Abstract
The effect of vermicompost added to a loam soil on the leaching behaviour of two herbicides (triclopyr and fluroxypyr) was examined. Mobility of the herbicides was assessed using disturbed soil columns under laboratory conditions. In both cases, the addition of vermicompost significantly increased the sorption of the compounds. For both, DT50 values were slightly higher in the amended soil, due to the increased adsorption. Rate constants (k) calculated according to pseudo-first order model were significantly lower in the case of triclopyr (very persistent), which led to a much lower degradation rate compared to fluroxypyr (persistent) in both unamended and amended soils. Values calculated for the experimental leaching index (ELI) in unamended and amended soils showed medium and high leachability for fluroxypyr (0.31 and 0.29) and triclopyr (0.72 and 0.70), respectively. Other index-based screening models (GUS, RLPI, LIX) also catalogue both herbicides as potential leachers. Results confirm that triclopyr and fluroxypyr may contaminate groundwater resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
According to the European Directive on groundwater protection against pollution (EC 2006), groundwater (GW) is the main source of fresh water in the European Union (EU). Many European citizens use GW as a source of drinking water, but unfortunately, its use is seriously threatened by the downward movement of some pesticides used in agriculture through the soil profile (Mouvet 2007). Plant protection products (PPPs), such as some pesticide, efficiently help farmers reduce damages caused by pests, diseases and weeds on crop yields. However, despite their benefit in crop production, the pollution of surface and GW by some PPPs (mainly herbicides) at unacceptable levels should be avoided because they can affect human health, especially those catalogued as endocrine disruptors (Mnif et al. 2011). Consequently, the EU has limited the concentrations of pesticides in GW (0.1 µg L−1 for individual pesticide and 0.5 µg L−1 for total pesticides) (EC 2006) to protect citizens from harmful effects.
Once on the soil, mobility and persistence are two of the main factors involved in the behaviour of a pesticide. Adsorption, degradation and movement are key processes in the fate of pesticides in the environment (Navarro et al. 2007; Durães et al. 2018). Pesticides are commonly leached through soil horizons by irrigation or rainwater. Physical–chemical properties of the pesticide, texture, organic matter content and permeability of the soil, volatilization rate, crop-root uptake, method/dose of pesticide application and climate change are key factors influencing the leaching of pesticides (Pérez-Lucas et al. 2019). Adsorption and desorption processes regulate the magnitude of leaching (Wauchope et al. 2002). Among the different properties of the soil, the content of organic carbon (OC) is considered as the main factor responsible on pesticide adsorption, degradation, and mobility in soil (Briceño et al. 2007; Sadegh-Zadeh et al. 2017). For this reason, the soil organic adsorption coefficient (KOC) is commonly used as an indicator of the relative potential mobility of pesticides through the soil. In addition, the determination of the disappearance half-life time (DT50) in the soil is a valuable tool for calculating a mobility index. Some authors have proposed different screening models to evaluate pesticide leaching potential (Demir et al. 2019; Pérez-Lucas et al. 2019). These methods, based on both the characteristics of soils and physico-chemical properties of pesticides, are easy to apply because pesticide mobility can be estimated with relatively few input data. However, soil properties such as pH and content of organic and inorganic colloids may alter the ionization of the pesticide resulting in different soil-pesticide interactions.
Laboratory studies using disturbed/undisturbed soil columns (OECD 2007; US EPA 2008; Katagi 2013) and field assays by means of suction outdoor lysimeter (Kordel and Klein 2006) are techniques commonly used to assess pesticides leaching potential. The former is useful to evaluate potential of pesticides for downward mobility, while the latter more reliably reflects the probable field behaviour. Disturbed columns are uniformly filled with sieved soil and consequently, this method is generally more reproducible than others to test pesticide mobility.
Among other pesticides, triclopyr (TC) and fluroxypyr (FR), members of the pyridine class of herbicides (Fig. 1), are widely used to control unwanted broadleaf weeds inducing auxin-like responses and resulting in disruption of plant cell growth. Drift or runoff from treated areas may be hazardous to aquatic organisms and non-target plants. Both are relatively non-toxic to terrestrial wildlife, but relatively toxic to freshwater fish and aquatic invertebrates showing high water solubility (> 6 g L−1) and Groundwater Ubiquity Score (GUS) index (2.4–2.9) (Lewis et al. 2016). Therefore, they are candidates to pollute GW resources. However, to the best of our knowledge, the leaching behaviour of both herbicides influenced by addition of organic amendments has not been reported. With this aim, laboratory studies were conducted to assess the effect of an earthworm humus (vermicompost) on the mobility of both herbicides using disturbed loam soil columns.
Materials and Methods
Analytical standards (≥ 98.5%) of TC and FR were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Chromatographic-grade acetonitrile and reagents (NaCl and CaCl2) were supplied by Scharlab (Barcelona, Spain).
A Calcaric regosol (IUSS 2006) taken from the Campo de Cartagena (Murcia, southeastern Spain) with loam texture (26.1% sand, 48.4% silt, 25.5% clay) was chosen for this study. Soil samples were collected from the surface (top 20 cm), air-dried, and sieved through a 2 mm mesh. The main physico-chemical properties of the soil (pH: 7.9; organic matter (%): 0.4; total nitrogen (%): 0.2; CaCO3 (%): 37.4, and cation exchange capacity: 23.9 meq/100 g) were determined by standard methods (Sparks 1996).
The earthworm humus, pH: 8.4; electrical conductivity (dS m−1): 2.1, organic matter (%): 34.4; total nitrogen (%): 1.3; humic acids (%): 14.2; fulvic acids (%): 5.8 used as organic amendment was supplied by Lombrimur (Murcia, Spain). For adsorption, degradation and leaching experiments, the appropriate amount of vermicompost was added to reach 0.8% of organic matter content in the amended soil.
Adsorption behaviour of herbicides was determined using a batch equilibrium method (OECD 2000). Briefly, 0.01 M CaCl2 was used to prepare standard solutions of TC and FR to maintain a constant ionic strength and reduce cation exchange. Triplicate 5 g soil samples were added to a 50 mL centrifuge tube containing 25 mL of herbicide solution (0.1 µg mL−1). The mixture was shaken until adsorption equilibrium was reached (24 h at 20 ± 1 °C). Finally, tubes were centrifuged at 4000 rpm for 10 min, and the supernatant was filtered through a nylon filter (0.22 μm). The amount adsorbed (Ca) was considered as the difference between Ci (initial concentration in solution) and Ce (concentration remaining after equilibration). The distribution coefficient (Kd) was obtained from the relationship between Ca and Ce (Kd = Ca/Ce). Finally, the KOC was calculated as (Kd × 100)/% OC.
Soil degradation experiments followed the OECD (2002) procedure. To evaluate herbicide persistence, soil samples (50 g, as oven dry weight basis) were placed in incubation flasks (n = 3). Afterwards, 1 mL of a methanol/water (10/90 v/v) solution containing 37.5 μg of TC and FR was applied to achieve an initial concentration of 0.75 μg g−1 of each. According to the bulk density (1.35 g cm−3) of the soil and assuming herbicides were uniformly distributed along the top 10 cm of the soil, the amount added (0.75 mg kg−1) was equivalent to an application rate of 1 kg ha−1. Then, all flasks (n = 3) were incubated in the dark at 21 ± 2 °C with the soil moisture at 30% of the field capacity. After 7, 15, 30 and 63 days of incubation, flasks from each treatment were taken and kept at 4 °C for 2–3 days until analysis. Water losses (> 10% of the initial value) were compensated weekly by addition of distilled water. Experimental data were fitted according to pseudo first-order kinetics: Ct = C0 e−kt → Ln Ct = Ln C0 − kt, where C0 is the initial concentration of the herbicide; k is the rate constant (day−1); Ct is the concentration of herbicide in the soil at a given time; and t is the post-application time (d).
Leaching behaviour was studied following OECD (2007) guidelines. Briefly, the movement of herbicides was studied in polyvinyl chloride (PVC) columns (40 cm length, 5 cm i.d.) filled with 300 g of soil. After the column was packed, air present in the column was removed by using 0.01 M CaCl2, and the water in excess was eliminated by gravity. Following this, 1 mL of a methanol/water solution (10 + 90, v/v) containing 225 µg mL−1 of each compound (spiking level = 0.75 µg g−1) was added to the top of each column. Twenty-four hours after herbicide application, compounds were leached by adding 600 mL of 0.01 M CaCl2 with a peristaltic pump. Leachates (50 or 100 mL day−1) were quantitatively collected at the bottom of the columns during nine days and then filtered through a nylon membrane filter (0.22 μm). Finally, columns were opened, and the soil separated in three segments of equal length. To avoid aqueous hydrolysis, herbicide residues were daily extracted from leachates. Experiments were carried out in triplicate avoiding direct light.
In all cases, water samples (10 mL) were added to a centrifuge tube and then extracted with 10 mL of acetonitrile by sonication (15 min at 0.5 cycles and 60% amplitude) using a 200 W sonic dismembrator (Dr. Hielscher GmbH, Stahnsdorf, Germany) followed by a salting-out step with 2 g NaCl. The tube was shaken and centrifuged for 5 min at 4000 rpm. Dried soil samples (10 g) were extracted with 20 mL of acetonitrile/water (1/1) by sonication and centrifuged at 4000 rpm during 10 min. In both cases, 2 mL of the supernatant were filtered (0.22 μm nylon membrane) and transferred into the chromatographic vial for high performance liquid chromatography (HPLC)-mass spectroscopy (MS) analysis.
TC and FR residues were determined using an HPLC system (1200 series, Agilent Technologies, Santa Clara, USA) equipped with a triple quadrupole mass spectrometer (G6410A series, Agilent Technologies) operating in electrospray ionization (ESI) positive ion mode. Separation was carried out using reversed phase C8 analytical column of 150 mm × 4.6 mm and 5 μm particle size (Zorbax Eclipse XDB-C8) maintained at 25 °C. The mobile phase consisted of acetonitrile (solvent A) and 0.1% (v/v) formic acid in water (solvent B). The gradient program was set as follows: 10% A, constant for 5 min, followed by a linear gradient to 100% A to 35 min. The flow rate was maintained constant (0.6 mL min−1), and the injected sample volume was 5 μL. Two single reaction monitoring (SRM) transitions were used for quantitation and confirmation of TC (SRM1: 256 → 210; SRM2: 256 → 146) and FR (SRM1: 255 → 209; SRM2: 255 → 181). SigmaPlot version 13 statistical software (Systat, Software Inc., San Jose, CA) was used for curve fitting and calculation of statistical parameters.
Results and Discussion
Linearity had R > 0.99 over the range assayed (10–1000 μg L−1). Both limits of detection (LODs) and quantification (LOQs) were calculated from the signal-to-noise (S/N) ratio of 3 and 10, respectively. LOQs were 0.08 μg L−1 (FR) and 0.12 μg L−1 (TC) in water and 0.13 (FR) and 0.19 (TC) μg kg−1 in soil. Average recoveries (n = 5) obtained for both herbicides in water (10 μg L−1 and 100 μg L−1) ranged from 83 to 103% with relative standard deviations (RSD) < 7.3%. In the case of soil, recoveries (100 µg kg−1 and 1000 µg kg−1) obtained varied from 81 to 93% and RSD < 4.8%.
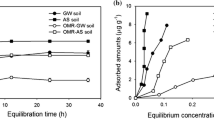
Calculated values of Koc in the amended (AS) and unamended (US) soil are shown in Fig. 2. In both cases, addition of vermicompost significantly (p < 0.05) increased the sorption of the herbicides. However, values below 2 indicate a limited retention capacity in the soil, with the consequent possibility of leaching.
Similar results (log KOC = 1.1–1.9) were found by other authors (MDAR 2017) in laboratory studies conducted with twelve soils and different contents of OM. Other works (Lehmann et al. 1990; Kah et al. 2007) point out that values of log KOC for FR, in different soils, vary between 1.7 and 2.2, also in the range calculated by the current study. The pKa values of FR (2.94) and TC (2.68) indicate that both herbicides are found anionically in the soil. Thereby, based on the soil pH (7.9), repulsion occurs with the negative charges present on the surface of clays and organic matter.
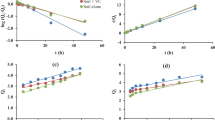
As observed in Table 1, data fit well to a first-order kinetics because linearity was observed after logarithmic transformation with coefficient of determination (R) ranging from 0.93 to 0.96, higher values than the threshold (R ≥ 0.7) suggested by Organisation for Economic Cooperation and Development (OECD 2002). The standard error of estimate (Sy/x) values were lower than 0.02 in the most unfavourable case.
Rate constants (k) calculated are significantly lower in the case of TC, which leads to a much lower degradation rate compared to FR in both soils. Consequently, DT50 (t½) values are 2–3 times higher for TC. For both herbicides, half-lives were slightly higher in the AS, due to the increased adsorption, although some authors indicate higher degradation rates for FR in AS due to the increase of microorganisms (Tao and Yang 2011). According to the classification proposed by Gravrilescu (2005), FR can be considered “persistent” and TC “very persistent”. Lehmann et al (1991) found a percentage of degradation for FR of 60% after 30 days, although after 366 days, the major residue corresponded to methoxypyridine, the main metabolite originated by degradation of the parent compound. Other studies provide DT50 values ranging from 39 days (forest soil) to 69 days (mineral soil) (Thompson et al. 2000), while Hu et al. (2014) found variable DT50 values varying from 27 to 43 days depending on the type of soil. Regarding TC, certain authors specify a DT50 in the soil of 138 days (Johnson et al. 1995).
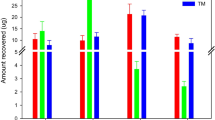
Distribution from soil and water for both herbicides applied to US and AS columns is depicted in Fig. 3a. In the case of the US, herbicides were found in leachates ranging from 17% (FR) to 40% (TC) of the initial mass in the soil. Addition of vermicompost weakly reduced the leaching of the herbicides. For FR, the mass leached from the AS decrease by 11% as compared to the value recovered from US. In the case of TC, the decline was only 5%. Only residues of TC and FR were found in the middle and bottom layers of the AS columns, although at very small levels, lower than 0.5% of the initial mass added to the columns.
Relative breakthrough curves (BTCs) of the herbicides applied to US and AS columns are shown in Fig. 3b. Relative BTCs show both herbicides leached quickly (maximum about 100–150 mL) in both cases. For AS, when 300 mL were recovered, the shape of the curve indicated some interaction with the inorganic and organic soil fractions, especially humic and fulvic acids. The amber colour of the AS leachates confirm the presence of dissolved organic matter (DOM), as demonstrated by the absorbance measurements (285 nm and 450 nm) (data not shown). A decrease in herbicide leaching was observed with the fall of the concentration of DOM in leachates. Although the extent to which DOM is involved in the transport process of pesticides is not well-known, the movement of herbicides may be enhanced by an increase in DOM moving through the soil by DOM-herbicide unions. Based on the pH of the study, both, humic and fulvic acids are expected to be more soluble in water and encourage herbicides to be in aqueous solution by the increase of the hydrophilicity as they serve as an aqueous solubility enhancer for most of the organic pollutants. However, the humin fraction may facilitate pesticide sorption by increasing reactivity and molecular stability, thus contributing to increased hydrophobic interactions with pesticides. According to Wanting et al. (2005), the presence of hydrophobic fractions of DOM promotes adsorption of some herbicides as atrazine, while hydrophilic fractions tend to inhibit adsorption.
The current study propose a new and simple index referred to as the experimental leaching index (ELI) using the following equation:
where MLIX is the total recovered mass (µg) / added mass (µg), VW is the total volume in leachates and MS is the mass of soil used. The ELI values are between 0 and 2 to facilitate a comparison between different pesticides. Classification criteria of leachability is as follows: ELI ≤ 0.1: Immobile; 0.1 > ELI ≤ 0.6: Transition; 0.6 > ELI ≤ 1.5: Mobile; 1.5 > ELI ≤ 2: Very mobile.
This index has been compared with others proposed by different authors such as the Groundwater Ubiquity Score (GUS), Relative Leaching Potential Index (RLPI) and Leachability Index (LIX). All are based on persistence and mobility parameters such as first-order rate constant (k), half-life (t½) and the soil organic adsorption coefficient (KOC):
GUS = log t1/2 · (4 - log Koc) (Gustafson 1989)
GUS > 2.8: Mobile; 1.8 > GUS < 2.8: Transition; GUS < 1.8: Immobile
RLPI = (KOC/t1/2) · 10 (Hornsby 1992)
RLPI ≤ 10: Mobile; 10 > RLPI < 2000: Transition; RLPI ≥ 2000: Immobile
LIX = exp (−k · KOC) (Spadotto 2002)
LIX = 1: Very mobile; 0.1 > LIX < 1: Mobile; 0 > LIX < 0.1: Transition; LIX = 0: Immobile
Table 2 shows the calculated values for the four indices used. The GUS values are slightly higher than the values contributed in literature (2.42 and 2.85 for FR and TC, respectively) (Lewis et al. 2016). Although both herbicides have relatively low KOC values, they can be considered as persistent compounds (DT50 > 100 days), especially TC (very persistent). Consequently, both herbicides can be classified as potential leachers according to the GUS index. According to LIX, the calculated values are near to 1, especially TC, indicating high leachability. Regarding RLPI, in both cases, the values are lower than 10. In this case, TC also presents a higher risk for downward movement through the soil. Finally, the proposed index (ELI) indicated that FR is leachable and TC very leachable.
Indices used catalogue both herbicides as potential leachers (medium–high leachability) in both AS and US. Consequently, they are serious candidates to contaminate GW resources. Further studies should be carried out to evaluate the effect of other organic amendments to decrease the leaching potential of TC and FR as well as the possible presence of degradation products in soil and leachates.
References
Briceño G, Palma G, Durán N (2007) Influence of organic amendment on the biodegradation and movement of pesticides. Crit Rev Environ Sci Technol 37:233–271
Demir AEA, Dilek FB, Yetis U (2019) A new screening index for pesticides leachability to groundwater. J Environ Manag 231:1193–1202
Durães N, Novo LAB, Candeias C, da Silva EF (2018) Distribution, transport and fate of pollutants. In: Soil pollution. Academic Press, London, pp 29–56
European Commission (EC) (2006) Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Off J Eur Union L 372:19–31
Gavrilescu M (2005) Fate of pesticides in the environment and its bioremediation. Eng Life Sci 6:497–526
Gustafson D (1989) Groundwater ubiquity score: a simple method for assessing pesticide leachability. Environ Toxicol Chem J 8:339–357
Hornsby AG (1992) Site-specific pesticide recommendations: the final step in environmental impact prevention. Weed Technol 6:736–742
Hu J, Wang T, Long J, Chen Y (2014) Hydrolysis, aqueous photolysis and soil degradation of fluroxypyr. Inter J Environ Anal Chem 94:211–222
IUSS Working Group WRB (2006) World reference base for soil resources 2006. World Soil Resources Reports No. 103. FAO, Rome
Johnson WG, Lavy TL, Gbur EE (1995) Sorption, mobility and degradation of triclopyr and 2,4-d on 4 soils. Weed Sci 43:678–684
Kah M, Beulke S, Brown CD (2007) Factors influencing degradation of pesticides in soil. J Agric Food Chem 55:4487–4492
Katagi T (2013) Soil column leaching of pesticides. Rev Environ Contam Toxicol 221:1–105
Kordel W, Klein M (2006) Prediction of leaching and groundwater contamination by pesticides. Pure Appl Chem 78:1081–1090
Lehmann RGJ, Miller R, Laskowski DA (1990) Fate of fluroxypyr in soil. Weed Res 30:383–388
Lehmann RGJ, Lickly LS, Lardie TS, Miller JH, Baldwin WS (1991) Fate of fluroxypyr in soil: 3. Significance of metabolites to plants. Weed Res 31:347–355
Lewis KA, Tzilivakis J, Warner DJ, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess 22:1050–1064
Massachusetts Department of Agricultural Resources (MDAR) (2017) Triclopyr. Executive Office of Energy and Environmental Affairs, Boston
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8:2265–2303
Mouvet C (2007) Pesticides in European groundwaters: biogeochemical processes, contamination status and results from a case study. In: Quevauviller P (ed) Groundwater science and policy: an international overview. RSC Publishing, London, pp 545–583
Navarro S, Vela N, Navarro G (2007) An overview on the environmental behaviour of pesticide residues in soils. Span J Agric Res 5:357–375
Organization for Economic Cooperation and Development (OECD) (2000) Guidelines for testing of chemicals, No 106, adsorption–desorption using a batch equilibrium method. Organization for Economic Cooperation and Development (OECD), Paris
Organization for Economic Cooperation and Development (OECD) (2002) Guidelines for testing of chemicals, No. 307, aerobic and anaerobic transformation in soil. Organization for Economic Cooperation and Development (OECD), Paris
Organization for Economic Cooperation and Development (OECD) (2007) Guidelines for testing of chemicals, No. 312, leaching in soil columns. Organization for Economic Cooperation and Development (OECD), Paris
Pérez-Lucas G, Vela N, El Aatik A, Navarro S (2019) Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In: Larramendy ML, Soloneski S (eds) Pesticides—use and misuse and their Impact in the environment. IntechOpen, London, pp 45–71
Sadegh-Zadeh F, Wahid SA, Jalili B (2017) Sorption, degradation and leaching of pesticides in soils amended with organic matter: a review. Adv Environ Technol 2:119–132
Spadotto CA (2002) Screening method for assessing pesticide leaching potential. Pestic Rev Ecotoxicol Meio Ambient 12:69–78
Sparks DL (1996) Methods of soil analysis. Part 3—chemical methods. Soil Science Society of America, Madison, pp 1085–1121
Tao L, Yang H (2011) Fluroxypyr biodegradation in soils by multiple factors. Environ Monit Assess 175:227–238
Thompson DG, Pitt DG, Buscarini TM, Staznik B, Thomas DR (2000) Comparative fate of glyphosate and triclopyr herbicides in the forest floor and mineral soil of an Acadian forest regeneration site. Can J For Res 30:1808–1816
United States Environmental Protection Agency (US EPA) (2008) Fate, transport and transformation test guidelines. Leaching studies. OPPTS 835.1240, EPA 712-C-08-010. Environmental Protection Agency, Washington DC
Wanting L, Jianming X, Yanzheng G (2005) Effects of dissolved organic matter from sewage sludge on the atrazine sorption by soils. Sci China Ser C Life Sci 48:57–66
Wauchope RD, Yeh S, Linders J, Kloskowski R, Tanaka K, Rubin B, Katayama A, Kördel W, Gerstl Z, Lane M, Unsworth JB (2002) Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Manag Sci 58:419–445
Acknowledgements
The authors are grateful to University of Murcia for financial support (Project 4711).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pérez-Lucas, G., Vela, N., Abellán, M. et al. Use of Index-Based Screening Models to Evaluate the Leaching of Triclopyr and Fluroxypyr Through a Loam Soil Amended with Vermicompost. Bull Environ Contam Toxicol 104, 497–502 (2020). https://doi.org/10.1007/s00128-020-02818-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02818-9