Abstract
Concentration and human health risks of heavy metals (Pb, Cu, Cr, Cd, As, Ni) in water, sediment, crab (Tympanotonus fuscatus) and periwinkle (Callinectes amnicola) were assayed in New Calabar River, Nigeria. Results revealed that concentration (mg/L) of the metals in water in the two seasons studied, ranged from 1.1E-2 ± 1.0E-3…for Cd to 2.90 ± 0.03…for Cu. In sediment, the concentration (mg/kg) ranged from 1.1E-2 ± 1.0E-3…for As to 5.17 ± 0.04… for Ni. In biota, the concentration (mg/kg) ranged from 0.01…for As in crab to 19.22…for Cu also in crab. Estimated human health risk from the metals contamination due to consumption of biota gave each of HQ and HI as < 1, indicating no risk except at Agip’s sample location where HI was > 1. Estimated human health risk due to dermal absorption through swimming, indicated no risk as each of HQ and HI was < 1. These make the river unpolluted except at Agip’s sample location.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Industrial and municipal wastes are indiscriminately disposed into surface water bodies in many developing countries including those in Sub-Saharan Africa because of weak environmental regulations (Arukwe et al. 2012; Inam et al. 2019). Hazardous chemicals may become enriched and biomagnified through the food chain, posing risks to aquatic organisms. In addition, when fish and other animals from such water are used for human consumption, periodic monitoring is needed to protect the health of those who consumed such fish and animals (Inam et al. 2019). The common environmental contaminants include toxic heavy metals, radionuclide, endocrine disrupting chemicals, toxic gases, plant nutrients and various oxygen consuming wastes (Inam et al. 2015). The sources of these contaminants include agricultural chemicals such as fertilizers, pesticides, and herbicides, atmospheric emissions from industries and households, leachates from waste dumps, community sewage, treated and untreated industrial effluents (Horsfall and Spiff 2013). Environmental pollution which used to be a peculiar problem of industrialised countries is now the problem of developing countries because of weak enforcement of environmental pollution control laws and regulations (Oketola and Fagbemigun 2013).
Heavy metals are some of the major chemical pollutants that have lasting effects on the natural balance in aquatic system. They are serious environmental problems in the marine environment, and sediment is the ultimate sink of these contaminants (Huang et al. 2009; Emara et al. 2015). These chemical pollutants are not biodegradable, so they can affect the environment adversely for a long time. Biodegradability means the ability for the pollutants to be removed from the environment through natural biological processes (Travizani et al. 2016). In the aquatic system, heavy metals tend to accumulate in the bottom sediment and become concentrated by biota. The process of accumulation of heavy metals by fish and aquatic animals through non dietary routes is called bioconcentration (Travizani et al. 2016). The toxicity of heavy metals can result in reduced mental and central nervous system functions, reduction in energy, damage to blood compositions, the lungs, kidneys, liver, and other vital organs (Onwali et al. 2014). There is every need to screen for these contaminants in aquatic environment in order to promote environmental protection.
The city of Port Harcourt is the major hub for oil and gas exploration, refining and related activities in Nigeria with an estimated population of about two million people (Inam et al. 2019). The city has witnessed rapid economic growth, development, urbanization, and exponential population growth. The New Calabar River which runs across the city of Port Harcourt receives all kinds of wastes discharged from point and non-point sources of industrial, domestic, trading and agricultural activities. This makes the river a dump for treated and untreated wastes (Inam et al. 2019). The wastes dumped in the river could lead to accumulation of toxic elements in the river. This could pose human health risks to the consumers who consume fish and other animals from the river and those who use the river for recreational and domestic purposes.
There are data and information on the concentration levels of some heavy metals in the New Calabar River and other rivers in the Niger Delta region. For example, Davies et al. (2006) reported the concentration levels of Cr, Pb and Cd in water, periwinkle (T. fuscatus) and sediment from Elechi Creek. They noted that the metals concentration levels were higher in sediment than in water and higher in periwinkle than in sediment. Similarly, Wokoma (2014) in his study on heavy metal burden in water, sediment and crab from Sombreiro River, reported that the concentration levels of the investigated metals (Cr, Cu, Ni and Zn) were higher in sediment than in water, with the concentration levels ranging from 0.02 ± 0.01 mg/kg Cr to 340.6 ± 106.21 mg/kg Zn in sediment, and 0.01 mg/L Cr to 0.99 ± 0.104 mg/L Zn in water. Accordingly, Onwali et al. (2014) investigated the concentration level of Pb in periwinkle and sediment samples from the Eagle Island River in Port Harcourt using atomic absorption spectrophotometer (AAS). They noted that the concentration level of Pb in the periwinkle and sediment was 0.91 ± 0.54 and 0.22 ± 0.19 mg/kg, respectively. However, data and information on the human health risk assessment of the heavy metal contamination are either insufficient or absent in the area. There is therefore every need to assess the human health risk of these heavy metals contamination in the New Calabar River.
The objectives of this study are to quantify the concentration levels of some heavy metals (Pb, Cu, Cr, Cd, As, Ni) in water, sediment, crab (Tympanotonus fuscatus) and periwinkle (Callinectes amnicola) from New Calabar River and to assess the human health risks associated with consumption of the biota and swimming in the river. The expected results shall provide adequate data on the concentrations of these heavy metals in the river system. The results shall also provide adequate information on the degree of pollution and human health risks associated with the consumption of the biota and dermal absorption through swimming.
Materials and Methods
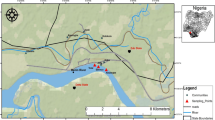
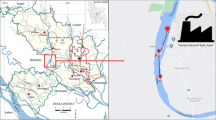
The New Calabar River is located on the eastern flank of the Niger Delta River system. It lies between latitudes 4° 45′ and 5° 00′ N and longitudes 6° 50′ and 7° 00′E. The river rises from Elele-Alimini where it is freshwater and non-tidal. At Aluu, the New Calabar River is freshwater and tidal where it is joined by a smaller river tributary which rises from Isiokpo and then empties into some creeks and lagoon bordering the Atlantic Ocean. Between Choba and Qgbogoro, a little distance downstream, the river turns brackish. It is part of the major river networks in the Niger Delta and has been routinely studied because of its vulnerability to contamination from domestic, industrial, agricultural, and natural runoffs from the densely populated and industrialized city of Port Harcourt (Horsfall and Spiff 2002; Onojake et al. 2017; Inam et al. 2019).
Water, sediment, crab (Tympanotonus fuscatus) and periwinkle (Callinectes amnicola) samples were collected in August 2016 and March 2017 representing wet and dry seasons, respectively. Samples were collected in five locations along the river course, between the entrance into the Greater Port Harcourt area (Abonema Wharf) and its exit from the urban area at Aluu. The sampling points were chosen based on their accessibility. The coordinates of the sampling points were recorded using global positioning system (GPS). The coordinates are as presented in Table 1. Water samples (500 mL per sampling point) were collected at a depth of 1 cm below the water surface in HNO3 pre-rinsed (1L) containers and 5 mL of concentrated HNO3 added immediately to minimize chemisorption. Five (5) water samples were randomly collected per each sample location, making 25 samples for the five locations and 50 for both the wet and dry seasons. Sediment samples were collected with the aid of a plastic trowel during low tide at 10–15 cm depth. Samples were randomly collected from five different points in each sample location and pooled together to obtain one composite sample per location, making five composite sediment samples for the five locations and ten for both the wet and dry seasons. Crab samples were caught directly from the river during low tide through a basket. Periwinkles were handpicked along the river bank, washed immediately with the river water to remove mud and algae. Each of the crab and periwinkle samples was randomly collected from five points per sample location and pooled together to obtain one composite sample (each of the crab and periwinkle) per sample location and five for the five locations. That is ten composite samples (each of the crab and periwinkle) were collected for both the wet and dry seasons. In each case, samples were properly labelled and kept in clean plastic containers and stored at 20 °C before taking to the laboratory for analyses.
Levels of the investigated heavy metals in the analysed samples were determined using UNICAM solar 969, AAS. A plot of absorbance of the working standards versus concentration of the metals gave the concentration of the test element from the equation:
where \({C}_{test}\)= concentration of the test element, \({A}_{test}\)= absorbance of the sample, \({C}_{std}\) = concentration of standard, \({A}_{std}\) = absorbance of standard, \(Df\) = dilution factor, \(Wt\) = weight of sample used.
For water sample from each location, 100 mL were filtered and acidified with 10 mL HNO3 and 10 mL 50% HCl solutions and evaporated to near dryness. This was transferred to a 100 mL volumetric flask and made up to the mark with deionised water and then aspirated into the flame of the AAS.
For sediment, 1 g was taken in a 250 mL conical flask, 15 mL of the mixture of perchloric, nitric and sulphuric acids in the ratio of 1:2:2 were added and heated in a fume chamber until dense white fumes appeared. The flask was allowed to cool and the content filtered into a 50 mL volumetric flask and made up to the mark with distilled water and then aspirated into the AAS.
Each of the biota was dried in an oven at 80 °C for 24 h and homogenised to powder form. Two (2) g of the powdered sample were taken in a beaker; 6 mL HNO3, 2 mL perchloric acid and 30 mL distilled water added and stirred. The beaker was heated, allowed to cool, the content filtered and made up to 50 mL with distilled water and then aspirated into the AAS.
Quality control of the AAS was guaranteed through the implementation of laboratory quality assurance and laboratory methods, including the use of standard operating procedures, calibrations with standards and analyses with reagent blanks. Samples were analysed in triplicates, all chemicals and reagents used were of analytical grade. The detection limit of the AAS for all the metals investigated in all media was \(1. 0 \times {10}^{-4}\) mg/L for water and \(1. 0 \times {10}^{-4}\) mg/kg for sediment and biota.
The exposure assessment refers to the determination of the magnitude, frequency, duration and route of exposure with respect to the chemical contaminants in question (USEPA 1992). A commonly applied exposure scenario in this case is the dermal absorbed dose (DAD). As noted by USEPA (2000) and Kang et al. (2006), the DAD values were obtained with the equation given below.
where DAD = dermal absorbed dose, \({C}_{W}\)= average concentration of heavy metal in water, SA = exposed skin area, KP = skin adherence factor, \({ABS}_{D}\) = dermal absorption factor, ET = exposure time, EF = exposure frequency, ED = exposure duration, CF = unit conversion factor for water, BW = average adult body weight, and AT = average time. The magnitude of the toxic effects was estimated in terms of hazard quotient (HQ) (Sharma 2009). This is the ratio of the single substance exposure level over a specified period to a reference dose for that substance derived from a single exposure period. This was evaluated by using the equation given below as described by USEPA (2000).
DAD = dermal absorbed dose (mg kg−1 day−1)), \({RfD}_{Dermal}\) = dermal reference dose (mg kg−1 day−1). HQ below one (1) implies that the level of exposure is not likely to cause any obvious adverse effects (USEPA 2000; Wang and Gardinal 2013). The HQ for each sample were summed up to obtain overall toxic risk, the hazard index (HI) as indicated in the equation below.
where i = the number of trace metals. If the calculated HI is less than one, then the non-carcinogenic adverse effect due to the exposure pathway or toxicant was assumed to be negligible.
The exposure assessment for ingestion of the biota (periwinkle and crab) was evaluated from the equations given below:
where CF = concentration (mg/kg) of the determined heavy metal in the sea animal, IR = ingestion rate (kg/day), FI = the fraction ingested (unit less), EF = exposure frequency (365 days/year), ED = exposure duration, BW = body weight, and AT = the averaging time (period over which exposure is average in days). \({RfD}_{o}\) = oral reference doses of the heavy metals. The actual risk was characterised using HQ as detailed in the second equation. HQ below one (1) implies that the level of exposure is not likely to cause any obvious adverse effects (USEPA 2000; Ijeoma et al. 2015).
The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, USA). Student’s t- test was used to determine the differences between the concentration levels of each of the analysed element in water as well as in sediment samples in the dry and wet seasons. P < 0.05 was considered the level of statistical significance.
Results and Discussion
The concentration levels of the heavy metals in water and sediment determined in this study are presented in Tables 2 and 3, respectively. In Table 2, the concentration levels of the heavy metals in water were in the order: Ni > Cu > Pb > Cr > Cd > As, while in sediment (Table 3), the order was: Ni > Cr > Cu > Pb > Cd > As. The results of t – test at p < 0.05 revealed that the concentration levels of most of the metals in water as well as in sediment samples from all locations were significantly higher in the dry season than in the wet season. This could probably be attributed to decrease in the volume of the river during the dry season as a result of evaporation.
The mean concentration level of Pb reported in water in each of the two seasons in this study (Table 2) was above the Nigerian Industrial Standard (NIS) limit of 0.01 mg/L Pb in water reported by SON (2007) and 0.05 mg/L Pb in water reported by USEPA (1992). The Pb concentration level in water recorded in this study was also above the value of 0.01 mg/L reported by Ideriah et al. (2012). This could be attributed to increased commercial and industrial activities around the river bank and the continuous inflow of domestic wastes into the river. The Pb level recorded in sediment samples from each sample location in this study was lower than the range (11.5 to 72.0 mg/L) reported by Iwegbue et al. (2018). It was above the recommended value of 2.2 mg/kg reported by WHO (2006). Pb is a cumulative poison that is associated with several health hazards like anemia and reproductive defects (Moore 1988). The highest desirable limit of Pb in drinking water specification is 0.01 mg/L.
From Table 2, Ni concentration level in water from each of the sample location recorded in this study was equally higher than the NIS and WHO recommended standards of 0.02 mg/L (SON 2007) and 0.07 mg/L (USEPA 1992), respectively. The high level of Ni observed in water at Choba study location might be due to the dredging activities there, while the lower level observed at Aluu may be attributed to low or no activity in the area. The Ni level in sediment from each sample location in this study was higher than those reported by Wokoma (2014).
Cu is essential for metabolism. High levels of Cu in human system are toxic and may cause liver damage. The mean concentration level of Cu in the water samples investigated in this study (Table 2) was lower than 21 mg/L reported by Ijeoma et al. (2015) and higher than 0.08 mg/L recorded by Ideriah et al. (2012). It was also higher than the maximum allowable limit of 1 mg/L recommended by NIS for aquatic medium (SON 2007). Dumping of industrial and municipal wastes indiscriminately into the water bodies in many developing countries including those in Sub-Saharan Africa because of weak environmental regulations (Arukwe et al. 2012; Inam et al. 2019), dumping of domestic wastes as well as washing of agrochemical fertilizers from agricultural farmlands into the water bodies by runoffs water when it rains, could be the potential sources of Cu in the studied river. This is because Cu is widely used in houses for piping water and as electrical conductor. In addition Cu is used in corrosion resistant and decorating painting. Besides, the compounds of Cu are used as chemical catalysts, wood preservatives, fungicides, antifungal paints, disinfectants, nutritional supplements, in fertilizers, in feeds, in petroleum refining as well as in printing inks and dyes. Indeed, large amounts of copper wire are used in telephones as well as in television sets, motors and generators (Locks et al. 2007).
In each of the seasons, Cr concentration level in water from Aluu as well as Choba sample locations recorded in this study (Table 2) was lower than the USEPA and NIS standards given as 0.05 mg/L (USEPA 1992; SON 2007). However, the Cr level in water recorded in Iwofa as well as the overall mean level for each of the season was higher. This could be due to anthropogenic inputs via industrial activities and domestic wastes. Cr in its hexavalent form is known as a carcinogen and implicated in many health effects in animals. The mean Cr level in water samples recorded in this study for each of the season is close to the range of 0.06 to 0.31 mg/L reported by Ideriah et al. (2012).
In the sediment, the Cr level recorded at Agip’s study location was as high as 5.11 ± 0.11 mg/kg during the wet season (Table 3). This study location is known for its high boating activities where illegally refined petroleum products are traded. In addition, there is a huge gutter in the Agip area where domestic wastes and sewage from the city are washed into the river during the wet season, thus increasing the Cr level. However, Cr level in sediment at Iwofe study location was higher during the dry the season than the wet season (Table 3). This could probably be attributed to decrease in the volume of the river due to evaporation as well as boating and other commercial activities prevalent at Iwofe during the dry season. In general, Cr level recorded in sediment samples from each of the sampling point in this study (Table 3), was lower than the range of 3.1 to 14.9 mg/kg reported by Iwegbue et al. (2018) and exceeded the WHO recommended standard of 0.5 mg/kg (WHO 2006).
The mean Cd concentration level in water recorded in this study was in agreement with the one reported by Wokoma (2014). The Cd level in water (Table 2) was also in close range with the NIS and WHO standards given as 0.003 mg/L (SON 2007) and 0.005 mg/L, respectively. Cd is a cumulative poison. It is very toxic to the human system. It is not an essential element in any biological processes (Manoj and Avinash 2012). Cd is believed to promote renal arterial hypertension. Elevated level may cause liver and kidney damages, or even anaemia, retarded growth, and death (Waalkes 2000). Cd level in sediment recorded in this study was in close agreement to those reported by Ideriah et al. (2012) and Wokoma (2014).
The mean Arsenic (As) concentration level in water recorded in this study was below the WHO prescribed provisional guideline and NIS standard for As in drinking water. The maximum permissible limit of As in water is 0.01 mg/L (WHO 2006; SON 2007). The As level recorded in sediment in this study was within the NIS recommended permissible limit of 0.01 mg/kg given by SON (2007).
The mean concentration levels of heavy metals in periwinkle (Callinectes amnicola) and crab (Tympanotonus fuscatus) samples determined in this study are as presented in Figs. 1 and 2, respectively. The levels of heavy metals recorded in this study for the periwinkle and crab samples varied across the two seasons. The concentration levels were in the order: Cu > Ni > Cr > Pb > As > Cd and Cu > Ni > Pb > Cr > Cd > As for periwinkle and crab, respectively. In Fig. 1, the mean concentration level of each of the investigated heavy metals in periwinkle ranged from 0.002 mg/kg Cd to 16.30 mg/kg Cu in the dry season and from 0.002 mg/kg Cd to 12.40 mg/kg Cu in the wet season. That is from Fig. 1, the mean concentration level of Cd in periwinkle was 0.002 mg/kg in the dry season as well as in the wet season. The mean concentration level of As was 0.012 mg/kg in the dry season and 0.006 mg/kg in the wet season. Ni level in the dry season was 5.63 mg/kg and 7.48 mg/kg in the wet season. Pb level was 6.88 mg/kg in the dry season and 7.49 mg/kg in the wet season. In the dry season, the mean concentration level of Cr in periwinkle was 7.37 mg/kg and 5.54 mg/kg in the wet season, while that of Cu was 16.30 mg/kg in the dry season and 12.40 mg/kg in the wet season. As presented in Fig. 2, the mean concentration level of each of the investigated heavy metals in crab ranged from 0.02 mg/kg Cd to 20.50 mg/kg Cu in the dry season and from 0.01 mg/kg As to 14.70 mg/kg Cu in the wet season. That is from Fig. 2, the mean concentration level of Cd in crab was 0.02 mg/kg in the dry season and 0.17 mg/kg in the wet season. The level of As was 0.28 mg/kg in the dry season and 0.01 mg/kg in the wet season. Ni was 9.10 mg/kg in the dry season and 7.24 mg/kg in the wet season. Pb was 7.34 mg/kg in the dry season and 8.16 mg/kg in the wet season. In the dry season, the mean concentration level of Cr in crab was 11.00 mg/kg and 9.36 mg/kg in the wet season, while that of Cu was 20.50 mg/kg in the dry season and 14.70 mg/kg in the wet season.
The concentration levels of most of the metals in both periwinkle and crab were higher in the dry season than in the wet season. This could probably be attributed to decrease in the volume of the river during the dry season as a result of evaporation. The mean concentration levels of Cr in each of the two animals recorded in this study were higher than those reported by Davis et al. (2006) and Chindah et al. (2009). The concentration level (0.002 mg/kg) of Cd in periwinkle recorded in this study for both the dry and wet seasons was in close agreement to those reported by Davis et al. (2006), Chindah et al. (2009) and Wokoma (2014).
The estimated human health risks due to dermal exposure to heavy metals in water through swimming are as presented in Table 4. From the table, the HQ of each of the metals in samples from each of the locations was less than one (< 1) in the two seasons. This implies that the level of exposure is not likely to cause any obvious adverse effects (USEPA 2000; Wang and Gardinal 2013). Accordingly, the HI of each sample was < 1, implying that non-carcinogenic adverse effect due to dermal exposure is negligible. However, gradual increases in cumulated HQ were observed in the sections of the river with increased human activities. These imply that human health risks could arise in the near future if action is not taken to curb the trend.
The estimated human health risks of the heavy metals due to consumption of the periwinkle (Callinectes amnicola) and the crab (Tympanotonus fuscatus) from the New Calabar River are as presented in Table 5. From the table, the HQ of each of the metals in the two animals from the two locations considered was < 1 in the two seasons. This implies that consumption of the two animals from the studied river is not likely to portray any toxic risk (USEPA 2000; Wang and Gardinal 2013). However, as seen in Table 5, the HI of crab from Agip sample location was greater than one (> 1) in the two seasons, while the HI of the crab from Iwofe was < 1 in the two seasons. Although the HI of crab from Iwofe was < 1, it was actually approaching one. Similarly, although the HI of periwinkle from the two respective sample locations of Agip and Iwofe was < 1, it was actually approaching one in the two seasons. This implies high contamination and long term bioaccumulation of the investigated heavy metals in the river (Inam et al. 2019). It could also be stated that, since the HI approaches 1, chronic human health risk cannot be ruled out.
From the results, it could be concluded that the investigated heavy metals occurred at varied concentration levels in the water, sediment, periwinkle and crab. In general, the concentration levels of the metals in the analysed samples were in the order: Ni > Cr > Cu > Pb > Cd > As. Pb, Ni and Cr levels in the water, periwinkle and crab were above their WHO permissible limits (WHO 2006). Cu level in water was equally above its WHO permissible limit. The human health risk assessment classifies the river as safe with regards to dermal exposure to the heavy metals through swimming as each of HQ and HI was < 1. Estimated human health risk from the metals contamination due to consumption of the two sea animals gave each of HQ and HI as < 1, indicating no risk except in Agip sample location where HI was > 1 in the two seasons. Further human health risk assessment of these and other heavy metals in other rivers in Nigeria’s Niger Delta is recommended to protect aquatic ecosystems and biodiversity in the area.
References
Arukwe A, Eggen T, Möder M (2012) Solid waste deposits as a significant source of contaminants of emerging concern to the aquatic and terrestrial environments—a developing country case study from Owerri, Nigeria. Sci Total Environ 438:94–102. https://doi.org/10.1016/j.scitotenv.2012.08.039
Chindah AC, Braide SA, Amakri J, Chikwendu SO (2009) Heavy metal concentrations in sediment and periwinkle Tympanotonus fuscatus in the ecological zones of Bonny River system, Niger Delta, Nigeria. Open Environ Pollut Toxicol J 1:93–106
Davies OA, Alison ME, Uyi HS (2006) Bioaccumulation of heavy metals in water, sediment and periwinkle (Tympanotonus fuscatus var radula) from Elechi Creek. Niger Delta Afri J Biotechnol 5(10):968–978
Emara M, Farag KS, Dawuh AA, Fathi M (2015) Assessment of heavy metals concentraton in water and edible tissue of Nile tilapia (Oreochromics nitoticus) from two fish farms irrigated with different water sources. Egypt Inter J Environ 4(1):105–115
Horsfall M, Spiff AI (2002) Distribution and partitioning of trace metals in sediments of the lower reaches of the New Calabar River, Port Harcourt. Nigeria Environ Monit Assess 78(3):309–326. https://doi.org/10.1023/A:1019991020048
Horsfall M, Spiff AI (2013) Principles of Environmental Pollution. Toxicology and Waste Management. Onyoma Research Publications, Port Harcourt, Nigeria, p 488p
Huang XF, Hu JW, Deng JJ, Li CX, Qin FX (2009) Speciation of heavy metals in sediments from Baihua and Aha lake, Asia- Pacific. J Chem Engin 4:635–642
Ideriah TJ, David DS, Ogbonna DN (2012) Distribution of heavy metals in water and sediment along Abonema shoreline. Nigeria Resources and Environment 2(1):33–40
Ijeoma FV, Andrew N, Clare W, Gillian M (2015) Assessment of physico-chemical properties and metal contents of water and sediments of Bodo Creek, Niger Delta. Nigeria J Toxicol Environ Chem 97(2):135–144
Inam EJ, Nwoke IB, Udosen ED, Offiong N (2019) Ecological risks of phenolic endocrine disrupting compounds in an urban tropical river. Environ Sci Pollut Res 26(21):21589–21597. https://doi.org/10.1007/s11356-019-05458-7
Inam E, Offiong N, Kang S, Yang P, Essien J (2015) Assessment of the occurrence and risks of emerging organic pollutants (EOPs) in Ikpa River Basin freshwater ecosystem, Niger Delta –Nigeria. Bull Environ Contam Toxicol 95(5):624–631. https://doi.org/10.1007/s00128-015-1639-9
Iwegbue CMA, Beecroft OV, Ogala JE, Egobueze FE, Tesi GO, Martincigh BS (2018) Concentrations and ecological risks of metals in some coastal creeks in the Niger Delta. Nigeria Afri J Aqua Sci 43(3):241–253
Kang J, Kondo F, Katayama Y (2006) Human exposure to bisphenol A. Toxicology 226:79–89. https://doi.org/10.1016/j.tox.2006.06.009
Locks K, Criel P, De Schanamphelenaene KAC, Van-Eckhout H, Janssen CR (2007) Influence of calcium, magnesium, sodium, potassium and pH on copper toxicity in barley (Horduem vulgaria). Ecotoxicity and Environ Safety. https://doi.org/10.1016/jecoenv.2006.1-014
Manoj K, Avinash P (2012) A review of permissible limits in drinking water. Indian J Occupat Environ Med 16(1):40–44
Moore M (1988) Haematological effects of lead. Sci Total Environ 71(419):431
Oketola AA, Fagbemigun TK (2013) Determination of nonylphenol, octylphenol and bisphenol- A in water and sediments of two major rivers in Lagos, Nigeria. J Environ Protect 4:38–45. https://doi.org/10.4236/jep.2013.47A005
Onojake MC, Sikoki FD, Omokheyeke O, Akpiri O (2017) Surface water characteristics and trace metals level of the Bonny/New Calabar River Estuary, Niger Delta. Nigeria Appl Water Sci 7(2):951–959. https://doi.org/10.1007/s13201-015-0306-y
Onwali DO, Ajuru G, Brown H, Amadi FB (2014) The concentration of Lead in Periwinkle (Tympanotonus fuscatus) and River sediment in Eagle Island River, Port Harcourt. Nigeria Amer J Environ Protect 2(2):37–40
Sharma VK (2009) Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: A review on occurrence, fate and treatment. J Environ Sci Health Part B 44:423–427
SON (Standard Organisation of Nigeria) (2007) Nigerian standard for drinking water quality, Nigerian Industrial Standard (NIS). ICS 13.060.20, Price group D @ SON, 554p
Travizani TH, Figueira RC, Riberio AP, Oreophilo CY, Major AP, Petti MA, Corbusier TN, Montone RC (2016) Bioaccumulation of heavy metals in marine organisms and sediments from Admirably Bay. King George Island, Antarctica. Marine Pollu Bull 755 - 756
USEPA (United State Environmental Protection Agency) (1992) Framework for ecological risk assessment EPA/630/R.92/001. Risk assessment forum, USEPA, Washington D C
USEPA (United State Environmental Protection Agency) (2000) Guidelines for assessing chemical contaminant data for use in fish advisories: Fish sampling and analysis. 3rd edition Washington DC
Wang C, Gardinali PR (2013) Detection and occurrence of microconstituents in reclaimed water used for irrigation -a potentially overlooked source. Anal Bioanal Chem 405(18):5925–5935. https://doi.org/10.1007/s00216-013-6799-z
WHO (World Health Organisation) (2006) Guidelines for drinking-water quality (electronic resource). Incorporating first addendum. 3rd edn, volume 1.Recommendations. Geneva. 491–493
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorganic Biochem 79:240–244
Wokoma OA (2014) Bioaccumulation of trace metals in water, sediment and crab (Callinectes) from Sombreiro River, Niger Delta. Nigeria Internal J Sci Technol Res 3(12):295–299
Acknowledgements
The authors are grateful to everyone that has contributed in one way or the other to the success of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uwah, E.I., Nwoke, I.B., Inam, E.J. et al. Human Health Risk Assessment of Heavy Metal Contamination in New Calabar River. Bull Environ Contam Toxicol 105, 317–324 (2020). https://doi.org/10.1007/s00128-020-02921-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02921-x