Abstract
Assessing the effects of phytoextraction on soil properties is important for successful implementation of this method. This study was conducted to evaluate the effects of phytoextraction by Sedum alfredii Hance on the availability of metals and improvement of the microbial community (biomass and structure) of a Cd, Zn and Pb contaminated soil. Phytoextraction significantly decreased the acid extractable, Mn/Fe oxide and organic matter bound fractions of Cd and Zn as well as the acid extractable Pb in the rhizosphere soil. Soil microbial biomass, total, bacterial, actinomycete, fungal, AM fungal, and protozoa phospholipid fatty acids (PLFAs) were significantly enhanced. The ratio of fungal to bacterial and gram-positive to gram-negative bacterial PLFAs were significantly changed. Redundancy analysis showed that microbial biomass and specific groups of PLFAs were negatively correlated with available metals while positively correlated with dissolved organic carbon/organic acids. In conclusion, phytoextraction by S. alfredii reduced available metal concentrations and improved soil microbial properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Over the past decades, heavy metal contamination of different soils has become a serious ecological problem in China. According to a nationwide soil survey conducted by the Environmental Protection Ministry of China, heavy metals are the main pollutants on farmland. Heavy metals are non-biodegradable and display long-term persistence, therefore they represent a permanent threat to ecosystems, to plant growth and to human health through the food chain (Chary et al. 2008). Thus, it is urgent to select appropriate methods to remediate the contaminated soils. Phytoextraction, the use of green plants to take up heavy metals from soil, is a promising technique that has the benefits of being in-situ, low cost and environmentally sustainable (Garbisu and Alkorta 2001; Sheoran et al. 2010).
The rhizosphere is a unique soil–root interface where physico-chemical properties and biogeochemical processes drastically differ from the non-vegetated soil (Kidd et al. 2009). In this microsphere, the root-induced changes of soil properties, especially pH and dissolved organic carbon (DOC) are recognized as critical factors influencing metal solubility (Kidd et al. 2009; Kim et al. 2010). Moreover, the bioavailability of heavy metals can be modified by root exudates. Among the root exudates, organic acids were reported to be responsible for dissolving the solid phase metals and making them available for plant (Cieśliński et al. 1998). The special conditions in the rhizosphere of hyperaccumulators such as metal replenishment from less available pools are critical for the uptake of heavy metals.
The removal of metals from soil is the basic aim of phytoextraction. Therefore, many studies were conducted to investigate the influence of hyperaccumulators on soil metal dynamics (Li et al. 2014; Martínez-Alcalá et al. 2016). Numerous methods were used to investigate the behavior of metals in soils. Sequential extraction has been widely used to investigate the effects of environmental changes on soil metal bioavailability and mobility. Among the different methods for sequential extraction, the procedure proposed by the Community Bureau of Reference (BCR) has been widely used in analysis of metal availability in soil (Anju and Banerjee 2010). In this present study, the BCR procedure was used to determine changes of metal availability and combined forms in the rhizosphere of Sedum alfredii during phytoextraction.
Although the effects of phytoextraction on soil metal removal have been extensively studied, it should be noted that the ultimate goal of soil remediation is not only to remove metals from soil but to improve soil quality (Epelde et al. 2009). Thus, relevant indicators reflecting soil quality should be chosen carefully. Soil microorganisms play a fundamental role in soil nutrient cycling. In addition, soil microbes are highly sensitive to changes in soil conditions. Therefore, microbial properties are increasingly being used to assess soil ecological health under the influence of heavy metals (Epelde et al. 2009; Jiang et al. 2010). Among the different microbial properties, microbial biomass carbon (MBC) gives an early indication of soil organic matter, which is the effective part of soil biomass and has major impacts on soil fertility (Ciadamidaro et al. 2014). Different groups of microorganisms have different patterns of phospholipid fatty acids (PLFAs), therefore analysis of PLFAs enables direct characterization of the microbial community (Azarbad et al. 2016). This method provides useful information on the responses of microbial structure to metal contamination (Pennanen et al.1996; Azarbad et al. 2016).
Sedum alfredii Hance originating from an ancient silver mining site of Zhejiang has a remarkable capacity to hyperaccumulate Cd and Zn as shown in our previous field investigation (Yang et al. 2014).Therefore, S. alfredii might be a good choice to be used in soil conservation and phytoextraction. However, the rhizosphere dynamics of S. alfredii during phytoextraction have not been fully understood. Few studies have been conducted to evaluate the effects of phytoextraction by S. alfredii on soil microbiological properties of highly contaminated soils by multiple metals (Cd–Zn–Pb). Information about the relationships between soil microbial and chemical properties such as different forms of metals and organic acids have not been elucidated. Therefore, we carried out a greenhouse experiment to study rhizosphere soil chemical properties (pH, organic acids, DOC and metal fractions) and microbial properties (MBC and PLFA) during 8 months growth of S. alfredii. Then, we explored the relationships between soil chemical and microbial properties. We hypothesized that S. alfredii growing in heavy metal contaminated soils could induce positive changes on the chemical and biochemical properties of metal contaminated soil.
Materials and Methods
The experimental soil was collected from a mining area in Zhejiang province, China. The basic physiochemical properties of the soil were pH 6.4, total organic C 16.3 g kg−1, total N 1.2 g kg−1, CEC 10.2 cmol kg−1, total Cd 70.7 mg kg−1, total Zn 8773 mg kg−1 and total Pb 17,651 mg kg−1. The shoots of S. alfredii were obtained from an ancient silver-mining site. The plant shoots were grown in Hoagland solution before being transferred to pots. The pot experiment had a completely randomized design that had two treatments such as unplanted control and planted in the pot. Each treatment was conducted with three replicates. Four uniform re-rooted S. alfredii seedlings were transplanted into each pot. The experiment was carried out in a greenhouse, whose temperature and humidity were maintained at 20/25°C night/day and about 70%, respectively. Soil water content was adjusted to 60% of water holding capacity. After 8 months growth, plants were harvested and soils were sampled. In the plant-grown pot, the root with adhering soil was dug up from the pot. The soil that remained in the pot was considered as the near-rhizosphere soil. Soil at about 1–5 mm from the root surface was collected by brush and treated as rhizosphere soil.
Soil pH was measured using a pH meter (Thermo 410C-01A, USA) according to the standard method NY/T1121.2-2006 (Zhao et al. 2017). Soil DOC was extracted according to the method of Jones and Willett (2006). DOC in the soil solutions was determined using a TOC analyzer (Shimadzu TOC-V CPH total organic carbon analyzer, Japan). The soil organic acids were extracted as described by Cieśliński et al. (1998). The extracts were injected into a reverse phase column (250×4 mm) with a flow rate of 0.6 mL min−1 and detected at 210 nm by HPLC (Agilent 1200, USA). The isocratic elution was Na2SO4 solution (100 mM). Soil Cd, Zn and Pb were fractionated with the BCR procedure (Pueyo et al. 2008). First step (acid-soluble fraction): soil was extracted with 0.11 mol L−1 acetic acid (v:w ratio = 40:1, 16 h). Second step (Mn/Fe oxide bound fraction): the residue from the first step was extracted with 0.5 mol L−1 hydroxylammonium chloride (v:w ratio = 40:1, 16 h). Third step (organic matter bound fraction): the residue from the second step was digested with H2O2 then extracted by 1 mol L−1 ammonium acetate (v:w ratio = 40:1, 16 h). Fourth step: the residue from the third step was digested with HNO3–HClO4–HF. To evaluate the accuracy of the procedure, reference material was used (GBW07437, soil for extractable trace elements) with the same procedure. Blank determination was done using the same procedure. The Cd, Zn and Pb contents in the solution and digestion were estimated using ICP-MS (PerkinElmer NexION 300X). The fumigation–extraction method was used to determine soil microbial biomass C (Vance et al. 1987). The concentration of organic C was measured by TOC analyzer (Shimadzu TOC-V CPH total organic carbon analyzer, Japan). Microbial biomass C was calculated by the difference between organic C of fumigated and non-fumigated soil calculated by a conversion factor of 0.45. The PLFAs were extracted, fractionated, and methylated as described by Wu et al. (2009). Fatty acid methyl esters were analysed in GC (Agilent N6890, USA) with MIDI peak identification software (Version 4.5, Newark, DE). The methyl nonadecanoate fatty acid (19:0) was added as an internal standard. Based on the literature (Frostegård and Bååth 1996), PLFAs were grouped into bacterial, fungal, actinomycete, AM fungal, and protozoa PLFAs.
All the data were expressed as mean with standard errors. One-way ANOVA was employed to determine the statistical differences in values among the rhizosphere, near-rhizosphere and bulk soils by using SPSS19.0. Redundancy analysis (RDA) was conducted to determine the correlations between soil microbial characteristics and soil chemical properties by using Canoco for Windows 4.5.
Results and Discussion
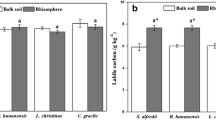
Chemical conditions such as pH in the rhizosphere of hyperaccumulators play an important role in metal accumulation and speciation (Kim et al. 2010). A significant decrease in pH in the rhizosphere of S. alfredii was found in our study (Table 1). This may in part be due to the significantly (p < 0.05) increased organic acids in the rhizosphere of S. alfredii (Table 1). After 8 months growth, three kinds of organic acids (lactic, tartaric and acetic acid) were found in rhizosphere soil. In contrast, only lactic acid and tartaric acid were detected in the near-rhizosphere and the unplanted soils. Rhizosphere induced acidification has been reported to play an important role in metal accumulation, such as in Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu (Jiang et al. 2010) and Rorippa globosa (Turcz.) Thell (Wei and Twardowska 2013). Growth of S. alfredii led to significantly (p < 0.05) higher DOC contents in the rhizosphere and near-rhizosphere soils after 8 months growth (Table 1). This may be due to the input of plant litter and root exudates. The increase in DOC in the rhizosphere of hyperaccumulators had previously been documented. Wei and Twardowska (2013) reported that DOC was significantly increased in the rhizosphere of R. globosa after phytoextraction of Cd contaminated soil. Growth of Brassica juncea in long-term heavy metal contaminated soil significantly increased the soil DOC relative to the control (Kim et al. 2010). Therefore, it can be concluded that rhizosphere acidification and increased DOC might be important for uptake of metals by S. alfredii.
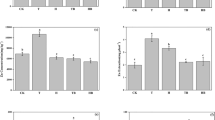
Phytoextraction of heavy metal contaminated soils have usually focused on the changes in the available metals. The metal fractions extracted by the BCR method at different steps represent different degrees of bioavailability (Anju and Banerjee 2010). The acid extractable, Mn/Fe oxides and organic matter bound fractions are considered to be available or potentially available. In this study, the acid extractable, Mn/Fe oxide bound and organic matter bound fractions of Cd and Zn was significantly (p < 0.05) decreased in the rhizosphere soil compared to those of the near-rhizosphere and unplanted soils (Figs. 1, 2). In addition, the acid extractable fraction of Pb was also significantly (p < 0.05) decreased (Fig. 3). S. alfredii has a high ability to take up metals from soil. The metal uptake by S. alfredii might be responsible for the removal of available metals found after phytoextraction. It has been reported that hyperaccumulators could provoke changes in the rhizosphere soil properties, which facilitate the uptake of metals (Kidd et al. 2009). Many rhizosphere experiments with hyperaccumulators show a clear decrease in metal availability in rhizosphere soils after phytoextraction (Li et al. 2014; Martínez-Alcalá et al. 2016). However, metal availability in soils depends on the uptake by plants and the replenishment from non-available pools. Thus, there is no general consensus as to whether hyperaccumulators enhance or diminish available metals in the rhizosphere. Contrasting results have also been reported, which showed metal bioavailability increased in the rhizosphere of hyperaccumulators (Kim et al. 2010).
The different fractions (acid extractable, Mn/Fe oxide bound, organic matter bound and residual form) of Zn extracted by the BCR procedure in rhizosphere, near-rhizosphere and unplanted soils. Bars represent standard errors. Different letters on the error bars indicate significant differences at p < 0.05
The different fractions (acid extractable, Mn/Fe oxide bound, organic matter bound and residual form) of Pb extracted by the BCR procedure in rhizosphere, near-rhizosphere and unplanted soils. Bars represent standard errors. Different letters on the error bars indicate significant differences at p < 0.05
The measurement of MBC can be used to assess the impact of soil metal contamination (Ciadamidaro et al. 2014). After 8 months of growth, the MBC of rhizosphere soil was significantly (p < 0.05) higher than that of the near-rhizosphere and unplanted soils (Table 2). Analysis of PLFAs showed that phytoextraction by S. alfredii noticeably enhanced the total PLFAs and specific groups (bacterial, actinomycete, fungal, AM fungal, and protozoa) of PLFAs (Table 2). The results of principal component analysis (PCA) showed that the rhizosphere soil was separated clearly from the near-rhizosphere and bulk soils by positive PC1 values (Fig. 4). In agreement with our results, previous studies also confirmed that plant growth could increase the MBC of the metal contaminated soils (Jiang et al. 2010; Ciadamidaro et al. 2014). The higher MBC sustained by plant growth likely occurred through rhizo-depositions. Positive correlations among MBC/PLFAs and soil DOC (organic acids) found in this study suggested that the input of labile carbon stimulated the growth of microorganisms (Fig. 5). It has been previously reported that metals have adverse effects on biomass of different groups of soil microbes. Liu et al. (2016) showed that Cu significantly reduced the total, bacterial, and fungal PLFAs in soil. Thus, in our study, the reduction of available metals may also contribute to the increase of microbial biomass. The negative correlations between MBC/PLFAs and metal concentrations support this hypothesis (Fig. 5).
The ratios of different groups of microbial PLFAs such as F/B and GP/GN were frequently used to indicate environmental change and functional shifts of the soil microbial community. High concentrations of metals were reported to have led to a reduction of F/B (Pennanen et al. 1996). In our study, significantly higher values of F/B were found in the rhizosphere and near-rhizosphere soils (Table 2). One explanation would postulate that fungi on average are more sensitive to different availability of metals than bacteria, facilitating their adaptation to decreased metal concentrations in the rhizosphere during phytoextraction. The negative correlation between the F/B and heavy metals found in this study support this view (Fig. 5). In addition, a higher value of F/B was reported to correlate with high contents of organic carbon (Stevenson et al. 2014). Similarly, positive correlations between F/B and soil DOC (organic acids) was found (Fig. 5). In this study, phytoextraction by S. alfredii decreased the GP/GN (Table 2). This finding indicated that the biomass of GN bacteria increased more than GP bacteria in the rhizoshere. This increase may be caused by GN bacteria utilizing a variety of C sources and quickly adapting to the environment (Chang et al. 2016). Thus, it is not surprising to find that GP/GN negatively correlated with DOC and organic acids (Fig. 5). Moreover, positive correlations between GP/GN and metal concentrations were found (Fig. 5). This is consistent with the results found by Garcia-Sanchez et al. (2015), showing that GP/GN was significantly higher in metal polluted soils. These findings indicated that the phytoextraction process alleviated the toxicity of metals to the microbial community.
Phytoextraction is a promising technology that uses hyperaccumulating plants to remove heavy metals from soils. However, several issues should be considered before this technology can be put into practice. One issue is the applicability under field conditions. In recent years, co-planting system (the growth of hyperaccumulator plant associated with crop) was introduced to practice agricultural production in the contaminated soils. Some research showed that co-planting could remove heavy metals from the contaminated soil by hyperaccumulators while restricting metal uptake by crops (Wu et al. 2007; Wang et al. 2015). S. alfredii is a relatively fast-growing and high-biomass hyperaccumulator, which has potential in the co-planting system, especially in South China, the main heavy metal contaminated area of China. The climate (temperature and seasonal precipitation) in South China is suitable for the growth of S. alfredii, which is natively occurring in South China. This study showed that S. alfredii growth improved soil quality by reducing soil available metals and increasing microbial biomass and thus will provide more suitable soil conditions for the survival of local flora and fauna. What’s more, S. alfredii is a perennial plant that could be harvest several times a year. In addition, the high amounts of phytoextraction residue need to be managed properly. To date, methods have been developed to deal with the phytoextraction residue, such as direct disposal or landfilling (Ribé et al. 2012). However, due to the limitation in site selection and the high leaching risk, the thermal conversion method has been adopted for phytoremediation residue disposal, such as pyrolyzed as biochar (Huang et al. 2018). It has been reported that the pyrolysis could decrease the metal bioavailability of phytoextraction residue and the resulting product is of low ecological risk (Wang et al. 2017; Huang et al. 2018). Thus, after harvesting the aboveground parts, pyrolysis might be used for the disposal of S. alfredii residue. If the environmental risk of S. alfredii biochar turns out to be low, then they may be used as efficient sorbents for heavy metals.
This work demonstrated that phytoextraction by S. alfredii significantly decreased the acid extractable, Mn/Fe oxides and organic matter bound fractions of Cd and Zn and acid extractable Pb in contaminated soil. In addition, growth of S. alfredii decreased the pH and increased DOC and organic acids in rhizosphere soil. In regards to microbial properties, phytoextraction by S. alfredii enhanced soil MBC, total and specific groups (bacterial, actinomycete, fungal, AM fungal, and protozoa) of PLFAs. Soil microbial properties were closely related to chemical properties such as available metal concentrations, pH, DOC and organic acids. In conclusion, phytoextraction by S. alfredii reduced metal availability by decreasing the available metal pool and improved soil microbial properties.
References
Anju M, Banerjee DK (2010) Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 78:1393–1402
Azarbad H, van Straalen NM, Laskowski R, Nikiel K, Röling WFM, Niklińska M (2016) Susceptibility to additional stressors in metal-tolerant soil microbial communities from two pollution gradients. Appl Soil Ecol 98:233–242
Chang E-H, Chen T-H, Tian G, Chiu C-Y (2016) The effect of altitudinal gradient on soil microbial community activity and structure in moso bamboo plantations. Appl Soil Ecol 98:213–220
Chary NS, Kamala CT, Raj DS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Ciadamidaro L, Madejón P, Madejón E (2014) Soil chemical and biochemical properties under Populus alba growing: three years study in trace element contaminated soils. Appl Soil Ecol 73:26–33
Cieśliński G, Van Rees KCJ, Szmigielska AM, Krishnamurti GSR, Huang PM (1998) Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 203:109–117
Epelde L, Becerril JM, Mijangos I, Garbisu C (2009) Evaluation of the efficiency of a phytostabilization process with biological indicators of soil health. J Environ Qual 38:2041–2049
Frostegård Å and Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soil 22:59–65
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol 77:229–236
Garcia-Sanchez M, Garcia-Romera I, Cajthaml T, Tlustos P, Szakova J (2015) Changes in soil microbial community functionality and structure in a metal-polluted site: the effect of digestate and fly ash applications. J Environ Manag 162:63–73
Huang H, Yao W, Li R, Ali A, Du J, Guo D, Xiao R, Guo Z, Zhang Z, Awasthi MK (2018) Effect of pyrolysis temperature on chemical form, behavior and environmental risk of Zn, Pb and Cd in biochar produced from phytoremediation residue. Bioresour Technol 249:487–493
Jiang J, Wu L, Li N, Luo Y, Liu L, Zhao Q, Zhang L, Christie P (2010) Effects of multiple heavy metal contamination and repeated phytoextraction by Sedum plumbizincicola on soil microbial properties. Eur J Soil Biol 46:18–26
Jones D, Willett V (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Kidd P, Barceló J, Bernal MP, Navari-Izzo F, Poschenrieder C, Shilev S, Clemente R, Monterroso C (2009) Trace element behaviour at the root–soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259
Kim K-R, Owens G, Naidu R, Kwon S-l (2010) Influence of plant roots on rhizosphere soil solution composition of long-term contaminated soils. Geoderma 155:86–92
Li Z, Wu L, Hu P, Luo Y, Zhang H, Christie P (2014) Repeated phytoextraction of four metal-contaminated soils using the cadmium/zinc hyperaccumulator Sedum plumbizincicola. Environ Pollut 189:176–183
Liu A, Cao H, Yang Y, Ma X, Liu X (2016) Combinational effects of sulfomethoxazole and copper on soil microbial community and function. Environ Sci Pollut Res 23:4235–4241
Martínez-Alcalá I, Bernal MP, de la Fuente C, Gondar D, Clemente R (2016) Changes in the heavy metal solubility of two contaminated soils after heavy metals phytoextraction with Noccaea caerulescens. Ecol Eng 89:56–63
Pennanen T, Frostegard A, Fritze H, Baath E (1996) Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl Environ Microbiol 62:420–428
Pueyo M, Mateu J, Rigol A, Vidal M, Lopez-Sanchez JF, Rauret G (2008) Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ Pollut 152:330–341
Ribé V, Nehrenheim E, Odlare M, Gustavsson L, Berglind R, Forsberg A (2012) Ecotoxicological assessment and evaluation of a pine bark biosorbent treatment of five landfill leachates. Waste Manag 32:1886–1894
Sheoran V, Sheoran AS, Poonia P (2010) Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: a review. Crit Rev Environ Sci Technol 41:168–214
Stevenson BA, Hunter DW, Rhodes PL (2014) Temporal and seasonal change in microbial community structure of an undisturbed, disturbed, and carbon-amended pasture soil. Soil Biol Biochem 75:175–185
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang S, Wei S, Ji D, Bai J (2015) Co-planting Cd contaminated field using hyperaccumulator Solanum nigrum L. through interplant with low accumulation welsh onion. Int J Phytoremed 17:879–884
Wang S, Gao B, Li Y, Ok YS, Shen C, Xue S (2017) Biochar provides a safe and value-added solution for hyperaccumulating plant disposal: a case study of Phytolacca acinosa Roxb. (Phytolaccaceae). Chemosphere 178:59–64
Wei S, Twardowska I (2013) Main rhizosphere characteristics of the Cd hyperaccumulator Rorippa globosa Thell. Plant Soil 372:669–681
Wu QT, Wei ZB, Ouyang Y (2007) Phytoextraction of metal-contaminated soil by Sedum alfredii H: effects of chelator and co-planting. Water Air Soil Pollut 180:131–139
Wu Y, Ding N, Wang G, Xu J, Wu J, Brookes PC (2009) Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma 150:171–178
Yang W, Li H, Zhang T, Sen L, Ni W (2014) Classification and identification of metal-accumulating plant species by cluster analysis. Environ Sci Pollut Res 21:10626–10637
Zhao X, Wang H, Peng H, Wang L, Lu X, Huang Y, Chen J, Shao T (2017) Buoyant ALG/HA/HGMs composite adsorbents for highly efficient removal of copper from aqueous solution and contaminated kaolin soil. J Chem Eng 327:244–256
Acknowledgements
This research supported by the National Natural Science Foundation of China (Grant No. 41501345), the Natural Science Foundation of Fujian Province (Grant No. 2015J01155), the Foundation for Distinguished Young Scholars of Fujian Agriculture and Forestry University (Grant No. XJQ201628) and the Foundation of Central Guidance for Local Development (Grant No. 2016L3004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W., Li, P., Rensing, C. et al. Changes in Metal Availability and Improvements in Microbial Properties After Phytoextraction of a Cd, Zn and Pb Contaminated Soil. Bull Environ Contam Toxicol 101, 624–630 (2018). https://doi.org/10.1007/s00128-018-2478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2478-2