Abstract
Mercury (Hg) methylation could occur in freshwater ecosystems with low or high salinity. However, few studies are available about the effects of salinity change on mercury(Hg) release and methylation. In-situ experiments using Suaeda heteroptera wetland soil column from the Liaohe estuary were performed to decipher how total mercury (THg) and methylmercury (MeHg) contents change under fluctuant salinity and wet and dry soil conditions. Salinity gradients were set to 0.50% (S1), 1.00% (S2), 1.50% (S3) and 1.80% (S4), and pure deionized water was used as a blank control (CK). Wet and dry soil conditions were set to full inundation condition (WD1) and naturally dried treatment (WD2). Results indicated that the highest THg and MeHg contents were found in surface and bottom soil when water salinity treatment was CK under WD1. THg and MeHg decreased with salinity under WD1. THg contents in overlying water varied from 0.854 to 1.243 µg L−1 under WD1 treatments and increased with salinity change. When under WD2 treatment, THg contents in both soil layers gradually decreased with rising salinity. Meanwhile, MeHg contents in both soil layers reached the lowest level at CK (1.666 μg kg−1and 2.520 μg kg−1) and increased gradually with the rising salinity. By comparison, THg content of the soil was much lower in WD1 than that in WD2. Under the WD1 condition, the MeHg contents and %MeHg decreased with rising salinity and showed significantly different in different salinity treatment, however, its showed an opposite trend with rising salinity under the WD2 condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury(Hg), as a global contaminant, can originate from both natural and anthropogenic sources (Driscoll et al. 2013). mercury(Hg) transport in the environment is complex due to interact biogeochemical processes and the propensity for transformation, remobilization, and biological uptake over various environmental geochemical conditions (Merritt and Amirbahman 2009). Furthermore, soil condition with poor oxygen and concomitant microbial activity can enhance MeHg production, which is the most bioaccumulative and toxic form of mercury(Hg) compounds (Eagles-Smith and Ackerman 2014). MeHg could effectively accumulate in fish bodies, and bring health risk to humans. Methylation rate of inorganic MeHg was negatively correlated with soil/water salinity, and it was more conducive to mercury(Hg) methylation in environments with low salinity (Hsu-Kim et al. 2013). It is favored for demethylation processes and MeHg is labile under high salinity (Kongchum et al. 2006). Another study showed that the presence of high concentrations of chloridecould prevent MeHg complex (CH3HgCl) conversion to other mercury(Hg) species (Sun et al. 2013). The ratio of MeHg to THg usually increased with salinity in surface estuarine sediment (Hollweg et al. 2009). Furthermore, uptake of inorganic mercury(Hg) and MeHg in fish increased with salinity (Dutton and Fisher 2011). However, Buckman et al. (2017) found no relationship between salinity and MeHg in fish bodies, sediments, or water. It implied that mercury(Hg) methylation and fate in estuary sediments were still with distinct knowledge gaps.
Liaohe estuary is flat and located in the lower reaches of the Liaohe River. Most of the water in the estuarine wetland comes from the river and tidal water input (Zheng et al. 2017a, b). Phragmites australis, Suaeda heteroptera, and mudflats are the main wetland types. Soil is saline here (Xiao and Yang 2015). Suaeda heteroptera is predominant here (Tao 2016). In recent years, the salinity of Liaohe estuary has changed significantly because the interaction between saltwater and freshwater has changed a lot, such as rising sea level and decreasing runoff. Salinity and water were responsible for Suaeda heteroptera wetland degeneration (Wang et al. 2010). Studies had indicated that the optimal salinity for Suaeda heteroptera growth was 1.5%. Probably, salinity affected not only the distribution of mercury(Hg) but also the synthesis and stability of MeHg. However, few are available on how salinity change affects mercury(Hg) methylation in estuarine wetland soil. In the present work, we explored the effects of salinity shift on mercury(Hg) to release and MeHg production based on in-situ microcosmos experiments.
Method and Materials
The topsoil (0–10 cm) and subsoil (10–20 cm) samples were collected in S. heteroptera wetlands in the Liaohe estuary, China, using a stainless-steel soil drill, which was cleaned by washing agent and 10% HCl before using. After removing the litter and shellfish in the soil, the samples were put into a self-sealing bag and brought back to the laboratory. All soil samples were naturally dried at room temperature, mixed and ground through 2 mm sieves and preserved at – 4℃ before organic matter, THg, and MeHg analysis. The physical and chemical properties of the test deposits are shown in Table 1.
Optimum growth salinity of S. heteroptera is 1.27%, and the ecological optimum threshold of salinity is 0.89%–1.65% (Cui et al. 2008). Therefore, the experimental submergence salinity was set at 0.50%, 1.00%, 1.50%, and 1.80%. Artificial seawater was prepared according to Li et al. (2018) and diluted to salinity of 0.50% (S1), 1.00% (S2), 1.50% (S3) and 1.80% (S4). Pure deionized water was used as a blank control (CK). No THg and MeHg were detected in artificial seawater.
The simulated culture device was shown as Fig. 1. The culture column had a height (h1) of 45 cm, an inner diameter (φ1) of 16 cm, and a round hole (φ2) of 0.5 cm at the bottom, and was connected with the hose. Two glass fibre (φ3=15 cm) were placed inside to prevent soil from blocking the hose, and quartz sand (250 g) was laid between the two glass fibres.
All topsoil and subsoil samples were ground and passed through a 2 mm sieves. One kilogram topsoil followed by one-kilogram subsoil was placed in a culture column according to natural soil generation horizons. Subsequently, artificial seawater at different salinity was poured into the corresponding culture column slowly. The total height of soil in the column was h2 = 30 cm, and depth of artificial seawater, h3, was set to 3 cm. The hoses were held with a valve to prevent artificial seawater outflow and air entrance. Three parallels were set for each salinity condition and soil were cultured at room temperature (20–25℃). Two hydrological scenes were set to simulate different hydrology conditions, which were full inundation condition (WD1) and naturally dried treatment (WD2). Under WD1 treatment, the top soil samples, sub soil samples of cultured soil and water samples were collected after 15 days, and the depth (h3) and salinity of artificial seawater in the column were maintained by deionized water during this period. Afterwards, we didn’t add any water to the column until the overlying water was naturally evaporated, and then, the soil samples were collected again.
The soil samples were freeze-dried, ground and sieved through a 100 mesh sieves for THg and MeHg analysis. Water samples (30 mL) collected through the hose were centrifuged at 3000 r min−1 for 15 min, and the supernatant was transferred into a conical flask (250 mL). Nitric acid (68%) was added to adjust pH to 2, and water samples were stored at 4℃ before determination.
THg contents in soil samples were extracted by H2SO4–HNO3–V2O5 digestion method described by Rasmussen et al. (1991). THg contents in water samples were extracted as described by Zhang et al. (2010). All forms of mercury(Hg) in soil and water samples were converted to Hg2+. Hg2+ in the digestion solution and was reduced to elemental mercury(Hg) by adding a drop of 20% NH2OH·HCl solution (V/V) before it was analyzed by cold vapor atomic absorption spectrometry (F732-V, Huaguang, Shanghai), the detection limits was 0.05 μg L−1. Precision and accuracy of the analytical methods were evaluated by comparing the THg concentrations in certified reference materials to the measured values. The expected and measured concentrations of THg in certified reference materials (GBW–07401) were (0.032 ± 0.004) and (0.033 ± 0.003) mg kg−1.
The MeHg in the freeze-dried soil was extracted and separated using the method described by Li et al. (2019). One gram of each sediment sample was weighed into a 50-mL polyethylene centrifuge tube. About 5 mL 6 mol L−1 HCl was added to extract all forms of Hg. The tube was left to stand overnight and then was ultrasonically cleaned for 2 h. The tube was then centrifuged at 3000 rpm for 15 min. The supernatant fluid was transferred into a 60-mL separating funnel and 2 mL of CH2Cl2 was added and then shaken for 30 min to extract MeHg into the CH2Cl2 phase. After standing for 15 min, the CH2Cl2 phase was transferred into a 100-mL heart-shaped bottle and an additional 2 mL of CH2Cl2 was added into the 60-mL separating funnel again to remove any remaining MeHg. The same steps were then repeated on the same sediment sample. Finally, 5 mL deionized water was added into the 100-mL heart-shaped bottle, which contained the 4 mL CH2Cl2 phase. The 100-mL heart-shaped bottle was placed in a 60 ℃ water bath and the CH2Cl2 was evaporated by rotary evaporator. The CH2Cl2 was drawn off and the MeHg was left in the water phase. The water phase in the heart-shaped bottle was transferred into a 50-mL glass tube. Finally, 1 mL 18 mol L−1 H2SO4 and 1 mL bromide agent were added into the glass tube, which played the role of oxidation and indicator, respectively. Before sample testing, a drop of hydroxylamine hydrochloride was added into the tube to deoxidize the residual bromide agent. The MeHg of soils and blanks was determined by a Tekran Model 2600, which was stable, and the detection limits was 0.005 ng L−1, the recoveries of MeHg were between 94.1 and 102.3%. MeHg was verified by IAEA–433. The MeHg content in the standard sample was 0.17 ± 0.07 mg kg−1, and the validation result was 0.16 ± 0.04 mg kg−1.
Scanning electron microscopy was used for soil microstructure analysis using a Hitachi S-4800 instrument.
All reagents used in the experiment were all of the Guaranteed reagent(GR). All vessels used in this study were soaked in 3 mol L−1 HNO3 for 24 h prior to use and rinsed five times with deionized water. THg and MeHg values of all samples were higher than the blanks or the minimum detection limit.
The (%MeHg) was calculated by (MeHg/THg), which was used to indicate the methylation rate of mercury(Hg) in soil.
The data obtained were processed by Microsoft Excel 2003, and statistical analyses were conducted by statistical package SPSS 12.0.
Results and Discussion
Rising salinity would release mercury(Hg) into the overlying water, and eventually, soil Hg content decreases in the soil. With WD1 treatment (Fig. 2a), THg contents reduced by 21%–32% and 16%–28% in the topsoil and subsoil respectively. The highest THg contents, 0.103 mg kg−1, and 0.101 mg kg−1, were observed under CK treatment either in the topsoil or the subsoil. The lowest THg content was observed when salinity was set to S3. THg contents in soils decreased with rising salinity and showed significantly different in different salinity treatment (Fig. 2a). THg contents in flooding water varied from 0.085 to 1.243 μg L−1 (Fig. 2b), and the lowest (0.085 μg L−1) and the highest (1.243 μg L−1) values were observed under CK and S3 treatments, respectively. THg contents in water samples increased significantly with salinity (Fig. 2b). Rising THg contents in water were much lower than the reduced mercury in soil. It could be seen that other factors in the soil also affected the change of mercury under different salinity treatments. Mercury could release into the atmosphere through the water–air interface, soil-air interface under wetting and drying conditions (Gardfeldt et al. 2003; Zhang et al. 2013). Guo (2019) demonstrated that mercury released into the pore water from soil was higher than that released into the flooding water.
Under WD2 treatment, after the soil was dried from flooded conditions (Fig. 4), THg contents in topsoil and subsoil reduced by 11%–19% and 5%–16%, respectively. Thus, the mercury(Hg) contents of soil during WD2 period were higher than that during WD1 period. THg contents in topsoil and subsoil had the highest values, 0.117 mg kg−1 and 0.115 mg kg−1, under S1 and CK treatment, respectively. When salinity was set to S4, THg contents in topsoil and subsoil decreased to 0.107 mg kg−1 and 0.101 mg kg−1, respectively. THg contents in soil decreased with rising salinity and showed significantly different in different salinity treatment (Fig. 3).
Salinity in Liaohe estuary was governed by both tide and river water input. Main salt compounds of seawater were chloride in Liaohe estuary, and salinity was constantly changing due to tidal currents and runoff flow. Sodium ions (Na+) could compete for adsorption sites with Hg2+ and replace it in the soil solution system, and finally yield more active Hg2+ in soil solution (Zheng et al. 2017a, b). In addition, chlorine ion (Cl−), complexing with Hg2+, could form complex compounds like HgCl20 and HgCl3− (Yin et al. 1997). As estimated by Kim et al. (2004), more Cl− could facilitate the conversion of Hg(OH)2 to HgCl2 and HgCl3−, and finally, lead to a Hg2+ reduction in the soil. Therefore, high salinity accelerated mercury(Hg) release from the soil, which was consequently fixed or adsorbed by OM into the soil solution. This complex reaction reduced THg contents of soil and made it more bioavailable. However, some different results were also reported that salinity had no significant effect on mercury(Hg) contents in mangrove sediments (Ding et al. 2011), which may due to the low pH value and OM of soils in mangrove wetland. When salinity of soil solutions was increased, it may change the desorption process of mercury(Hg) from soil, through the property of themselves.
In the Liaohe estuary, tidal currents input more water than runoff in the dry season, and it could raise water salinity greatly. It was very likely to promote the release of mercury(Hg) from soil in Suaeda heteroptera wetland. In the flood season, salinity becomes low and had little effect on mercury(Hg) release from soil.
THg contents in flooding water can directly reflect interactions between overlying water and sediment. The bioavailability and toxic effect of mercury(Hg) might be enhanced while mercury(Hg) entered overlying water through diffusion from soil. In this study, interfaces or phases that interact with the overlying water (soil and air), might be the source of mercury(Hg) in overlying water. However, previous studies showed that more than 95% of the mercury(Hg) in the air was gaseous elemental mercury(Hg), and the effect on mercury(Hg) contents in overlying water was minimal (Yan and Feng 2011). These results suggested that the release of mercury(Hg) from soil was the unique mercury(Hg) source of flooding water.
Significantly lower THg contents were observed in WD1 period when compared with the WD2 period. This might be due to higher mercury(Hg) flux in flooding period (Liang et al. 2014), which could facilitate the transport of mercury(Hg) within the soil column from the deep layer to the flooding water. Moreover, the decomposition of OM might also contribute to mercury(Hg) release under WD1 period. Thus, flooding was conducive to mercury(Hg) to release from soil. This result agrees well with that from previous researches (Liang et al. 2014; Wang et al. 2015). After the flooding water naturally dried, the THg contents of topsoil and subsoil samples increased again. However, different results were observed in Yellow River Delta wetlands by Liu et al. (2017). It could be explained by the fact that sunlight played an important role in the emission of mercury from soil in the wild (Moore and Carpi 2005). Under weak light condition, the THg of flooding water might be adsorbed by soil again.
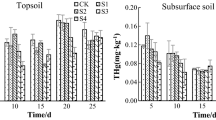
The content of MeHg in the soil showed a diametrically opposed change rule with rising salinity of flooding water under WD1 and WD2 treatment conditions. Under WD1 treatment, the highest MeHg contents were observed in the topsoil and subsoil (4.073 μg kg−1 and 4.865 μg kg−1) under CK condition.The MeHg contents and %MeHg decreased with salinity and showed significantly different in different salinity treatment (Fig. 4a, b).
After the soil was naturally dried(WD2), MeHg contents in the topsoil and subsoil reached the highest when the salinity of flooding water was 1.80% and 1.50% respectively (3.674 μg kg−1 and 5.414 μg kg−1). The MeHg contents and %MeHg showed significantly difference in different salinity treatment (Fig. 4c, d). Under different salinity of flooding water, the MeHg content in the subsoil (10–20 cm) was higher than that in the topsoil (0–10 cm).
Soil ventilation condition changed with the wet-dry rotation environment, which could affect soil redox conditions. Mercury(II) in the soil could be converted to MeHg by microorganisms under aerobic conditions (Raposo et al. 2008). Although we didn’t detect the species and quantity of methylation microorganism in this study, Benoit (2003) found that biological methylation played a dominant role in the synthesis of MeHg. Under flooding condition, most bacteria in soil was the anaerobic species, which could induce mercury(Hg) methylation effectively in soil (Martin-Doimeadios et al. 2004). When the flooding water naturally dried, The content of MeHg in the soil was also higher than that in the test soil. It was speculated that there were some aerobic microorganisms which played a leading role in mercury methylation in the soil (Xiang et al. 2014). Although it was generally believed that an anaerobic environment was more conducive to mercury methylation, the study of mercury methylation in aerobic environment should not be ignored.
The MeHg could be detected in soil under WD1 treatment conditions and higher than the test soil, which indicated that the MeHg could be synthesized under different salinity conditions. A similar observation by Oliveira et al. (2015) that methylation microorganism, such as Sulfur reduction bacteria (SRB), could survive from freshwater to marine ecosystems including hypersaline environments. In this study, MeHg production in soil was affected by the bioavailability of mercury(II) for methylation and by the activity of Hg-methylating microbes, both of which were significantly affected by salinity of flooding water. The uncharged HgCl2 in low salinity conditions was more easily diffused through the plasma membrane to the cytoplasm, where methylation occurs, compared to [HgCl4]2− and [HgCl3]− in high-salt environments, which were not conducive to mercury(Hg) methylation (Barkay et al. 1997). In addition, the protoplasm of methylation microorganism could separate from the cell wall under high salinity conditions, resulting in cell death or activity decline, thus reducing the degree of mercury methylation (Boyd. et al. 2017).
After the flooding water naturally dried, the methylation rate of mercury increased with rising salinity (Fig. 4d), which indicated that the inhibitory effect of salinity on methylation was specially favored under reducing conditions. With the drying of the flooding water, the soil will gradually enter the aerobic environment, and the Hg-methylating microbes will gradually die. However, the metabolite of methylating microbes, methylcobalamin (VB12), could convert the dissolved Hg2+ to MeHg (Ridley et al. 1977). In the drying process, most of the dissolved Hg2+ will be reabsorbed by the organic matter in the surface soil under low salinity conditions, thus reducing the methylation rate of mercury(Hg). In addition, some aerobic and facultative anaerobic microbes resistant to Hg could degrade MeHg, resulting in the decrease of MeHg content in the soil (Barkay and Wagner-döbler 2005). Under high salinity conditions, due to the influence of sodium ions (Na+), there were still a large number of dissolved Hg2+ available for methylation in pore water.
Studies had also found that the methylation activity of mercury(Hg) in seawater is usually lower than that in fresh water (Berman and Bartha 1986). Some studies observed a significant inverse correlation between the sediment salinity and MeHg formation (Compeau and Bartha 1984). Furthermore, some studies suggested that MeHg stability reduces when salinity increases, while the demethylation process is favored (Kongchum et al. 2006). However, there are studies that suggest the opposite, that MeHg is more stable in salty water (Whalin et al. 2007). Another study observed that MeHg as a percentage of total Hg increased with salinity in surface estuarine sediment (Hollweg et al. 2009). Because the methylation of mercury in wetland soil was affected by many factors, different wetland ecosystem had their unique physical and chemical properties. Therefore, the change of salinity had different effects on mercury methylation in different wetland ecosystem and may be an important threat that requires further study.
Topsoil was characterized by high soil OM, which could promote the activity of Hg-methylating microbes. However, the existence of dissolved OM could modify Hg2+ species by complexing mercury(Hg) into porewater. This could reduce mercury(Hg) bioavailability and adversely affect MeHg production. MeHg contents in topsoil were lower than those in subsoil, but there were significant outlier MeHg contents at 7–9 cm (Wang et al. 2015). Recent studies indicated that the superficial fraction of soil (< 16 cm) was more conducive to the methylation of mercury(Hg) (Correia and Guimaraes 2016). Mercury(Hg) methylation in sediments was governed by many factors such as OM characteristics, Eh, pH, temperature, salinity, sulfate or sulfide concentrations, microbial diversity and activity (Graham et al. 2012). Under natural conditions, combined effects of salinity, flooding conditions, and complicating environmental factors may have more pronounced effects on mercury(Hg) release and MeHg production in soil of Suaeda heteroptera wetlands.
This paper investigated that the rising salinity would promote the release of mercury(Hg) from Suaeda heteroptera wetland soil under wetting and drying conditions. The full inundation condition could promote the release of mercury(Hg) from soil to flooding water. After the flooding water naturally dried, the mercury(Hg) in flooding water also could be adsorbed by soil again, resulting in the increase of THg contents of soil.
The results showed that MeHg could be synthesized by methylation microorganism under different salinity conditions, but the variation trend of MeHg with salinity was different because of the redox conditions. The low salinity was conducive to MeHg production in soil under flooding conditions, and the MeHg contents and %MeHg were decreased with rising salinity. After the flooding water naturally dried, the high salinity could improve the %MeHg and accelerate MeHg synthesis. Although topsoil was characterized by high soil OM, the MeHg content was higher in subsoil than those in topsoil.
References
Barkay T, Wagner-Döbler I (2005) Microbial transformations of mercury: potentials, challenges, and achievements in controlling mercury toxicity in the environment. Adv Appl Microbiol 57:1–52. https://doi.org/10.1016/S0065-2164(05)57001-1
Barkay T, Gillman M, Turner RR (1997) Effects of dissolved organic carbon and salinity on bioavailability of mercury. Appl Environ Microbiol 63(11):4267. https://doi.org/10.1002/(SICI)1097-0290(19971105)56:3%3c345:AID-BIT13%3e3.0.CO;2-F
Benoit JM (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. ACS Symp 835:262–297. https://doi.org/10.1021/bk-2003-0835.ch019
Berman M, Bartha R (1986) Levels of chemical versus biological methylation of mercury in sediments. Bull Environ Contam Toxicol 36:401–404. https://doi.org/10.1007/BF01623527
Boyd ES, Yu RQ, Barkay T, Hamilton TL, Baxter BK (2017) Effect of salinity on mercury methylating benthic microbes and their activities in Great Salt Lake. Utah Sci Total Environ 581–582:495–506. https://doi.org/10.1007/s00128-018-2326-4
Buckman K, Taylor V, Broadley H et al (2017) Methylmercury bioaccumulation in an urban estuary: delaware River USA. Estuaries Coasts 40(13):1358–1370. https://doi.org/10.1007/s12237-017-0232-3
Compeau G, Bartha R (1984) Methylation and demethylation of mercury under controlled redox, pH and salinity conditions. Appl Environ Microbiol 48(6):1203–1207. https://doi.org/10.1016/0141-4607(84)90089-1
Correia RRS, Guimaräes JRD (2016) Impacts of crab bioturbation and local pollution on sulfate reduction, Hg distribution and methylation in mangrove sediments, Rio de Janeiro. Braz Mar Pollut Bull 109(1):453–460. https://doi.org/10.1016/j.marpolbul.2016.05.028
Cui BS, He Q, Zhao XS (2008) Researches on the ecological thresholds of Suaeda salsa to the environmental gradients of water table depth and soil salinity. Acta Ecol Sin 28:1408–1418 (in Chinese)
Ding ZH, Wu H, Liu JL (2011) Distribution of Hg in mangrove trees and its implication for Hg enrichment in the mangrove ecosystem. Appl Geochem 26:205–212. https://doi.org/10.1016/j.apgeochem.2010.11.020
Driscoll CT, Mason RP, Chan HM (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. https://doi.org/10.1021/es305071v
Dutton J, Fisher NS (2011) Salinity effects on the bioavailability of aqueous metals for the estuarine killifish Fundulus heteroclitus. Environ Toxicol Chem 30:2107–2114. https://doi.org/10.1002/etc.600
Eagles-Smith CA, Ackerman JT (2014) Mercury bioaccumulation in estuarine wetland fishes: evaluating habitats and risks to coastal wildlife. Environ Pollut 193:147–155. https://doi.org/10.1016/j.envpol.2014.06.015
Gardfeldt K, Sommar J, Ferrara R (2003) Evasion of mercury from coastal and open waters of the Atlantic Ocean and the Mediterranean Sea. Atmos Environ 37(1):73–84. https://doi.org/10.1016/s1352-2310(03)00238-3
Graham AM, Aiken GR, Gilmour CC (2012) Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ Sci Technol 46(5):2715–2723. https://doi.org/10.1021/es203658f
Guo HJ (2019) Distribution characteristics and bioavailability of mercury based on diffusive gradients in thin films technique in water of Nansi Lake. University of Jinan, Jinan (in Chinese)
Hollweg TA, Gilmour CC, MasonR P (2009) Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Marire Chem 114(3):86–101. https://doi.org/10.1016/j.marchem.2009.04.004
Hsu-Kim H, Kucharzyk KH, Zhang T (2013) Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Technol 47:2441–2456. https://doi.org/10.1021/es304370g
Kim CS, Rytuba JJ, Brown JGE (2004) EXAFS study of mercury(II) sorption to Fe- and Al-(hydr)oxides: II. Effects of chloride and sulfate. J Colloid Interface Sci 270(1):9–20. https://doi.org/10.1016/j.jcis.2003.07.029
Kongchum M, Devai I, Delaune RD (2006) Jugsujinda total mercury and methylmercury in freshwater and salt marsh soils of the Mississippi river deltaic plain. Chemosphere 63:1300–1303. https://doi.org/10.1016/j.chemosphere.2005.09.024
Li H, Zheng DM, Ma HC (2018) Simulation of total mercury content variability in wetland sediments in the Liaohe Estuary. J Agro-Environ Sci 37(4):774–779 (in Chinese)
Li H, Zheng DM, Yang JS (2019) Salinity and redox conditions affect the methyl mercury formation in sediment of Suaeda heteroptera wetlands of Liaoning province, Northeast China. Mar Pollut Bull 142:537–543. https://doi.org/10.1016/j.marpolbul.2019.03.066
Liang P, Zhang C, Yang YK (2014) A simulation study of mercury release fluxes from soils in wet-dry rotation environment. J Environ Sci 26:1445–1452. https://doi.org/10.1016/j.jes.2014.05.010
Liu RH, Liu SX, Wang J (2017) Change of mercury and methylmercury in Yellow River Delta wetlands from autumn to summer. Acta Sci Circum 37(1):272–279 (in Chinese)
Martin-Doimeadios R, Tessier E, Amouroux D (2004) Mercury methylation/demethylation and volatilization pathways in estuarine sediment slurries using species-specific enriched stable isotopes. Mar Chem 90(1–4):107–123. https://doi.org/10.1016/j.marchem.2004.02.022
Merritt KA, Amirbahman A (2009) Mercury methylation dynamics in estuarine and coastal marine environments—a critical review. Earth Sci Rev 96:54–66. https://doi.org/10.1016/j.earscirev.2009.06.002
Moore C, Carpi A (2005) Mechanisms of the emission of mercury from soil: role of UV radiation. J Geophys Res 110(D24):1–9. https://doi.org/10.1029/2004JD005567
Oliveira DCD, Correia RR, Marinho CC (2015) Mercury methylation in sediments of a Brazilian mangrove under different vegetation covers and salinities. Chemosphere 127(127):214–221. https://doi.org/10.1016/j.chemosphere.2015.02.009
Raposo JC, Ozamiz G, Etxebarria N (2008) Mercury biomethylation assessment in the estuary of Bilbao (North of Spain). Environ Pollut 156(2):482–488. https://doi.org/10.1016/j.envpol.2008.01.017
Rasmussen PE, Mierle G, Nriagu JO (1991) The analysis of vegetation for total mercury. Water Air Soil Pollut 56(1):379–390. https://doi.org/10.1007/BF00342285
Ridley W, Dizikes L, Wood J (1977) Biomethylation of toxic elements in the environment. Science 197(4301):329–332. https://doi.org/10.1126/science.877556
Sun RG, Wang D, Zhang Y (2013) Photo-degradation of monomethylmercury in the presence of chloride ion. Chemosphere 91(11):1471–1476. https://doi.org/10.1016/j.chemosphere.2012.12.013
Tao W (2016) Study on growth ecological stoichiometry relation of Suaeda heteropterain Liaohe River Estuary wetland. Dalian Ocean University, Dalian (in Chinese)
Wang Y, Liu RH, Gao HW (2010) Degeneration mechanism research of Suaeda heteroptera wetland of the Shuangtaizi Estuary National Nature Reserve in China. Procedia Environ Sci 2:1157–1162. https://doi.org/10.1016/j.proenv.2010.10.124
Wang XY, He CF, Sun RG (2015) Releases and methylation of soil mercury in water-level fluctuating zone of the three gorges reservoir region. Environ Chem 34(1):172–177 (in Chinese)
Whalin L, Kim E, Mason R (2007) Factors influencing the oxidation, reduction, methylation and demethylation of mercury species in coastal waters. Mar Chem 107:278–294. https://doi.org/10.1016/j.marchem.2007.04.002
Xiang YP, Du HX, Shen H (2014) Dynamics of total culturable bacteria and its relationship with methylmercury in the soils of the water level fluctuation zone of the Three Gorges Reservoir. Sci Bull 59(24):2966–2972. https://doi.org/10.1007/s11434-014-0324-4
Xiao Y, Yang JS (2015) Soil organic carbon mineralization and its relation with salinity in coastal wetland of Liao-he estuary. Chin J Ecol 34(10):2792–2798 (in Chinese)
Yan HY, Feng XB (2011) Research development on water/air exchange flux of mercury. Environ Chem 30(1):92–96 (in Chinese)
Yin Y, Allen HE, Huang CP (1997) Kinetics of mercury(II) adsorption and desorption on soil. Environ Sci Technol 31(2):496–503. https://doi.org/10.1021/es9603214
Zhang ZS, Sun XJ, Wang QC (2010) Recovery from mercury contamination in the second Songhua River. China Water Air Soil Pollut 211(1–4):219–229. https://doi.org/10.1007/s11270-009-0294-3
Zhang C, Song L, Wang DY (2013) Mercury speciation transformation in soil of the water-level-fluctuating zone in the Three Gorges Area under alternative dry-wet condition. Chin J Appl Ecol 24(12):3531–3536 (in Chinese)
Zheng DM, Yang JS, Li H (2017a) Mercury and methylmercury concentrations and their influencing factors in soils of different types of wetlands of Liaohe Estuary. Chin J Ecol 36(4):1067–1071 (in Chinese)
Zheng SA, Han YL, Li XH et al (2017b) A simulation study on the effect of salinity on the fractions distribution of exogenous mercury in the wastewaterirrigated area of Tianjin City. China Environ Sci 37(5):1858–1865 (in Chinese)
Acknowledgements
We acknowledge Zheng Dongmei, Professor of the University of Shenyang, China, for her help in interpreting the significance of the results of this study.
Funding
Under the auspices of National Natural Science Foundation of China (41571085), Liaoning Province Key R & D Plan Guidance Project (2019JH8/10200024) and The Program for Innovative Talents of Liaoning Higher Education Institution (LR2016078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Zheng, D., Zhang, X. et al. Total and Methylmercury of Suaeda heteroptera Wetland Soil Response to a Salinity Gradient Under Wetting and Drying Conditions. Bull Environ Contam Toxicol 104, 778–785 (2020). https://doi.org/10.1007/s00128-020-02874-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02874-1