown. Cd, Cr and K had a strong linear correlation with the tissue pool weight, especially Cd with r = 0.94, while the Sc had a strong negative correlation tissue pool weight, Cd, Cr and K. Zn had positive correlation with As and Na had a positive correlation with Co, but negative with Cu; K had a positive correlation with Br.
The PCA tests was performed with the following characteristics: all values were normalized by logarithm, missing data within each group were filled with the average values of each group, elements not relevant to environmental issues (i.e. Na and K) were excluded, as they are abundant in marine environments, in addiction Ba, Ca, Hg and Ni were excluded due to the low number of results in both regions and/or the fact that they were below the LoQ; Cu element was excluded due the non-normal distribution. For the PCA analysis was considered enough factors to explain 75.8% of the data variance. Despite the recommendation considering factorial loads above 0.4 for each component (Field 2009, Nory 2018), the chemical elements with the highest factor load values, in module, were considered as load factors.
The exploratory PCA grouped the chemical elements into three main factors explaining 75.8% of the total data variance. The case projection graphs exhibited in Fig. 2 indicate that Component 1, under higher loads, and component 2, under less loads, were responsible for grouping the elements that separate Botany and Ferraz station areas as not impacted and possibly impacted, respectively (Santos et al. 2006). Elements widely known as potentially toxic (Santos et al. 2006; Negri et al. 2006; Fávaro et al. 2012; Majer et al. 2014) were more grouped and located closer to Ferraz Station when compared to the Botany region. Thus, the concentrations of the most common elements in environmental contamination studies presented in Table 2 and the t test applied to verify differences between means are in line with the PCA results.
Component 1, which explains 43.5% of the total data variance, associates Br, Cr, Pb and Zn to the Ferraz Station region (impacted) with factor load values of 0.34, 0.38, 0.37 and 0.42, respectively. In addition, Cr, Pb and Zn are well grouped in the Ferraz Station area (Fig. 2). Table 4 presents a comparison of results reported by other authors for other species and sampling sites concerning elements with relevant load factors for this PCA component.
Br concentrations were higher at the Ferraz Station area (300 ± 60 mg kg−1) compared to the non-impacted Botany region (220 ± 02 mg kg−1). Br is present in seawater at around 67 mg kg−1 and in sediment at around 76 mg kg−1 in both study regions, respectively (Fávaro et al. 2012). Differences in concentrations may be related to the availability of this element within the S. neumayeri food chain as is more evident due the positive correlation with K (Table 3) (Grotti et al, 2008). There is evidence that S. neumayeri naturally accumulates Br, given the high concentrations detected at both sites, since Br concentrations are generally high in benthic organisms, according to other assessments carried out with Perna perna mussels (Seo et al. 2013). In addition, Br concentration in the present study was detected at the same order of magnitude as in L. variegatus sea urchins (Alves et al. 2017a). Since Br concentrations in S. neumayeri are statistically different between the sampling points, further studies with a higher number of individuals are required in order to verify whether this difference is anthropic in origin.
Regarding Cr, concentrations at the Ferraz Station region were practically two-fold than those detected in the Botany region (0.8 ± 0.2 mg kg−1 and 0.5 ± 0.1 mg kg−1, respectively). The observed values were of the same order of magnitude as those observed in S. neumayei sea urchins in the Terra Nova region (Grotti et al. 2008), and lower than in A. ingens sea urchins from the Casey Station region (Duquesne and Riddle 2002). A. ingens sea urchins also exhibited higher Cr concentrations in the contaminated region (more than two-fold higher than in the non-impacted region, at Casey Station, while the macroalgae D. menziensii, probably a food source for S. neumayeri sea urchins (McClintock 1994), contained almost threefold higher Cr content at the Ferraz Station region compared to the non-impacted Botany region, probably due to anthropic coal and oil burning and the production of non-ferrous metals (Santos et al 2005; Trevisani et al. 2018). Shinagawa (2016) reported that Cr sedimental content at Ferraz Station is threefold higher than in the control region (Table 4), which may be the result of the 2012 fire. Based on the results of this study, S. neumayeri sea urchins bioaccumulated higher Cr contents at Ferraz Station region compared to the preserved Botany area, in addition Cr had a positive correlation with the weight of tissue pool (gonads and digestive tract) and a negative with Sc (Table 3), which may be a consequence of the greater Cr content in the environment (Table 2). Cr bioaccumulation trends observed herein are similar to that observed for L. variegatus sea urchins on the coast of Brazil, considered adequate biomonitors (Alves et al. 2017a, b). Based on this, the present study suggests that S. neumayeri may prove to be adequate Cr biomonitors.
Zn, detected at 80 ± 10 and 120 ± 30 mg kg−1 at the Botany and Ferraz Station areas, respectively, was detected at same order of magnitude as in S. neumayeri and A. ingens sea urchins in other studies (Table 4) (Duquesne and Riddle 2002; Majer et al. 2014). Zn concentrations in Ferraz station region sediments ranged from 100 to 148 mg kg−1 before the 2012 fire (Favaro et al. 2012). Shortly after the fire, the concentration of this metal increased to 2800—3500 mg kg−1 (Shinagawa 2016). Studies conducted on L. ellipctica bivalves, considered good biomonitors (Negri et al. 2006), indicate higher Zn concentrations in impacted sites, but did not hypothesize on anthropic sources of this element (Majer et al. 2014). The aforementioned authors assessed the possible Zn increases at the Ferraz Station due to the 2012 fire and suggested continuous biomonitoring evaluations. Trevisani et al. (2018) concluded that the amount of Zn in benthic organisms may be related to the availability of this element in sediment.
Pb values in S. neumayeri sea urchins were of 1100 ± 400 and 1700 ± 1100 ng g−1 at the Botany and Ferraz Station areas, respectively. However, this difference was non-significant (p > 0.05), which may suggest that the Pb content is more related to natural biological variability (Amaro et al. 2015; Cabrita et al. 2017). In 2013, one year after the 2012 fire, a study concerning the bioaccumulation of toxic elements in S. neumayeri sea urchins was carried out, at Pb concentrations were about 40-fold higher in relation to the non-impacted sampling area (Table 4) (Majer et al. 2014) suggesting acute contamination, probably related to the fire. Shinagawa (2016) evaluated sediments and reported higher Pb concentrations at Ferraz Station compared to the non-impacted region (Table 4). Higher Pb concentrations were also detected at Casey Station, which is considered contaminated. Although the results of this study do not point to Pb contamination, the other biomonitoring studies presented in Table 4 suggest possible anthropic Pb sources, such as coal and oil combustion (Majer et al. 2014; Trevisani et al. 2018). On the other hand, the result of this study for Pb suggests its spread throughout the collection sites, especially in the preserved region since significant increases in Pb concentrations S. neumayeri sea urchins were observed.
PCA Component 2, which explains 23.1% of the total data variance, positively associated Cd, Rb, with factor load factors of 0.46 and 0.47, respectively, while Sc was negatively associated, with a factorial load of -0.42. Rb (6.4 ± 0.4 and 6.0 ± 0.4 mg kg−1) and Sc (8 ± 1 and 8 ± 4 ng g−1) at the Botany and Ferraz Station areas, respectively, were not significantly different (p < 0.05) by the t test. An indication that these elements are isolated is noted in Fig. 2, which may suggest that the evaluated sea urchins probably accumulate these elements naturally, regardless of the study region conditions.
Cd concentrations were of 4700 ± 1700 and 3300 ± 1300 ng g−1 at the Botany and Ferraz Station areas, respectively, the same order of magnitude reported by Grotti et al. (2008) for other regions (Table 4). The significant difference between means (p = 0.01) suggests potential S. neumayeri contamination by this element in the Botany region, as this metal is not essential. In contrast, the PCA diagram (Fig. 2) indicates that this difference is probably not associated to the study regions. In addition, Cd contents at the Ferraz station were almost threefold higher compared to the results reported by Majer et al. (2014) (Table 4) for the same species and region. Some studies in another antarctica regions suggest soil contamination by Cd, while also considering that this element may be associated to the benthic community food chains (Zhang et al. 2009; Majer et al. 2014).
The exploratory PCA Factor 3, which explains 9.3% of the total data variance, associates only Cu to the Ferraz Station region. Cu concentration were of 5500 ± 2500 and 6700 ± 2400 ng g−1 at the Botany and Ferraz Station areas, respectively, with a factor load factor of − 0.67. On the other hand, according to the t-test, no significant differences between the means of both regions were detected. However, the high coefficient of variation may suggest a natural variability of this element in the studied sea urchins. The low number of analyzed individuals must also be considered, which can lead to high coefficient of variation values in biological samples. Majer et al. (2014) reported concentrations of around 34 mg kg−1 of Cu in soil close to the location of the Ferraz Station ruins. The authors suggested that this element may be accessible to the local ecosystem due to seasonal melting. Another study associated Cu to the sediment conditions of the Admiralty Bay region, where it may be associated to chalcopyrite and rock erosion (Machado et al. 2001; Trevisani et al. 2018).
The present study contributed towards knowledge of the mineral composition of S. neumayeri sea urchins, indicating that this species is rich in Na and K compared with other marine organisms.
Br, Cr, Cs, K, Se and Zn were higher at the Ferraz Station, considered an impacted site, compared to the less impacted control Botany region. This indicates a certain degree of environmental impacts in the former, especially concerning Cr and Zn, which may be related to the 2012 fire. The other analyzed metals were not significantly different between the two study regions. The observed differences were confirmed by statistical comparisons between means.
The PCA assessment, in addition to comparative studies with other organisms, suggest the possibility of passive biomonitoring for Cr and Zn, indicating that this organism may be a good passive biomonitor candidate for coastal environments. However, studies in active biomonitoring need to be made to confirm if S. neumayeri can be used as biomonitor for other elements. In addition, the Comandante Ferraz Station region, despite the 2012 fire, did not show any signs of significant contamination by potentially toxic metals, except for Cr.
References
Alves MB, Emerenciano AK, Bordon IC, Silva JRMC, Fávaro DIT (2017) Biomonitoring evaluation of some toxic and trace elements in the sea urchin Lytechinus variegatus (Lamarck, 1816) in a marine environment: northern coast of São Paulo (Brazil). J Radioanal Nucl Chem 316(2):781–790
Alves MB, Emerenciano AK, Bordon IC, Silva JRMC, Fávaro DIT (2017) Assessment of toxic and trace elements in the sea urchin Sterechinus neumayeri in the Antarctic marine environment. 2017 International Nuclear Atlantic Conference - INAC 2017. http://repositorio.ipen.br/bitstream/handle/123456789/28316/24151.pdf?sequence=1&isAllowed=y
Amaro E, Padeiro A, de Ferro AM, Mota AM, Leppe M, Verkulich S, Hughes KA, Peter H, Canário J (2015) Assessing trace element contamination in Fildes Peninsula (King George Island) and Ardley Island, Antarctic. Mar Pollut Bull 97:523–527
Bode P (1996) Instrumental and organizational aspects of a neutron activation analysis laboratory. Interfaculty Reactor Institute, Delft
Brasil (2014). Ministério de Meio Ambiente. Portaria n.445, de 17 de dezembro de 2014. Diário Oficial da União, Brasília, 18 de dezembro de 2014, seção 1, p.126–127. <http://www.icmbio.gov.br/portal/images/stories/biodiversidade/fauna-brasileira/avaliacao-do-risco/PORTARIA_N%C2%BA_445_DE_17_DE_DEZEMBRO_DE_2014.pdf>
Brasil (2015). Ministério do Meio Ambiente. Portaria n.98 de 28 de abril de 2015. Diário Oficial da União, Brasília, 29 de abril de 2015, seção 1, p.86. <http://www.icmbio.gov.br/portal/images/stories/biodiversidade/fauna-brasileira/portarias/p_mma_98_2015_altr_p_445_2014.pdf>
Brey T, Gutti J (1991) The genus Sterechinus (Echinodermata: Echinoidea) on the Weddel Sea shelf and slope (Antarctica): distribution, abudance and biomass. Polar Biol 11:227–232
Cabrita MT, Padeiro A, Amaro E, dos Santos MC, Leppe M, Verkulich S, Hughes KA, Peter H, Canário J (2017) Evaluating trace element bioavailability and potential transfer into marine food chains using immobilised diatom model species Phaeodactylum tricornutum, on King George Island, Antarctica. Mar Pollut Bull 121(1–2):192–200
Catharino MGM, Vasconcelos MBA, Sousa ECPM, Moreira EG, Pereira ACDS (2008) Biomonitoring of Hg, Cd, Pb and other elements in coastal regions of São Paulo state, Brazil, using the transplanted mussel Perna perna (Linnaeus, 1758). J Radioanal Nucl Chem 278:547–551
Duquesne S, Riddle MJ (2002) Biological monitoring of heavy-metal contamination in coastal Waters off Casey Station, Windmill Islands East Antarctica. Polar Biol 25:206–215
Fávaro DIT, Silva PSC, Mazzilli BP, Cavallaro GPM, Taddei MHT, Berbel GBB, Braga ES (2012) Sediment geochemistry in Admiralty Bay (Antarctica): trace, rare earth elements and radionuclides. Braz Antarct Res 5:11–24
Field A (2009) Descobrindo a estatística usando o SPSS, 2nd edn. Artmed, São Paulo
Grotti M, Soggia F, Lagomarsino C, Riva SD, Goessler W, Fracesconi KA (2008) Natural variability and distribution of trace elements in marine organisms from Antarctic coastal environments. Antart Sci 21(1):39–51
IAEA (2010) Latin American regional proficiency test on the determination of trace elements and radionuclides in algae, soil, and spiked water. International Atomic Energy Agency, Vienna
INMETRO (2017) Guidance on validation of analytical methods: guidance document. DOQ-CGCRE-008, Revisão 06. http://www.inmetro.gov.br/credenciamento/organismos/doc_organismos.asp?torganismo=calibensaios
King CK, Riddle MJ (2001) Effects of metal contaminants on the development of the common Antarctic sea urchin Sterechinus neumayeri and comparisons of sensitivity with tropical and temperate echinoids. Mar Ecol Prog Ser 215:143–154
Machado A, Lima EF, Chemale Júnio F, Liz JD, Ávila JN (2001) Química mineral de rochas vulcânicas da Península Fildes (Ilha Rei George). Antárt Rev Bras Geoc 31:299–306
Majer AP, Petti MAV, Corbisier TN, Ribeiro AP, Theophilo CYS, Ferreira PAdeL, Figueira RCL (2014) Bioaccumulation of potentially toxic trace elements in benthic organisms of Admiralty Bay (King George Island, Antarctica). Mar Pollut Bull 79:321–325
Marcovecchio JE (2004) The use of Micropogonias furnieri and Mugil liza as bioindicators of heavy metal pollution n La Plata river estuary, Argentina. Sci Total Environ 323:219–226
Markert B, Wappelhorst O, Weckert V, Herpin U, Siewers U, Friese K, Breulmann G (1999) The use of bioindicators for monitoring the heavy-metal status of the environment. J Radioanal Nucl Chem 240(2):425–429
McClintock JB (1994) Trophic biology of antarctic shallow-water echinoderms. Mar Ecol Prog Ser 111:191–202
Meissner M (1900) Echinoideen. Hamburger Magalhaensische Sammelreise. In: Ergebnisse der Hamburger Magahaensischen Sammelreise 1892/93, Bd 1 (1896–1907). L. Friedrichsen, Naturhistorisches Museum in Hamburg, Hamburg, pp 1–18. https://archive.org/stream/ergebnissede118961907hamb#page/n385/mode/2up
Negri A, Burns K, Boyle S, Brinkman D, Webster N (2006) Contamination in sediments, bivalves and sponges of McMurdo Sound, Antarctica. Environ Pollut 143:456–467
Nory RM (2018) Identificação de fontes de contaminação urbana em poeiras de túneis da cidade de São Paulo por meio de caracterização elementar e isotópica. 2018:185 (Master Degree in Nuclear Technology) – Instituto de Pesquisas Energéticas e Nucleares – IPEN-CNEN/SP. São Paulo. Available in: http://www.teses.usp.br. Access: May 2019.
Ribeiro AP, Figueira RCL, Martins CC, Silva CRA, França EJ, Bícego MC, Mahiques MM, Montone RC (2011) Arsenic and trace metal contents in sediment profiles from the Admiralty Bay, King George Island, Antarctica. Mar Pollut Bull 62:192–196
Santos IR, Silva Filho EV, Schaefer EGR, Albuquerque Filho MR, Campos LS (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar Pollut Bull 50:85–194
Santos IR, Silva Filho EV, Schaefer C, Sella SM, Silva CA, Gomes V, Passos MJdeACR, Ngan PV (2006) Baseline mercury and zinc concentrations in terrestrial and coastal organisms of Admiralty Bay, Antarctica. Environ Pollut 140:304–311
Seo D, Vasconcellos MBA, Saiki M, Catharino MGM, Moreira EG, Sousa ECPM (2013) Evaluation of the levels of Br, Cl, K, Mg, Mn and V in Perna perna mussels (LINNAEUS, 1758: MOLLUSCA, BIVALVIA) collected in coast of São Paulo, Brazil. International Nuclear Atlantic Conference. Recife. https://inis.iaea.org/collection/NCLCollectionStore/_Public/45/084/45084475.pdf?r=1
Shinagawa EH (2016) Avaliação de teores de metais e arsênio na Estação Antártica Comandante Ferraz após incêndio de 2012. pp.116 (Master degree in Oceanografia química) Instituto Oceanográfico, Universidade de São Paulo, São Paulo. https://www.teses.usp.br/teses/disponiveis/21/21137/tde-20022017-165501/publico/Dissertacao_Shinagawa_Edgar_Corrigida.pdf
Skoog DA, West DM, Holler FJ, Crouch SR (2014) Fundamentals of analytical chemistry, 9th edn. Brooks/Cole, Belmont
Trevisani TH, Pettia MAV, Ribeiro AP, Corbisier TN, Figueira RCL (2018) Heavy metal concentrations in the benthic trophic web of Martel Inlet, Admiralty Bay (King George Island, Antarctica). Mar Pollut Bull 130:198–205
Zhang ZS, Lu XG, Wang QC, Zheng DM (2009) Mercury, cadmium and lead biogeochemistry in the soil–plant–insect system in Huludao City. Bull Environ Contam Toxicol 83:255–259. https://doi.org/10.1007/s00128-009-9688-6
Acknowledgements
This study was part of the PROANTAR project, process: 421728/2017-5, entitled “Isolation and comparison of the echinochromes of tropical and Antarctic sea urchins and their potential pharmacological and therapeutic applications” in partnership with the Evolutionary Histophysiology Laboratory, Department of Cellular Biology and Development, belonging to the Biomedical Sciences Institute, at the University of São Paulo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alves, M.B., Emerenciano, A.K., da Costa Bordon, I.C.A. et al. Biomonitoring Assessment of Toxic and Trace Elements in Sterechinus neumayeri Sea Urchins from the Comandante Ferraz Station in Antarctica. Bull Environ Contam Toxicol 107, 11–19 (2021). https://doi.org/10.1007/s00128-021-03307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03307-3