Abstract
Mercury is a globally distributed, persistent environmental contaminant that affects the health of many taxa. It can suppress the immune system, which often plays a role in defense against parasites. However, there have been few investigations of whether mercury affects the abilities of animals to resist parasitic infection. Here, we exposed zebra finches to a lifetime dietary exposure of methylmercury (1.2 μg/g wet weight) and experimentally infected them with coccidian parasites to examine the effect of methylmercury exposure on parasitic infection. The mercury-exposed birds did not have an altered immune response (heterophil:lymphocyte ratio) nor a reduced ability to clear the infection. However, mercury-exposed birds tended to have higher parasite loads at the time when we expected the greatest immune response (2–3 weeks post-infection). Although mercury did not greatly influence the infection-course of this parasite in captivity, responses may be more accentuated in the wild where birds face additional immune challenges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Wildlife populations face many natural and anthropogenic stressors. The effects of natural stressors, such as infection by parasites, may be accentuated when they co-occur with anthropogenic stressors, such as pollution. Contaminants, including metals, can affect the immune system (de Jong et al. 2017), which may render individuals less able to overcome infection by parasites. Parasites, in turn, may reduce body condition, alter behavior, or otherwise increase the impact of contaminants. Likely as a consequence of these types of interactions, concentrations of metals have been correlated positively with incidence of parasites in some bird species (Bichet et al. 2013). Also, treatment with anti-parasitic medication eliminated the deleterious effect of organochlorine pollutants on reproductive success in gulls (Bustnes et al. 2006). Despite recent attention to these interactions, little experimental work has been reported. We address this gap by studying the course of an experimental infection (coccidian parasite) in a songbird that was exposed to a contaminant (methylmercury, hereafter MeHg).
Mercury (hereafter Hg) is a globally distributed and persistent toxicant that poses a health risk to a wide variety of taxa, including wildlife and humans (Wiener et al. 2003). MeHg tends to biomagnify up food webs, but until recently was considered a threat mainly to aquatic wildlife such as piscivorous birds. It is now clear that Hg can also affect terrestrial organisms, including songbirds that eat invertebrates (Cristol et al. 2008). In birds, Hg can suppress the immune system, which often plays a pivotal role in defense against parasites. Specifically, exposure to environmentally relevant levels of Hg delayed the proliferation of B-lymphocytes in zebra finches (Taeniopygia guttata) (Lewis et al. 2013), and Hg-exposure is associated with immunosuppression in a variety of other bird species (Kenow et al. 2007; Hawley et al. 2009; Fallacara et al. 2011). While there is mounting evidence of immunosuppressive effects of Hg in diverse avian models, it is also clear that different forms and exposure levels of Hg can cause either suppression or stimulation of animal immune systems (Treagan 1979).
Coccidian parasites infect epithelial cells of the intestine in all vertebrate taxa (Roberts and Janovy 2005), including many avian species; the genus Eimeria infects primarily domestic poultry, and wild game birds and waterfowl, while the genus Isospora is common in songbirds (Horak et al. 2004). Organisms become infected with coccidians when they ingest sporulated oocysts, often through feces. Infection lasts from 0.5 to 3 months (Filipiak et al. 2009). There are few published studies on coccidians in birds other than domestic chickens, but it is clear that they induce notable immune responses in several species (Saks et al. 2006; Lemus et al. 2010; Pap et al. 2011).
In this study we investigated whether MeHg exposure altered the abilities of captive zebra finches to resist or clear infection after experimental exposure to a coccidian of the genus Isospora. We hypothesized that MeHg-exposed birds would have suppressed immune responses and take longer to clear the coccidian infection than birds fed control diets.
Materials and Methods
Finches were housed individually in small (30 × 23 × 40 cm) cages on a 14:10-hour light:dark cycle at ~ 20°C. We provided ad libitum pelletized food, as well as vitamins and a limited amount of lutein carotenoid (10% of recommended concentration in water, FloraGLO Lutein, Kemin Industries, Des Moines, IA, USA). We divided the room in half with a sheet of plastic to separate uninfected and infected treatments and entered each side through separate doors. Researchers changed gloves, coats, and foot coverings to reduce risk of infecting control birds.
We exposed 32 adult zebra finches (n = 15 males, n = 17 females) to MeHg by mixing aqueous MeHg-cysteine into their nutritionally complete pelletized diet (“fruit blend for very small birds”, Zupreem, Mission, KS, USA) to a concentration of 1.2 µg/g (on wet weight basis, equivalent to 1.4 µg/g dry weight). MeHg-cysteine was made by dissolving MeHg-chloride (13%, Sigma–Aldrich, Saint Louis, MO, USA) in 100% ethanol and combining in a 1:99 ratio with degassed deionized water containing a 2× molar excess of cysteine to create a 40 ppm stock solution. Food was then prepared by diluting the stock solution to the desired concentration, mixing in with food, and then homogenizing in a rock tumbler for 30 min. The control treatment (n = 19 males, n = 11 females) received the same pellets with only water and cysteine added. The Hg treatment included developmental exposure, from egg through adulthood, by dosing parents of our subjects prior to breeding, and then continuing our subjects on the same dose after they hatched and throughout their lives. The dose of Hg was selected because it represents the upper end of the range of songbird prey items found at industrially-contaminated sites (Varian-Ramos et al. 2014). Before and after experimentally infecting the birds with coccidians, we collected a blood sample to determine the average blood Hg concentration for each bird. We analyzed the blood samples, and every batch of food, using a Direct Hg Analyzer (DMA-80, Milestone, Shelton, CT, USA). Quality control of both diet preparation and blood analysis has been described in detail elsewhere (Varian-Ramos et al. 2014). Briefly, the DMA-80 was calibrated approximately every 2 months and certified standard reference materials and blanks were run with every batch. The recovery of standard reference materials and spiked samples was 99–102%. The relative percent difference for the duplicates included with approximately every 20 blood samples was 7.5 ± 1.4%.
We collected coccidian oocysts from a naturally infected zebra finch in our outdoor zebra finch colony. We confirmed the identification of the coccidian to genus using a compound microscope at 100× (Duszynski and Wilber 1997). All oocysts were maintained in 2% potassium dichromate at room temperature for 7–10 days to allow for sporulation and at 4°C thereafter. To prepare the inoculation solution, we washed oocysts in distilled water by placing 1–2 mL of the fecal-dichromate solution in 15 mL test tubes, filling the remaining space with distilled water, centrifuging (IEM Clinical with 13 cm rotor) at 1300 rcf for 10 min, and then removing the supernatant until 1 mL of water and the pellet remained. We then added fresh distilled water and repeated this process five times to remove all potassium dichromate from the solution.
We orally inoculated half of the birds in each dietary treatment with a constant solution of sporulated Isospora oocysts to produce four treatment groups in a factorial experimental design: control/uninfected, Hg/uninfected, control/infected, and Hg/infected. We used a 20-gauge metal animal-feeding needle attached to a 1 mL syringe to administer approximately 100 oocysts in 100 µL of distilled water. Birds in the uninfected treatment group were given 100 µL of distilled water at the same time. When we monitored the birds prior to the experiment, we found 15 of 38 to already be shedding oocysts. To clear any pre-existing coccidian infections prior to inoculation, we had orally medicated all birds with 0.375 mg per 100 µL sulfadimethoxine (“Albon”, Pfizer, New York, NY, USA) for 10 days. Any birds still infected were removed from the study and the rest (16 in each dietary treatment) were given 7 days to clear medication prior to experimental infection.
To determine the onset and intensity of infection, we collected fecal samples from each bird for the first 5 days after inoculation, and then weekly for 6 weeks. We collected fecal samples at peak sporulation time, between 1800 and 2000 h (based on a pilot study in our lab and published data: Brawner and Hill 1999; Misof 2004; Filipiak et al. 2009). In general, oocysts appear in the feces within 6 days of infection (Roberts and Janovy 2005), but have been observed to appear in passerine species from 3 to 9 days after infection (Filipiak et al. 2009).
To determine the number of oocysts, each fecal sample was maintained in 2% potassium dichromate. We aliquoted 1 mL of homogenized fecal-dichromate mixture into a 15 mL glass centrifuge tube. We filled the remainder of the centrifuge tube with 14 mL of Sheather’s sugar flotation solution and placed a glass cover slip on top. We centrifuged the tube as above and then placed the cover slip onto a glass microscope slide and dried for 10 min. To count the total number of oocysts per slide, we used a compound microscope at 100× magnification and systematically scanned each slide while using a hand counter to tally each oocyst (a method with a published error rate of 7%, Duszynski and Wilber 1997). To calculate the number of oocysts per gram of feces, we determined the dry weight of the feces after using a centrifugal evaporator (Savant Speed Vac, Thermo Fisher Scientific, Waltham, MA, USA). If oocysts were too numerous to count accurately, we made new slides, following the procedures above, with smaller aliquots ranging from 20 to 500 µL. We then corrected for the smaller quantity of feces when calculating oocysts per gram. We sampled the uninfected treatments too, and found none had become infected (data not shown).
To assess the state of the immune system we measured the ratio of heterophils to lymphocytes (H:L). Heterophils are granulated phagocytic cells from the innate immune system that increase in response to physiological perturbations and are early modulators of the inflammatory response (Norris and Evans 2000). Lymphocytes are the circulating T and B cells of the acquired immune system and increase in response to infection, including by coccidian parasites (Rose et al. 1979). We collected 10 µl of blood to make the blood smears from each bird on the same days that we collected fecal samples. All birds were bled within 5 min of being collected from their housing cages; therefore, it is unlikely that our handling methodology affected H:L ratios (Davis 2005). We stained the blood smears using DipQuick stain (Jorgensen Laboratories, Loveland CO, USA). We used oil immersion on a compound microscope to identify heterophil, eosinophil, monocyte, and lymphocyte white blood cells. The slide was scanned systematically and each white blood cell was identified and tallied. A total of 100 white blood cells were counted and identified on each slide (Houwen 2000). Two observers, who had been trained for 3 h by a veterinary technician, did all counts and inter-observer count agreement rate was determined to be 88% for heterophils and 96% for lymphocytes based on recounts of five birds.
All analyses were performed using IBM SPSS Software version 20 (Armonk, NY, USA), with p < 0.05 as the criterion for significance. We used a repeated-measures ANOVA to test whether the change in H:L ratio, from before to after inoculation with the coccidian, was affected by Hg-dosing. In this analysis, ln-transformed H:L ratio (averaged over all relevant weeks of sampling) was the dependent variable, treatment (infected or not) was the among-subjects fixed factor, and stage of the experiment (pre-exposure or exposed) was the within-subjects fixed factor. We further explored differences in post-inoculation H:L ratios in a two-way ANOVA with dose and infection status as fixed factors.
Prior to analysis we improved the normality of the coccidian parasite counts using a Box-Cox transformation (Swaddle et al. 1994). Similar to above, we analyzed the change in coccidian parasite load (oocyst/g feces) using a repeated-measures ANOVA with time since inoculation as the within-subject factor and Hg treatment (dosed or control) as the among-subject fixed factor. Coccidian parasites have a genetically limited number of asexual reproductive cycles, which most likely occur early in the infection. During this time, newly formed merozoites are destroying old epithelial cells and invading new cells and the immune system is actively responding. Macrophages are engulfing freed merozoites while T cells are being recruited to the invaded cells (Roberts and Janovy 2005; Lemus et al. 2010). We were interested in the immune response to coccidians in the presence of Hg; thus it is useful to examine the parasite load during the time that the highest level of immune activity was most likely occurring (i.e., during the second and third weeks of infection). Therefore, we also conducted a repeated-measures ANOVA within a narrower time window of 7–21 days post-inoculation.
Results and Discussion
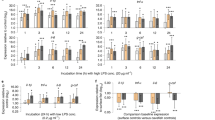
The Hg dosing was highly effective in creating a blood-Hg difference greater than three orders of magnitude (mean ± SD µg/g wet weight: controls = 0.006 ± 0.001, Hg-dosed = 21.31 ± 4.35). All birds orally inoculated with oocysts became infected with coccidians (Fig. 1). All but two started releasing oocysts in their feces 3 days after inoculation, with the remaining two releasing oocysts 1–2 days after inoculation. The infection typically lasted < 6 weeks, with only six birds still releasing oocysts at that date.
When tested across all times sampled, there was no evidence that birds exposed to Hg changed more than controls in H:L ratio from pre- to post-experimental infection (repeated-measured Greenhouse Geisser F 1,59 = 0.256, p = 0.615; pre-infection mean (± SD) H:L ratios: control/uninfected = 0.54 ± 0.29, Hg/uninfected = 0.43 ± 0.23, control/infected = 0.41 ± 0.27, Hg/infected = 0.43 ± 0.43; post-infection mean (± SD) H:L ratios: control/uninfected = 0.33 ± 0.12, Hg/uninfected = 0.39 ± 0.26, control/infected = 0.41 ± 0.21, Hg/infected = 0.51 ± 0.47). Considering only the control birds, the shift in H:L ratios after treatment was not affected by whether the treatment was coccidian or no infection (repeated-measures Greenhouse Geisser F 1,28 = 0.019, p = 0.742). Within uninfected birds only, there was no indication that Hg exposure affected H:L ratios (F 1.27 = 0.107, p = 0.747; Table 1).
Considering the entire infection period (3–42 days after treatment), the course of parasite loading (oocyst/g feces) did not differ between control and Hg-exposed birds (time-by-treatment interaction, repeated-measures Greenhouse Geisser F 4.76,142.7 = 0.977, p = 0.431, Fig. 1). Additionally, there was no consistent difference in overall parasite load between Hg-exposed and control birds, when considering this entire time period (F 1,30 = 1.45, p = 0.238). However, during the period when infection was predicted to have the largest effect (7–21 days), parasite load tended to be higher in Hg-exposed birds (F 1,30 = 3.16, p = 0.086; Fig. 1).
Although parasite load was not statistically significantly different between treatments across the entire period of infection, we did find a trend towards greater parasite loads in Hg-exposed birds from 7 to 21 days after infection. Specifically, during the period when the biggest immune response was expected, Hg-exposed birds tended to shed more oocysts, but the difference was not statistically significant. This could indicate a slower or weaker immune response in the Hg-exposed birds and should be examined further. MeHg can suppress the immune system as measured by induced proliferation of splenic B cells in zebra finches (Lewis et al. 2013); however, the measure of immune function we employed here, heterophil: lymphocyte ratio, did not differ with either Hg or infection status, leaving us unable to conclude whether immunosuppression might have been detected had we used other measures.
There is evidence that Hg dampens immune responses and is associated with higher parasite loads. Injection of mercuric chloride in mice caused increased parasitic burdens of Leishmania major (Bagenstose et al. 2001). There was a positive correlation between Hg and acanthocephalan intensities in gulls (Sagerup et al. 2009), and nematode abundance in ducks (Wayland et al. 2001), despite the fact that Hg concentrations were considered below toxicological concern for both studies. Similarly, blood-Hg levels were positively associated with the intensity of Leucocytozoon infection in adult loons (Weinandt 2006). Therefore, we had predicted that birds exposed to MeHg would have a suppressed cell-mediated immune response and resulting higher parasite loads. In fact, we detected no response of H:L ratios to Hg dosing or coccidian infection. We have previously shown suppression of in vitro B-cell proliferation in response to similar Hg exposure in this same colony of zebra finches (Lewis et al. 2013). The lymphocytes counted in the H:L ratios are circulating B and T cells of the cell-mediated immune response, the primary responder to a coccidian infection. We had expected the H:L ratios of Hg-exposed birds to be higher due to suppression of lymphocytes by Hg and an increase in heterophils resulting from the increased glucocorticoid levels often observed in birds under increased amounts of stress (Davis et al. 2008). The lack of differences between treatment groups in H:L ratio could indicate that this indirect metric is not an appropriate indicator of immune response when examining parasite-contaminant interactions or that the zebra finches in benign captive conditions were not sufficiently stressed by either the parasitic infection or the dietary Hg. Because we isolated the parasite from our own colony, and approximately half of the birds in the study showed signs of recent infection prior to the study, it is possible that the immune systems in our finch population were not highly responsive to this endemic parasite. While it would be desirable to identify the mechanism underlying any effect a contaminant, in this case we directly measured the functional response by documenting the numbers of coccidian oocysts shed. Follow-up research should focus on components of the immune system previously documented to respond to Hg in songbirds, such as swelling response to PHA (Hawley et al. 2009) or induced proliferation of splenic lymphocytes (Lewis et al. 2013).
Coccidian parasites can alter the physiology of birds, inhibiting the uptake of essential dietary components, such as carotenoids (Brawner et al. 2000; McGraw and Hill 2000; Horak et al. 2004) and causing a decrease in serum albumin, triglyceride, and vitamin E, as well as body mass and size of secondary sex characters (Buchholz 1995; Horak et al. 2004; Costa and Macedo 2005). Coccidian infections can also negatively affect feather growth during molt in house sparrows (Passer domesticus, Pap et al. 2011). Our results suggest that while exposure to Hg did not increase the likelihood or duration of an experimental coccidian infection, it likely resulted in a greater shedding of parasites at the height of immune response to infection.
Although our study indicated only marginal effects of Hg-exposure on the course of the coccidian parasite, we predict that these effects could be more severe in free-living birds that must cope with additional energetic and immunological challenges that our captive zebra finches did not face. This prediction is based upon the assumption that free-living birds would face unpredictable food availability, direct stress from risk of predation, and a more diverse array of parasites and diseases, all of which could make it more difficult to invest resources in overcoming an acute infection by coccidians. As an example, when researchers attempted to measure the effect of Hg on the immune response to an injection of PHA in songbirds, Hg exposure correlated with immunosuppression in the field (Hawley et al. 2009), but immunosuppression was not detected among captive-raised birds in the lab with even higher exposure to Hg (Caudill et al. 2015). This study demonstrates the importance of directly measuring the impact of contaminants on parasites or disease, rather than relying on the proxy measure of complex and poorly understood immune mechanisms underlying such responses.
References
Bagenstose LM, Mentink-Kane MM, Brittingham A, Mosser DM, Monestier M (2001) Mercury enhances susceptibility to murine leishmaniasis. Parasite Immun 23:633–640. doi:0.1046/j.1365-3024.2001.00427.x
Bichet C, Scheifler R, Coeurdassier M, Julliard R, Sorci G, Loiseau C (2013) Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS One 8:e53866. https://doi.org/10.1371/journal.pone.0053866
Brawner WR III, Hill GE (1999) Temporal variation in shedding of coccidial oocysts: implications for sexual-selection studies. Can J Zool 77:347–350. https://doi.org/10.1139/z98-207
Brawner WR III, Hill GE, Sundermann CA (2000) Effects of coccidial and mycoplasmal infections on carotenoid-based plumage pigmentation in male house finches. Auk 117:952–963. https://doi.org/10.1642/0004-8038(2000)117[0952:EOCAMI]2.0.CO;2
Buchholz R (1995) Female choice, parasite load and male ornamentation in wild turkeys. Anim Behav 50:929–943. https://doi.org/10.1016/0003-3472(95)80095-6
Bustnes JO, Erikstad KE, Hanssen SA, Tveraa T, Folstad I, Skaare JU (2006) Anti-parasite treatment removes negative effects of environmental pollutants on reproduction in an Arctic seabird. Proc R Soc Lond B 273:3117–3122. https://doi.org/10.1098/rspb.2006.3687
Caudill MT, Spear EL, Varian-Ramos CW, Cristol DA (2015) PHA-Stimulated immune-responsiveness in mercury-dosed zebra finches does not match results from environmentally exposed songbirds. Bull Environ Contam Toxicol 94:407–411. https://doi.org/10.1007/s00128-015-1472-1
Costa FJV, Macedo RH (2005) Coccidian oocyst parasitism in the blue-black grassquit: influence on secondary sex ornaments and body condition. Anim Behav 70:1401–1409. https://doi.org/10.1016/j.anbehav.2005.03.024
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335. https://doi.org/10.1126/science.1154082
Davis AK (2005) Effect of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76:334–338. https://doi.org/10.1648/0273-8570-76.4.334
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. https://doi.org/10.1111/j.1365-2435.2008.01467.x
de Jong ME, Scheiber IB, van den Brink NW, Braun A, Matson KD, Komdeur J, Loonen MJ (2017) Indices of stress and immune function in Arctic barnacle goslings (Branta leucopsis) were impacted by social isolation but not a contaminated grazing environment. Sci Tot Environ 601:132–141. https://doi.org/10.1016/j.scitotenv.2017.05.183
Duszynski DW, Wilber PG (1997) A guideline for the preparation of species descriptions in the Eimeriidae. J Parasitol 83:333–336
Fallacara DM, Halbrook RS, French JB (2011) The effects of dietary methylmercury on immune function and hematology in American kestrels (Falco sparverius). Environ Toxicol Chem 30:1320–1327. https://doi.org/10.1002/etc.494
Filipiak L, Mathieu F, Moreau J (2009) Caution on the assessment of intestinal parasitic load in studying parasite-mediated sexual selection: the case of blackbirds coccidiosis. Int J Parasitol 39:741–746. https://doi.org/10.1016/j.ijpara.2008.11.005
Hawley DM, Hallinger KK, Cristol DA (2009) Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18:499–503. https://doi.org/10.1007/s10646-009-0307-4
Horak P, Saks L, Karu U, Ots I, Surai PF, McGraw KJ (2004) How coccidian parasites affect health and appearance of greenfinches. J Anim Ecol 73:935–947. https://doi.org/10.1111/j.0021-8790.2004.00870.x
Houwen B (2000) Blood film preparation and staining procedures. Lab Hematol 6:1–7
Kenow KP, Grasman KA, Hines RK, Meyer MW, Gendron-Fitzpatrick A, Spalding MG, Gray BR (2007) Effects of methylmercury exposure on the immune function of juvenile common loons (Gavia immer). Environ Toxicol Chem 26:1460–1469. https://doi.org/10.1897/06-442R.1
Lemus JA, Vergara P, Fargallo JA (2010) Response of circulating T-lymphocytes to a coccidian infection: insights from a parasitization-vaccination experiment. Funct Ecol 24:638–645. https://doi.org/10.1111/j.1365-2435.2009.01681.x
Lewis CA, Cristol DA, Swaddle JP, Varian-Ramos CW, Zwollo P (2013) Decreased immune response in zebra finches exposed to sublethal doses of mercury. Arch Environ Contam Toxicol 64:327–336. https://doi.org/10.1007/s00244-012-9830-z
McGraw K, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc Lond B 267:1525–1531. https://doi.org/10.1098/rspb.2000.1174
Misof K (2004) Diurnal cycle of Isospora spp. oocyst shedding in Eurasian blackbirds (Turdus merula). Can J Zool 82:764–768. https://doi.org/10.1139/z04-054
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26. https://doi.org/10.1093/beheco/11.1.19
Pap PL, Vagasi CI, Czirjack GA, Titilincu A, Pintea A, Osvath G, Fulop A, Barta Z (2011) The effect of coccidians on the condition and immune profile of molting house sparrows (Passer domesticus). Auk 128:330–339. https://doi.org/10.1525/auk.2011.10142
Roberts L, Janovy J Jr (eds) (2005) Foundations of parasitology. McGraw Hill, Boston
Rose ME, Hesketh P, Ogilvie BM (1979) Peripheral blood leucocyte response to coccidial infection: a comparison of response in rats and chickens and its correlation with resistance to reinfection. Immunology 36:71–79
Sagerup K, Savinov V, Savinova T, Kuklin V, Muir DCG, Gabrielsen GW (2009) Persistent organic pollutants, heavy metals and parasites in the glaucous gull (Larus hyperboreus) on Spitsbergen. Environ Poll 157:2282–2290. https://doi.org/10.1016/j.envpol.2009.03.031
Saks L, Karu U, Ots I, Horak P (2006) Do standard measures of immunocompetence reflect parasite resistance? The case of greenfinch coccidiosis. Funct Ecol 20:75–82. https://doi.org/10.1111/j.1365-2435.2006.01068.x
Swaddle JP, Witter MS, Cuthill IC (1994) The analysis of fluctuating asymmetry. Anim Behav 48:986–989. https://doi.org/10.1006/anbe.1994.1327
Treagan L (1979) A survey of the effect of metals on the immune response. Biol Trace Elem Res 1:141–148
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS One 9(4):e95674. https://doi.org/10.1371/journal.pone.0095674
Wayland M, Gilchrist HG, Dickson DL, Bollinger T, James C, Carreno RA, Keating J (2001) Trace elements in king eiders and common eiders in the Canadian Arctic. Arch Environ Contam Toxicol 41:491–500. https://doi.org/10.1007/s002440010276
Weinandt ML (2006) Conservation implications of common loon (Gavia immer) parasites: black flies, haematozoans, and the role of mercury. MS thesis, Northern Michigan University, Michigan, USA
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology. CRC Press, Boca Raton, pp 409–463
Acknowledgements
This work was supported by a National Science Foundation grant (IOS-1257590) as well as the American Ornithologists’ Union, Virginia Academy of Science, Williamsburg Bird Club. We thank M. Whitney, C. Varian-Ramos, M. Caudill, C. Taylor, and J. Marioneaux for assistance. All protocols were approved by the Institutional Animal Care and Use Committee at the College of William & Mary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebers Smith, J.H., Cristol, D.A. & Swaddle, J.P. Experimental Infection and Clearance of Coccidian Parasites in Mercury-Exposed Zebra Finches. Bull Environ Contam Toxicol 100, 89–94 (2018). https://doi.org/10.1007/s00128-017-2246-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2246-8