Abstract
The soil samples of old Zawar mine sites were sandy texture, basic, electric conductivity range from 16 to 59 dSm−1 with a high content of heavy metals of Zn, Pb, Cd, and Fe, indicating poor soil-health. Two bacterial isolates Pseudomonas aeruginosa HMR1 and P. aeruginosa HMR16 (GenBank-accession-number KJ191700 and KU174205, respectively), differed in the Phylogenetic tree based on 16S-rDNA sequences. HMR1 isolate showed the high potential of Plant growth-promoting attributes like IAA, Phosphate-solubilization, Exopolysaccharide production, and Proline activities at high concentration of Zn augmented nutrient media after 24 h, followed by HMR1 + HMR16 and HMR16. Both isolates were survived at 100 ppm Zn (w/v) concentration, followed by Pb, Cd, and Fe. Linear RL value from Langmuir and Freundlich isotherms revealed that the suitable condition of Zn adsorption by HMR1 was at pH8 with 40°C. The value of r2 from pseudo-second-order kinetics and Transmission-Electron-Microscopic analysis confirmed Zn adsorption by HMR1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Heavy metal pollution is mainly a consequence of the speedy growth of industrialization, intensive use of the chemical in agriculture, mining (El-Meihy et al. 2019), fly ash deposits (Pandey 2020) including geogenic sources (Paul et al. 2020a). Toxic metals act as a brutal factor for the entire ecosystem, soil health, and human health via contaminating agricultural products (Wuana and Okieimen 2011; Dwivedi et al. 2013; Bolan et al. 2014). The extraction of economically valuable minerals for the development of human beings creates pressure on mining activity. Simultaneously, targeted minerals, other toxic heavy metals (THM) are emerging on the land surface (El-Baz et al. 2015; El-Meihy et al. 2019). The agency for toxic substance and disease registry listed the priority of hazardous compounds in the year 2019 and reported that arsenic is at first number followed by lead, while cadmium, cobalt, and zinc were at 7, 51, and 75 numbers respectively (Glick 2015). Mitigation of THM from soil has gain attention across the globe, and the mechanism for its removal is based on physical, chemical, and biological processes (Pandey and Singh 2019; Upadhyay et al. 2021). An eco-friendly and sustainable approach is a promising remarkable tract to the mitigation of THM mediated by microbes (Gupta and Diwan 2017; Kour et al. 2019). Microbes mediated THM removal has a distinguished mechanism like extracellular accumulation/precipitation, cell surface adsorption, and intracellular accumulation (Singh et al. 2011; Singh and Srivastava et al. 2016; Paul et al. 2020b). Biosorption of heavy metals depends on various criteria such as the concentration of bacterial biomass, initial metal concentration, temperature, pH, types (essential and non-essential) and retention time, etc. (Fathollahi et al. 2021). Microbes, such as bacteria, have the potential to produce exopolysaccharides (EPS), and other biochemical compounds can play a significant role in the biosorption of THM (Upadhyay et al. 2011; Gupta and Diwan 2017). Removal of THM also occurs through cell-sorption by living or dead microbes (Igwe and Abia 2006). Soil bacteria can produce different kinds of Phyto-stimulating hormones such as indole-3-acetic acid (IAA), which enhances metabolism for growth and adaptation, even under adverse conditions (such as heavy metal, salinity, drought, acidic pH) (Upadhyay and Singh 2014; Upadhyay et al. 2019). The beneficial bacterial species can produce phytohormones and organic acids (Upadhyay et al. 2009). The phytohormone and organic acids can induce plant-growth performance through phytostimulation, nutrient mobilization, mineralization (Kour et al. 2019). Organic acids of bacteria also sequestrate THM via rising mobilization of the element through altering the pH and redox reaction (Osmolovskaya et al. 2018). Native bacterial species have coping mechanisms against the surrounding environment in old mining sites, having potential survivability under heavy metal concentration and plant growth-promoting (PGP) attributes (Mishra et al. 2017). Therefore, the aims of the present work are (i) the screening of the potential heavy metal removal native bacterial isolate, (ii) PGP-attributes, and (iii) confirmation of heavy metal removal by adsorption kinetics.
Material and Methods
Soil samples (100 gm) collected in an autoclaved plastic bag from old mining sites of Zawar mine, [Latitude-24.3540034; Longitude-73.733064] Udaipur, India, during month of June and September, 2019. Soil-texture, water holding capacity, bulk density (BD), particle density (PD), pH, and ECe were analyzed as earlier described (AFNOR 1996; Upadhyay et al. 2012; Upadhyay et al. 2021). Soil samples were oven-dried at 600°C in a muffle furnace and sieved through a 2 mm governorates sieve. 0.5gm of the dried sieved sample digested with 1:3:3 ratios of concentrated HNO3, H2SO4, and H2O2, respectively (Saison et al. 2004). The volume of the digested sample maintained by distilled water with gentle heating, and samples were analyzed for heavy metals (Zn, Pb, Cd, and Fe) by atomic absorption spectrophotometer (AAS, Ecil Model-4141) as described earlier (Diwedi et al. 2014; Paul et al. 2014; Srivastava et al. 2014). The samples were analyzed for heavy metals with calibration of AAS by respective heavy metal solution. The experiment was repeated three times for each analysis.
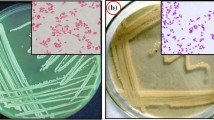
Two bacterial isolates, HMR1, and HMR16 were taken in the present study, which was isolated from soil samples of old mining site of Zawar mine on Nutrient agar medium (Bhojiya and Joshi 2012) and demonstrated its morphological and biochemical characters (Bhojiya and Joshi 2015). The isolates were routinely revived on Nutrient agar medium at 37 ± 2°C and stored at − 20°C in glycerol. 16S-rDNA partial sequence of bacterial isolates analyzed through alignment by using the on-line BLAST tool of GenBank database. The 16S-rDNA-sequences submitted to GenBank with accession number KJ191700 (Pseudomonas aeruginosa) and KU174205 (Pseudomonas aeruginosa) for HMR1 and HMR16 bacterial isolates, respectively. The 16S-rDNA-sequences, subjected to the CLUSTAL-W program (Boratyn et al. 2013), and the evolutionary distance pattern computed using the method of Maximum Composite Likelihood (Tamura et al. 2004). The phylogenetic tree, designed by MEGA-X (Kumar et al. 2018).
Both the isolates were analyzed for their PGP-attributes such as IAA, Phosphate-solubilizing, EPS-production, and Proline activity (µg mg−1 protein) as earlier described (Upadhyay et al. 2011, 2019). The bacterial isolate inoculates in a nutrient broth medium supplemented with heavy metals. 1 mL of supernatant mixed with 2 mL of glacial acetic acid and ninhydrin for 1 h at 100°C, 4 mL of toluene added after cooling, and absorbance was recorded at 520 nm to estimate Proline activity. Isolates were screened for heavy metals (Zn, Pb, Cd, Fe) resistance on nutrient medium (as earlier described method for salinity; Upadhyay et al. 2009) supplemented with different concentration (25, 50, 75, and 100 ppm) of ZnSO4·7H2O, Pb (NO3)2, Cd (NO3)2·4H2O), FeSO4·7H2O, respectively, on the plate (Teclua et al. 2008; El-Baz et al. 2015; Alnaimat et al. 2017). The isolate HMR1 chosen for further study based on their maximum PGP attributes and high tolerance against heavy metals behavior.
Adsorption of zinc by isolate HMR1 was studied through the isotherm model of Langmuir and Freundlich. Kinetics of the sorption process observed by Langmuir isotherm and the amount of heavy metal uptake evaluated as a mathematical Eq. (1) (Meitei and Prasad 2014).
where, q = amount of zinc uptake (mg/g); V = volume of zinc solution; Ci = initial concentration of zinc; Cf = final concentration of zinc; S = amount of bio-sorbent (g).
The Langmuir isotherm in terms of linear form calculated by plotting 1/q vs 1/Cf. The kinetic deviation between adsorbed and desorbed molecule can be evaluated by Eq. 2
where qe = amount of adsorbate adsorbed per unit amount of adsorbent at equilibrium; KA = adsorption coefficient (a measure of adsorption energy); qm = limiting the adsorbing capacity of adsorbent in monolayer adsorption. The linear form of Eq. 2 is representing as Eq. 3.
where Ce = equilibrium concentration (mg L−1); qe = amount adsorbed per gram of adsorbent at equilibrium (mg g−1); qmax = Langmuir constant related to the maximum adsorption capacity, and b is the energy of adsorption. The value of qmax and b obtaining by intercept and slope.
Equilibrium parameter observed as separation factor (RL) calculated as Eq. 4 (Hall et al. 1966). Favorable isotherm sorption represents the range of RL from 0 to 1 and RL = 0 refers to the irreversible adsorption process.
Freundlich isotherm evaluates to observe the effectiveness of adsorbent mediated free energy change and derived Eq. 5.
where, qe = amount adsorbed (mg g−1); Ce = equilibrium concentration (mg L−1); Kf = adsorption coefficient. The model can be linearized logarithmically as Eq. 6. Thus, a straight line plot exists between log qe and log Ce. Kf and n value calculated from the slope and intercept of the line graph.
The Zn-adsorption of the HMR1 mechanism was analyzed with the experimental results, with pseudo-first and pseudo-second-order kinetic models, which can imply representing by the following Eq. 7.
Integrating the above equation and applying boundary conditions, t = 0 to t = t and qt = 0 to qt = qe, the equation becomes Eq. 8.
where, qe and qt = adsorption capacity at equilibrium and at time t, respectively (mg g−1); K1 = rate constant of pseudo-first-order adsorption (min−1). The constant of pseudo-first-order kinetics obtained from the plot between \(\mathrm{log}( {q}_{e}-{q}_{t}\)) vs t. The value of intercept is equal to the log qe.
The pseudo-second-order assumes that the rate of sorption is proportional to the square of the number of unoccupied sites (Ho 2006), which can represent as equation-9.
Integrating the Eq. 9 (t = 0, q = 0, and t = t, q = qt) yields the following expressions as Eq. 10.
where K2 = equilibrium rate constant of the pseudo-second-order model (g mg−1 min−1). The slope and intercept of the plot t/q vs t were used for the analysis of qe and K2, respectively.
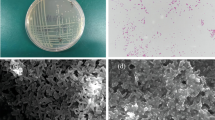
The bacterial isolate was incubated for 48 h in Zn supplemented medium and harvested by centrifugation (1000 rpm for 5 min) and washed two times with phosphate saline buffer (pH 7.2). The harvested cells were subjected to Zn-localization via TEM (Tecnai 20, Philips, Holland) as per the methods (Jain et al. 2020a). Bacterial cells were grown in a medium without Zn taken as control.
Results and Discussion
The Physico-chemical analysis of soil revealed that all soil-samples were sandy, pH range from 7.4 to 8.2, BD from 1.22 to 1.88, PD from 1.88 to 2.46, ECe (dSm−1) from 16 to 59 (Table S-1). Zn, Pb, Cd, and Fe observe in the soil samples (mg/Kg) with range1397 to 1876, 1005 to 1629, 100 to 261, and 3201 to 3865, respectively (Table S-1). This result indicates that the soil is less suitable for crop cultivation, so there is an urgent need to minimize the soil’s brutal effects. Keeping these views, the suitable native bacterial isolates HMR1 and HMR16 further examined for their natural tolerance mechanism against heavy metals. The kinds of the literature suggested that the significant performance of native bacterial isolates for mitigation of environmental problems such as detoxification of xenobiotic compounds (Singh et al. 2015) and THM adsorption (Mishra et al. 2017; Tripathi et al. 2018).
In the present study, HMR1 and HMR16 survived with higher Zn-concentrations than Pb, Cd, and Fe (data not shown). P. putida was screened for their heavy metals (Zn+2, Cd+2, Co+2, Ni+2, Cu+2, and Pb+2) tolerance as reported earlier (Bhojiya and Joshi 2016a). Similarly, salinity tolerance rhizobacteria screened for their salt [NaCl (w/v)] tolerance as reported earlier (Upadhyay et al. 2009; Upadhyay and Singh 2014). On the other hand, both the isolates and their consortium (HMR1 + HMR16) exhibits PGP-attributes like IAA production, P-Solubilization, EPS-production, and Proline activity. IAA is an essential PGP-hormone, which regulates plant growth-performance, and also it works in a stressful environment (Upadhyay et al. 2019). The P-solubilizing microbes, releasing organic acids and mobilizing or mineralizing trapped phosphate and minerals in soil (Bhojiya and Joshi 2016b). The rhizobacteria such as Pseudomonas, Bacillus, Arthobacter, etc., reported for their P-solubilizing activity in different soil-nature (Upadhyay et al. 2012; Jain et al. 2020b). The maximum PGP-attributes and Proline activity observed in HMR1 followed by the consortium (HMR1 + HMR16) and HMR16 at a higher concentration of Zn (Table 2). 27% rise in IAA (µg mg−1 protein) and 80% in Proline activity (µg mg−1 protein) was recorded in HMR1 at 100 and 75 ppm zinc concentration (w/v), respectively (Table1). Proline mediates coping mechanisms against the stressful environmental condition in the living organism, like in PGPR (Upadhyay et al. 2019) and plants (Dwivedi et al. 2014 and Paul et al. 2014). The EPS is a gift of nature for several organisms to perform various functions. The EPS-producing bacteria can enhance their intake or bind metal (Upadhyay et al. 2011; Gupta and Diwan 2017). Perez et al. (2008) demonstrated that the THM were significantly bio-adsorbed by EPS-producing Paenibacillus jamilae. 16S-rDNA sequencing revealed that both the isolates were Pseudomonas aeruginosa but differed in their sequence as observed in the phylogenetic tree (Fig. 1). Jain et al. (2020b) demonstrated that Zn tolerant PGPR mitigates Zn-toxicity in maize plant. Similarly, salt (NaCl) tolerant PGPR alleviates salinity stress in the wheat plant (Upadhyay et al. 2012). Based on high PGP-attributes and survivability against high Zn-concentration, HMR1 uses for further adsorption study.
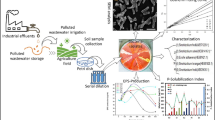
The analyzed experimental results for adsorption isotherm to explain the sorption process mechanism of the bacterial isolate. Isotherm kinetics for Zn adsorption by HMR1 at fixed pH (pH 6, pH 7, and pH 8) with different temperatures (36, 38, and 40°C) illustrated in Fig. 2. Among different pH and temperature conditions, the maximum adsorption capacity (mg/g) (qmax) founds with Langmuir isotherm, which was 263.15 mg/g at pH 6 and temperature 36°C. In the present study, the value of least square regression for this isotherm reported 0.97, which represents a good fit of Langmuir isotherm (Table S-2). Significant adsorption capacity (217.39 mg/g) was observed at pH 7 with temperature 36°C, while the adsorption capacity declined at temperatures 38°C and 40°C i.e. 125 mg/g. The lowest value of qmax (mg/g) i.e. 57.8 mg/g was found for Zn adsorption at pH 8 with 40°C. The constant b obtained from the Langmuir isotherm explains the affinity of heavy metals towards the sorbent. The maximum value of constant b (0.009), obtained at pH 6 with the temperature of 40°C shows the highest affinity towards the Zn-sorption. The monolayer biosorption consists of sorption of sorbate on a fixed number of identical sites and can represent through a linear model of Langmuir isotherm.
This model considers sorption as a chemical phenomenon. The shape of the Langmuir isotherm can expressed by calculating the RL factor (Separation factor). The RL value assumes the nature and feasibility of the sorption process and shows the significance of the reaction. If RL > 1, it is unfavorable, if RL = 1, it is linear, if 0 < RL < 1 it is favorable, and it is irreversible if RL = 0. The RL value obtained from Langmuir isotherm (Table S-3) for pH 6, pH 7, and pH 8 at different temperatures suggests that the adsorption process is favorable in all experimental conditions. It is linear (RL = 1) at pH 8 for 75 ppm and 100 ppm at 38°C, and RL = 1 also obtained for 50 ppm, 75 ppm, 100 ppm at 40°C, a similar approach was earlier reported by Wang et al. (2012). The Freundlich isotherm is applicable for the adsorption process, which occurs at the heterogeneous surface (Ayawei et al. 2015). This isotherm explains the surface heterogeneity, the exponential distribution of active sites, and their energies (Ayawei et al. 2015). The value of the Freundlich constant i.e. Kf and n estimate the adsorption-capacity and adsorption-intensity, respectively. The value of Kf and n based on the linear form of the Freundlich equation. The value of constant n found in a range of 1 to 2.43 in the present experimental study shows significant Zn-adsorption by HMR1. The value of constant n > 1 may be due to the heterogeneity in the distribution of active sites on the surface or any factors, which shows a fall in the adsorbent-adsorbate interaction (McKay et al. 1980). The Kf value was found maximum for adsorption at pH 6 on temperature 36°C, however, the least square regression value found 0.55 for the same, which indicates the lack of fit for Freundlich isotherm. On the other hand, at pH 8 with 40°C, the Kf and least square regression value found 13.18 mg/g and 0.99, which represented the most suitable adsorption process in agreement with Freundlich isotherm.
The experimental data of Zn-adsorption by HMR1 fitted to the pseudo-first-order and pseudo-second-order kinetics expressions (Table 2). The value of constant K1 for Zn-adsorption at pH 6 with temperature 36°C found to the maximum for initial Zn concentration of 75 ppm. The r2 value of 0.80 suggests that it is appropriate to use the pseudo-first-order kinetic expression to represent the sorption of Zn by HMR1. For Zn adsorption at pH 6 with temperature 38°C, the maximum value of K1 was found 0.09, while at 40°C the maximum value of K1 was found with an initial Zn concentration of 25 ppm (Fig. S-1). The lower r2 value for Zn adsorption at 38°C and 40°C suggests that it is inappropriate to use the pseudo-first-order-kinetic expression. A pseudo-first-order-kinetic expression for Zn adsorption at pH 7 was found appropriate at 40°C for an initial Zn concentration of 25 ppm (Fig. S-2). The r2 value from pseudo-first-order-kinetic expression at pH 8 for each reaction’s temperature and initial Zn concentration was found in a range of 0.70 to 0.018, which does not recommend this kinetic expression to represent sorption of Zn by HMR1 (Fig. S-3). Pseudo-second-order kinetic expression represents the multi-step sorption process that includes an initial rapid phase and the later slower phase that proceeds towards saturation (Kumar 2006). For the present experimental study pseudo-second-order rate, constant K2 was found to decrease with an increase in the initial concentration of Zn. It is attributed due to the multi-step sorption process that involves the initial rapid phase and later slower phase that proceed toward saturation. The higher r2 value of pseudo-second-order kinetics at different pH and temperature conditions confirms its applicability to express the adsorption of Zn by HMR1.
In the present study, TEM analysis revealed similar observation as isotherm and kinetics study, isolate HMR1 can remove Zn with high concentration through both adsorption and absorption mechanism as shown in Fig. 3. Transmission Electron Micrographs of P. aeruginosa strain HMR1 taken before and after the zinc treatment experiments established the presence of zinc deposits on the bacterial surface and inside the cells. Bacteria exhibit different tolerance mechanisms against heavy metal stress. The distribution of heavy metals in bacterial cells is also dependent on the type of mechanism to combat heavy metal stress. In the present study, the distribution of Zn within the cells and on the cell surface of P. aeruginosa may indicate that zinc forms a metal complex intracellularly, and also the heavy metal is regulated by ion efflux pumps present in the cell surfaces.
Conclusion
Mining activities are essential to fulfill the demands of natural resources across the globe. After harvesting natural resources from mining sites have less useful for their economic concern. Lands of old mining sites can apply to forestry, agricultural purposes, and ornamental plants. But with the higher concentration of THM become a challenge to utilized old mining sites. To combat this issue, an eco-friendly and sustainable approach based on the applications of suitable microbes that remove THM as well exhibits PGP-attributes. In the present study, P. aeruginosa- HMR1 isolate showed IAA-production, P-solubilization, EPS-production, and Proline activities with higher Zn-concentration. HMR1 showed the maximum amount of Zn adsorption at pH 8 with 40°C, revealed by Langmuir and Freundlich isotherms. Pseudo-second-order kinetics and TEM analysis confirmed the adsorption of Zn by HMR1. Thus, the present finding demonstrates the P. aeruginosa-HMR1 can be beneficial for the restoration of land for agricultural purposes.
References
AFNOR (1996) Quality of soil. Soils, sediments, mise en solution Totale par Attaque Acide. AFNOR, Paris
Alnaimat S, Shattal SA, Althunibat O, Alsbou E, Masha R (2017) Iron (II) and other heavy-metal tolerance in bacteria isolated from rock varnish in the arid region of Al-Jafer basin, Jordan. Biodiversitas 18(3):1250–1257. https://doi.org/10.13057/biodiv/d180350
Ayawei N, Ekubo AT, Wankasi D, Dikio ED (2015) Adsorption of congo red by Ni/Al-CO3: equilibrium, thermodynamic and kinetic studies. Orient J Chem. https://doi.org/10.13005/ojc/310307
Bhojiya AA, Joshi H (2012) Isolation and characterization of zinc tolerant bacteria from Zawar Mines Udaipur, India. Int J Env Engg Manag 3(4):239–242
Bhojiya AA, Joshi H (2015) Identification of Pseudomonas using Probabilistic identification of Bacteria (PIB) Software. Res J Recent Sci 4:14–18
Bhojiya AA, Joshi H (2016a) Heavy metal tolerance pattern of Pseudomonas putida Isolated from heavy metal contaminated soil of Zawar, Udaipur (India). Int J Innov Knowl Concepts 4(1):58–64
Bhojiya AA, Joshi H (2016b) Study of potential plant growth-promoting activities and heavy metal tolerance of Pseudomonas aeruginosa HMR16 Isolated From Zawar, Udaipur. India Curr Trends Biotechnol Pharm 10(2):161–168
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T et al (2014) Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J Hazard Mater 266(4):141–166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Boratyn GM (2013) BLAST: a more efficient report with usability improvements. Nucl Acids Res 41(W1):W29–W33. https://doi.org/10.1093/nar/gkt282
Dwivedi GK, Upadhyay SK, Mishra AK, Singh AK (2013) Hyper accumulation of cadmium in Phyllanthus amarus L. A medicinal plant. Ind J Life Sci 3(1):21–26
Dwivedi GK, Upadhyay SK, Mishra AK, Singh AK (2014) Hyper accumulation of cadmium in Solanum nigrum L. and their effects on phyto-chemicals and antioxidant enzymatic activities. Int J Pharm Sci Res 5(4):1424–1430. https://doi.org/10.13040/IJPSR.0975-8232.5(4).1424-30
El-Baz S, Baz M, Barakate M, Hassani L, El Gharmali A, Imziln B (2015) Resistance to and accumulation of heavy metals by actinobacteria isolated from abandoned mining areas. Sci World J 2015:761834. https://doi.org/10.1155/2015/761834
El-Meihy RM, Abou-Aly HE, Youssef AM, Tewfike TA, El-Alkshar EA (2019) Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ Exp Bot 162:295–301. https://doi.org/10.1016/j.envexpbot.2019.03.005
Fathollahi A, Khasteganan N, Coupe SJ, Newman AP (2021) A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 268:129290. https://doi.org/10.1016/j.chemosphere.2020.129290
Glick BR (2015) Beneficial plant–bacterial interactions. Springer, Cham
Gupta P, Diwan B (2017) Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5(2):212–223. https://doi.org/10.1021/i160018a011
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(3):681–689. https://doi.org/10.1016/j.jhazmat.2005.12.043
Igwe JC, Abia AA (2006) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:1167–1179. https://doi.org/10.4314/ajb.v5i11.43005
Jain D, Kour R, Bhojiya AA, Meena RH, Abhijeet S, Ranjan MS, Deepak R, Ameta KD (2020a) Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci Rep 10:13865. https://doi.org/10.1038/s41598-020-70846-w
Jain D, Shivani, Bhojiya AA, Singh H, Daima HK, Mohanty SR, Stephen BJ, Singh A (2020b) Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front Chem 8:778. https://doi.org/10.3389/fchem.2020.00778
Kour R, Jain D, Bhojiya AA, Sukhwal A, Sanadhya S, Saheewala H, Jat G, Singh A, Mohanty SR (2019) Zinc biosorption, biochemical and molecular characterization of plant growth promoting zinc tolerant bacteria. 3 Biotech 9(421):1–17. https://doi.org/10.1007/s13205-019-1959-2
Kumar VK (2006) Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J Hazard Mater 137(3):1538–1544. https://doi.org/10.1016/j.jhazmat.2006.04.036
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
McKay G, Otterburn MS, Sweeney AG (1980) The removal of colour from effluent using various adsorbents-III. Silica: rate processes. Water Res 14(1):15–20. https://doi.org/10.1016/0043-1354(80)90037-8
Meitei MD, Prasad NV (2014) Adsorption of Cu (II), Mn (II) and Zn (II) by Spirodela polyrhiza (L.) Schleiden:equilibrium, kinetic and thermodynamic studies. Ecol Eng 71:308–317. https://doi.org/10.1016/j.ecoleng.2014.07.036
Mishra J, Singh R, Arora NK (2017) Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front Microbiol 8:1706. https://doi.org/10.3389/fmicb.2017.01706
Osmolovskaya N, Dung VV, Kuchaeva L (2018) The role of organic acids in heavy metal tolerance in plants. Bio Comm 63(1):9–16. https://doi.org/10.21638/spbu03.2018.103
Pandey VC (2020) Fly ash properties, multiple uses, threats, and management: an introduction. In: Pandey VC (ed) Phytomanagement of fly ash. Elsevier, Amsterdam, pp 1–34. https://doi.org/10.1016/B978-0-12-818544-5.00001-8
Pandey VC, Singh V (2019) Exploring the potential and opportunities of recent tools for removal of hazardous materials from environments. In: Pandey VC, Bauddh K (eds) Phytomanagement of polluted sites. Elsevier, Amsterdam, pp 501–516. https://doi.org/10.1016/B978-0-12-813912-7.00020-X
Paul S, Upadhyay SK, Eugenia LP (2014) Accumulation of arsenic in radish (Raphanus sativus L.), and their effects on growth and antioxidant activities. Int J Pharm Sci Res 5(8):3536–3543. https://doi.org/10.13040/IJPSR.0975-8232.5(8).3536-43
Paul S, Singh V, Chauhan PK, Srivastava AK, Upadhyay SK (2020a) Assessment of carrot growth performance with inoculation of AsT-PGPR under arsenic infested zone. G- J Environ Sci Technol 7(6):78–84
Paul S, Upadhyay SK, Singh N (2020b) Geogenic source of arsenic and their effect on vegetable seed germination. Trop Plant Res 7(1):110–116. https://doi.org/10.22271/tpr.2020.v7.i1.015
Perez JAM, Garcıa-Ribera R, Quesada T, Aguilera M, Ramos-Cormenzana A, Monteoliva-Sanchez M (2008) Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J Microbiol Biotechnol 24:2699–2704. https://doi.org/10.1007/s11274-008-9800-9
Saison C, Schwartz C, Morel JL (2004) Hyperaccumulation of metals by Thlaspi caerulescens as affected by root development and Cd-Zn/Ca-Mg interactions. Int J Phyto 6:49–61. https://doi.org/10.1080/16226510490439981
Singh MP, Srivastava AK (2016) Decolorization of synthetic textile dye and enzymes production by improved strains of Pleurotus species. Cell Mol Biol 62:145
Singh JS, Pandey VC, Singh DP (2011) Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric Ecosyst Environ 140:339–353. https://doi.org/10.1016/j.agee.2011.01.017
Singh G, Upadhyay SK, Singh MP (2015) Dye-decolorization by native bacterial isolates, isolated from sludge of carpet industries Bhadohi-India. GJ Environ Sci Technol 2(6):81–85
Srivastava AK, Vishwakarma SK, Pandey VK, Singh MP (2014) Direct red decolorization and ligninolytic enzymes production by improved strains of Pleurotus using basidiospore derived monokaryons. Cell Mol Biol 60(5):15–21
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101(30):11030–11035. https://doi.org/10.1073/pnas.0404206101
Teclu D, Tivchev G, Laing M, Wallis M (2008) Bioremoval of arsenic species from contaminated waters by sulphate-reducing bacteria. Water Res 42:4885–4893. https://doi.org/10.1016/j.watres.2008.09.010
Tripathi M, Upadhyay SK, Kaur M, Kaur K (2018) Toxicity concerns of hexavalent chromium from tannery waste. J Biotechnol Bioeng 2(2):40–44
Upadhyay SK, Singh DP (2014) Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol 17(1):288–293. https://doi.org/10.1111/plb.12173
Upadhyay SK, Singh DP, Saikia R (2009) Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr Microbiol 59:489–496. https://doi.org/10.1007/s00284-009-9464-1
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21(2):214–222. https://doi.org/10.1016/S1002-0160(11)60120-3
Upadhyay SK, Singh JS, Saxena AK, Singh DP (2012) Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol 14(4):605–611. https://doi.org/10.1111/j.1438-8677.2011.00533.x
Upadhyay SK, Saxena AK, Singh JS, Singh DP (2019) Impact of native ST-PGPR (Bacillus pumilus; EU927414) on PGP traits, antioxidants activities, wheat plant growth and yield under salinity. Clim Change Environ Sustain 7(2):157–168
Upadhyay SK, Ahmad M, Srivastava AK, Abhilash PC, Sharma B (2021) Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 267:129216. https://doi.org/10.1016/j.chemosphere.2020.129216
Wang X, Guo Y, Li Y, Han M, Zhao J, Cheng X (2012) Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J Environ Anal Toxicol 2:7. https://doi.org/10.4172/2161-0525.1000154
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 1:1–6. https://doi.org/10.5402/2011/402647
Acknowledgements
The first author is grateful for utilizing the facilities of Mewar University, India, and the authors are thankful to the Sophisticated Instrumentation Centre for Applied Research and Testing—SICART, Sardar Patel Centre for Science and Technology, Gujarat, India for providing equipment and technical supports. Dr. Upadhyay, acknowledges the Department of Environmental Science, V.B.S. Purvanchal University for providing facilities.
Author information
Authors and Affiliations
Contributions
AAB: Conceptualization, Visualization, Methodology, Formal analysis, Writing-original draft. HJ, SKU & VVP: Result and Discussion, Writing-original draft. DJ, SKU, AKS & VVP: Analyzed data. VCP: Review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that have appeared to influence the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhojiya, A.A., Joshi, H., Upadhyay, S.K. et al. Screening and Optimization of Zinc Removal Potential in Pseudomonas aeruginosa-HMR1 and its Plant Growth-Promoting Attributes. Bull Environ Contam Toxicol 108, 468–477 (2022). https://doi.org/10.1007/s00128-021-03232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03232-5