Abstract
Biosorption has gained increased attention as a reliable and proven technology for the remediation of industrial effluents rich in chromium. The present study was planned to isolate potential fungi from effluents contaminated sites and assess their efficiency for the absorption and reduction of chromium. Two species of Aspergillus and a species of Trichoderma which were isolated from contaminated sites and exhibited resistance to 10 mM of chromium on agar were chosen for the study. A biosorbent was designed by growing these fungal isolates on luffa sponge under shaken condition. The absorption and reduction of chromium, by the designed biosorbent was determined by Atomic Absorption Spectrophotometry and UV Visible Spectrophotometer. Actively growing fungi on luffa sponge showed better absorption (21%–25%) and reduction (28%–35%) capacity when compared to heat killed biosorbent in all fungi tested within 24 h of incubation. Interestingly, there was a liner increase in the absorption and reduction (85%–100%) of chromium by the biosorbent designed by using A. niger.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chromium being one of the toxic heavy metals which is often used as a raw material in various industries such as electroplating, tanning, metal finishing, petroleum refining, wood processing, nuclear power plant, textile and automobile parts (Pal 1997; Smith et al. 2002). Even though chromium exists in various valence state, the most stable ones in the environment are in the form of trivalent (Cr-III) and hexavalent (Cr-VI) (Smith et al. 2002). Chromium in its trivalent form serves as a micro nutrients in human diet and is non-toxic, whereas, hexavalent form is reported for its toxicity having mutagenic, teratogenic and carcinogenic effect on human (Marsh and McInerney 2001). Removal of hexavalent chromium from the environment or its reduction to less harmful trivalent form is very much essential in the present scenario.
Conventional methods such as ion exchange, precipitation, chemical reduction, electrolysis have been employed to reduce the Cr (VI) concentration in the effluent. Although, these techniques have proven effective in removing chromium ions, they have their own significant disadvantages such as high cost, high energy requirement, and generation of secondary sludge which are often difficult to dewater, and these techniques might not work effectively as the concentration of metal ions falls below parts per million range (Brady and Tobin 1995).
Hence, there is always a need for the development of a low cost, effective and ecofriendly method to remediate the heavy metal pollution in the environment. In recent days, biosorption by microbes has been suggested to be a potential alternative method to the conventional techniques for the removal of chromium (Bishnoi and Garima 2005). Among the microorganisms, fungi have gained much interest, due to its large surface area and cellular composition which play a significant role in metal binding (Smily and Sumithra 2017). Fungi being fragile, its application in industries are limited. These limitations are overcome by entrapping the fungal biomass within an inert material. In recent days, application of natural agro-waste material, the luffa sponge (the dried fruit of Luffa cylindrica), is in limelight to immobilize fungal hyphae (Iqbal and Edyvean 2007) due its fibrous network.
The present study reports the efficiency of Aspergillus niger, Aspergillus terreus and Trichoderma longibrachiatum immobilized on luffa sponge, in absorbing and reducing hexavalent chromium.
Materials and Methods
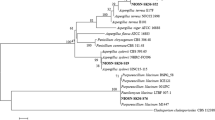
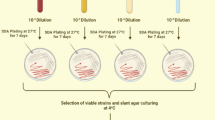
Soil, water and sewage samples were collected from various metal contaminated sites across South India (Hemambika et al. 2011). For the initial isolation of chromium resistant fungi, the collected samples were screened on Potato Dextrose Agar (PDA) medium amended with 5 mM of chromium (K2Cr2O7- Sigma), by serial dilution and standard spread plate methods (Shazia et al. 2013). These plates were incubated at 28°C for 6 days. Fungi grown on these plates had tolerance to chromium up to 5 mM. Resistant fungal isolates were identified both by characterizing the morphology, microscopic structures and further confirmed by molecular methods by gene sequencing the ITS regions of the ribosomal DNA (rDNA) (Iskandar et al. 2011).
The isolates exhibiting resistance to 5 mM of chromium were further tested for their metal tolerance towards higher concentration of chromium (5–50 mM) on PDA plates by agar well diffusion method (Hemambika et al. 2011). The inoculated plates were incubated at 28°C for 6 days. The fungal isolates grown on these plates were considered to be tolerant to higher concentration of chromium and preserved for future use.
The selected fungal isolates were mass cultured by inoculating them in Potato Dextrose Broth and growing under shaken condition for 4 days at 37°C. The fungal mat separated and repeatedly washed with deionized water to remove the media content, dried at 60°C for 24 h. Both metabolically active and inactive biomass were used for present study.
The selected fungal isolates were immobilized on luffa sponge (Luffa cylindrica) discs as described by Iqbal and Edyvean (2007). Both metabolically active and inactive immobilized fungal isolates were used to evaluate their absorption and reduction of chromium.
Different concentration of chromium (1–10 mM) were dissolved in 100 mL double distilled water and sterilized. Five grams of free/immobilized and metabolically active/inactive fungal biomass were mixed with sterile metal solution under aseptic condition (Acosta-Rodríguez et al. 2015; Morales-Barrera and Cristiani-Urbina 2008) and incubated for various period of time (1–25 days) at pH 7.0 and temperature 37°C. The total chromium concentration was determined by Hydride Generation Atomic Absorption Spectrophotometry (HG AAS) (Visoottiviseth and Ponviroj 2007) and hexavalent chromium was determined by diphenylcarbazide method (Sukumar 2010).
The small cylindrical column (2 × 30 cm) was packed with 35 g of fresh biomass with the help of sterile distilled water. The air bubbles in the column were removed by passing 25 mL of sterile distilled water through the column (Murthy and Marayya 2011). The fungal biomass in the column was activated by passing through 50 mL of 0.5 mM chromium solution and was allowed to stand overnight. The column was run with 100 mL of 1 mM chromium solution at a flow rate of 3 mL/min. The run down samples were collected and the residual chromium concentration was measured.
The changes in the vibrational frequency of the functional group on the surface of the free and immobilized fungal biomass was determined by Fourier transform infrared spectroscopy (FTIR). Infrared spectra were obtained by placing the homogenous mixture of KBr (1 g) and 2% of biosorbent (before and after biosorption) along with mineral oil in between the KBr plates of Fourier Transformer Infrared Spectrometer- (Perkin Elmer- Spectram two, USA). The obtained spectrum was compared with a reference library to identify the functional groups.
The surface morphology of untreated and chromium treated fungal biomass was observed under Scanning Electron Microscope (SEM- LEICA LEO 440, Germany). About 0.1 g of designed biosorbent (fungi immobilized on luffa sponge) was mounted on double sided tape and placed on a special stage of SEM (X-MAS 20 mm 2, Cambridge, UK). The samples were coated with a thin layer of gold to avoid sputtering. The scanning electron micrograph of the samples was obtained at ×100 magnification.
Data were subjected to Students’s “t-test” using GraphPad Prism 8 statistical program. Difference between means were considered significant at values of P < 0.05.
Results and Discussion
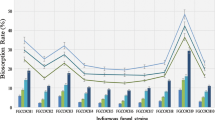
Twenty different fungal isolates belonging to various genera exhibited their resistance towards 5 mM of Chromium during initial round of screening. These isolates were further subjected to second round of screening by increasing the metal concentration. The results clearly showed that three species, namely Aspergillus niger, Aspergillus terreus and Trichoderma longibrachiatum were resistant to 10 mM of chromium (Fig. 1). Ezzouhi et al. (2009) reported that isolates of Penicillium and Aspergillus exhibited resistance to 10–15 mM of chromium on agar medium whereas, Zafar et al. (2007) reported that isolates of Aspergillus were resistant to 6 mM of chromium in their studies.
The absorption and reduction of the chromium by metabolically active/ inactive and free/immobilized fungal biomass were evaluated by mixing with various concentration of chromium solution (1–10 mM). The results clearly indicated that immobilized fungal biomass which is metabolically active was performed better in absorbing and reducing the chromium than the other forms.
The biosorbent prepared with A. niger (immobilized on luffa sponge) was selected for further study as it exhibited better absorption and reduction of chromium when compared to the biosorbent materials prepared with A. terreus and T. longibrachiatum. The biosorbent prepared with A. niger was able to absorb 98.9% of chromium from 1 mM solution of chromium. However, its absorption efficiency was reduced to 29% when increasing metal ions up to 10 mM at 24 h of incubation period (Fig. 2). At lower metal ion concentration in the solution, the metal ions interact with the binding sites present on the cell wall of the biosorbent material facilitating the maximum absorption, whereas at higher concentration more metal ions are left un- absorbed in the solution due to the saturation of the metal binding biosorbent material.
Time plays a crucial role in absorption or reduction of chromium ion by the designed biosorbent. As the metal ion concentration increases to 10 mM from the basal concentration (1 mM) the time taken for its absorption and reduction was also increased. The designed biosorbent effectively reduced hexavalent chromium within 25 days of incubation period. Liu et al. (2007) reported that biosorption of chromium in Mucor racemosus occurred in three different phases. During the first phase, 50% of metal ions were sequestered from a solution of 100 mg/L of chromium. The second phase is the slow process where Cr (III) start to appear in the solution within 1–8 h of contact time in the solution and at last phase the total Cr (VI) were removed completely within 24 h of contact time.
However, in the present study, 50% reduction of chromium was attained within 5 days of incubation period from a solution of 10 mM concentration and further reduction continued in a much slower rate till 25 days (Fig. 3). Morales and Cristiani (2008) isolated Trichoderma inhamatum from tannery effluent, which brought about complete reduction of hexavalent chromium from a solution of 2.4 mM concentration within 8 days of incubation period.
It was observed that, the activated cells were able to absorb 66% of chromium and effectively reduced 31% of hexavalent chromium (Fig. 4). However, Mungasavalli et al. (2007) reported that A. niger immobilized in a polysulfone matrix effectively absorbed 90% of chromium from a solution of concentration 10 mg/L with in 11 min of contact time, which is much lesser than in A. niger isolated in the present study.
The functional groups involved in the absorption of the metal ion was determined by FTIR analysis. The FTIR spectra of the immobilized fungal biomass was obtained before and after the absorption process. The results showed significant variation in the surface area of the functional groups after absorption of chromium.
The band around 3430 cm −1 is indicative of the existence of the –OH and –NH groups, whereas the C=O groups stretching at the band 1500 cm−1 demonstrated the presence of –COOH. The band in 1440 cm−1 is representative of –CH stretching including –CH3 and CH2 functional groups attributed to fatty acids found in fungal membrane phospholipids. The band in the region of 1220 cm −1 is the indication of C–OH bond (Fig. 5). It is well documented that fungal cell wall is composed of chitin molecules- long chain polymer of N-acetylglucosamine. The identified functional groups, (i.e.) –OH, –NH and –CH, in the present study provide further evidence that these groups are among few of the main constituents of N- acetylglucosamine, the main component of fungal cell wall.
The surface morphology of the designed biosorbent and its absorption efficiency was further determined by Scanning electron microscopic study. The micrograph of the untreated designed biosorbent revealed a uniform and luxuriant growth of fungi along the surface of the fibrous network of the luffa sponge (Fig. 6a). However, the surface morphology of the treated biosorbent material was altered with the deposition of chromium on the entire surface of the designed biosorbent material validating the efficacy of absorption of chromium by A. niger grown on luffa sponge (Fig. 6b).
Industrial effluents have been contributed to contaminate environmental components with various heavy metals. Microbes thriving in such components are well adopted with available nutrients by developing a variety of resistance mechanisms. The present study was focused on exploiting such microbes (fungi) having resistance for a heavy metal, chromium, by isolating from various metal-contaminated environmental samples. Among them, a filamentous fungus, Aspergillus niger, was identified as one such potential microbe showing high resistance for chromium. This fungus was exploited further by immobilizing its filamentous mycelium on an agro-waste material, luffa sponge. This intricately designed “biosorbent” showed increased level of absorption and reduction of chromium when compared with that of free fungal biomass at optimized conditions. This laboratory based preliminary work presented here can further be carried forward to treat large scale industrial effluents.
References
Acosta-Rodríguez I, Arévalo-Rangel DL, Cárdenas-González JF, Moctezuma-Zárate M, Martínez-Juárez VM (2015) Hexavalent chromium (VI) removal by Penicillium sp. IA-01. In: Advances in bioremediation of wastewater and polluted soil, vol. 8, pp 165–192.
Bishnoi NR, Garima N (2005) Fungus- an alternative for bioremediation of heavy metal containing wastewater: a review. J Sci Ind Res 64:93–100
Brady JM, Tobin JM (1995) Binding of hard and soft metal ions to Rhizopus arrhizus biomass. Enzyme Microb Technol 17:791–796
Ezzouhri L, Castro E, Moya M, Espinola F, Lairini K (2009) Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier Morocco. Afr J Microb Res 3(2):35–48
Hemambika B, Rani MJ, Kannan VR (2011) Biosorption of heavy metals by immobilized and dead fungal cells: a comparative assessment. J Ecol Nat Environ 3(5):168–175
Iqbal M, Edyvean RGJ (2007) Ability of loofa sponge-immobilized fungal biomass to remove lead ions from aqueous solution. Pak J Bot 39(1):231
Iskandar NL, Zainudin NAIM, Tan SG (2011) Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci 23(5):824–830
Liu T, Li H, Li Z, Xiao X, Chen L, Deng L (2007) Removal of hexavalent chromium by fungal biomass of Mucor racemosus: influencing factors and removal mechanism. World J Microbiol Biotechnol 23:1685–1693
Marsh TL, McInerney MJ (2001) Relationship of hydrogen bioavailability to chromate reduction in aquifer sediments. Appl Environ Microb 67:1517–1521
Morales-Barrera L, Cristiani-Urbina E (2008) Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water, Air, and Soil Pollut 187(1–4):327–336
Mungasavalli DP, Viraraghavan T, Jin YC (2007) Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloid Surf A 301(1–3):214–223
Murthy KSR, Marayya R (2011) Studies on the removal of heavy metal ions from industrial effluents using Ammonium PyrrolidineDithioCarbamate (APDC) loaded polyurethane foams (PUF). World Appl Sci J 12(3):358–363
Pal N (1997) Reduction of hexavalent chromium to trivalent chromium by Phanerochaete chrysosporium. Situ On-site Bioremediat 2:511–517
Shazia I, Uzma GRS, Talat A (2013) Bioremediation of heavy metals using isolates of filamentous fungus Aspergillus fumigatus collected from polluted soil of Kasur Pakistan. Int Res J Biol Sci 2(12):66–73
Smily JRMB, Sumithra PA (2017) Optimization of chromium biosorption by fungal adsorbent, Trichoderma sp. BSCR02 and its desorption studies. Hayati J Biosci 24(2):65–71
Smith WA, Apel WA, Petersen JN, Peyton BM (2002) Effect of carbon and energy source on bacterial chromate reduction. Bioremediat J 6:205–215
Sukumar M (2010) Reduction of hexavalent chromium by Rhizopus oryzae. Afr J Environ Sci Technol 4(7):412–418
Visoottiviseth P, Ponviroj N (2007) Selection of fungi capable of removing toxic arsenic compounds from liquid medium. Sci Asia 27:83–92
Zafar S, Aqil F, Ahmad I (2007) Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol 98(13):2557–2561
Acknowledgements
This work was financially supported by Ministry of Environment, Forest and Climate change (MOEF)- No. 1-39/2012-CT, New Delhi, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sriharsha, D.V., Lokesh Kumar, R. & Janakiraman, S. Absorption and Reduction of Chromium by Fungi. Bull Environ Contam Toxicol 105, 645–649 (2020). https://doi.org/10.1007/s00128-020-02979-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02979-7