Abstract
Mercury (Hg) methylation is often elevated at the terrestrial–peatland interface, but methylmercury (MeHg) production at this “hot spot” has not been linked with in situ biotic accumulation. We examined total Hg and MeHg levels in peat, invertebrates and tissues of the insectivore Sorex cinereus (masked shrew), inhabiting a terrestrial–peatland ecotone in northern Minnesota, USA. Mean MeHg concentrations in S. cinereus (71 ng g−1) fell between concentrations measured in spiders (mean 70–140 ng g−1), and ground beetles and millipedes (mean 29–42 ng g−1). Methylmercury concentrations in S. cinereus increased with age and differed among tissues, with highest concentrations in kidneys and muscle, followed by liver and brain. Nearly all Hg in S. cinereus was in the methylated form. Overall, the high proportional accumulation of MeHg in peat at the site (3.5% total Hg as MeHg) did not lead to particularly elevated concentrations in invertebrates or shrews, which are below values considered a toxicological risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) and methylmercury (MeHg) are contaminants of significant environmental concern due to high bioaccumulative potential and neurological health impacts at low concentrations in both humans and wildlife (e.g., Eagles-Smith et al. 2016). This includes impacts in remote ecosystems since gaseous Hg emissions have significant long-range transport potential (Schroeder and Munthe 1998). Mercury deposited to terrestrial systems either accumulates in soils or is re-emitted to the atmosphere, but small amounts may become hydrologically active and be transported as complexes with dissolved or particulate organic matter into aquatic and wetland ecosystems (e.g., Oswald et al. 2014). These inputs, as well as direct atmospheric deposition of Hg, may accumulate in anoxic soils or sediment where Hg methylating microbial activity is greatest, resulting in MeHg accumulation (Hsu-Kim et al. 2013). Methylmercury production is particularly elevated in wetlands because of their persistent wet, anoxic conditions, which provide ideal habitat for anaerobic microbes, including sulfate reducers and methanogens that are responsible for Hg methylation (Gilmour et al. 2013). Peatlands, a type of wetland, have been noted for high MeHg concentrations relative to total Hg concentrations (e.g., Tjerngren et al. 2012). Higher MeHg production in peatlands occurs in areas with groundwater upwelling, at the upland forest–peatland interface, or following significant water table fluctuations, which serve to introduce or recycle important terminal electron acceptors that fuel microbial activity (Branfireun et al. 1996; Mitchell et al. 2008; Coleman Wasik et al. 2015).
Studies conducted on aquatic and terrestrial food webs have found that Hg concentrations increase with trophic level, (e.g. Rimmer et al. 2010) and that Hg can be transferred from the aquatic environment to adjacent terrestrial food webs (e.g. Cristol et al. 2008; Kwon et al. 2015). Gann et al. (2015) found that Hg concentrations in spiders living in riverine wetlands of Texas and Louisiana, USA were elevated enough to physiologically impact nestling birds. Spiders may thus be an important link between aquatic and terrestrial food webs, serving as a transfer point for toxins from emerging aquatic insects to enter the systems of the spiders’ terrestrial predators (Speir et al. 2014; Wyman et al. 2011). Cristol et al. (2008) compared blood total Hg concentrations in both aquatic and terrestrial birds, finding that Hg concentrations in many terrestrial-feeding species of birds were in the same range as aquatic birds because of the influence of spiders as terrestrial prey, which have relatively high MeHg content. Recent work has associated increased MeHg production in peatlands to Hg accumulation in mosquito larvae (Coleman Wasik et al. 2012), suggesting possible ecological risk to peatland insectivores such as small mammals.
While there is a growing literature on ecologically important levels of Hg in terrestrial arthropods (e.g., Gann et al. 2015) and birds (e.g., Cristol et al. 2008), reports on MeHg bioaccumulation in insectivorous small mammals are rare. Shrews, for example, are voracious eaters, and thus may bioaccumulate Hg at greater rates than other species (Sánchez-Chardi et al. 2009). Shrews may be useful biomonitors for Hg pollution in terrestrial ecosystems because they inhabit limited ranges, have consistent feeding habits, are easily collected, live short lives, and reproduce quickly (Petkovsek et al. 2014; Talmage and Walton 1991). Shrews consume large amounts of prey relative to their mass and thus require large amounts of water, leading them to prefer moist habitats (McCay and Storm 1997). Shrews are commonly found in peatlands throughout North America, but are not necessarily peatland specialists (Nordquist 1992). To date, MeHg uptake in peatland shrews has not been accounted for despite their role as prey for a variety of avian and terrestrial predators, including owls and long-tailed weasels (Whitaker 2004).
In earlier work in small upland forest and peatland-dominated watersheds in northern Minnesota, USA, Mitchell et al. (2008) found that the greatest accumulation of MeHg was at the upland forest–peatland ecotone. We sought to extend this work by investigating whether the MeHg “hot spots” observed at the upland forest–peatland interface lead to significant MeHg uptake by invertebrates or the masked shrew, Sorex cinereus, inhabiting and/or foraging within the ecotone. The immediate study region has no point sources of Hg pollution and thus the relatively low concentrations of Hg observed in peatland soils of the region are likely entirely derived from atmospheric deposition (Kolka et al. 2011). Thus, an additional purpose of this work was to assess whether MeHg accumulation in an atmospherically-contaminated peatland of the region could lead to in situ MeHg bioaccumulation in invertebrates or shrews to levels significant enough to threaten local terrestrial and avian predators.
Materials and Methods
This study took place within the upland–peatland ecotone of the “S7” watershed, located at the Marcell Experimental Forest, north of Grand Rapids, MN in north-central Minnesota, USA (47°31′21″N, 93°28′7″W; Fig. 1). The S7 watershed has an overall area of 7.0 ha and is comprised of a 4.9 ha upland forest and a 2.1 ha bog peatland (Sebestyen et al. 2011). There is approximately 18 m of relief in the upland forest with a mean slope of 10°. Surface soils are sandy loams, overlain by a thin, organic-rich O horizon and underlain by low permeability glacial till, leading to predominantly interflow runoff from upland to peatland (Haynes and Mitchell 2012). Upland overstory vegetation mainly consists of mature quaking aspen (Populus tremuloides), sugar maple (Acer saccharum), and paper birch (Betula papyrifera) (Sebestyen et al. 2011). The peatland is an oval-shaped, treed bog dominated by Sphagnum species at the surface, with an overstory dominated by black spruce (Picea mariana), with paper birch (Betula papyrifera), yellow birch (B. alleghaniensis), and speckled alder (Alnus rugosa) at the upland ecotone. Peat depths are up to 7 m in the central bog, but peat is 0.10 to ~ 1 m deep at the upland ecotone where this study was focused. The climate is continental with a mean annual precipitation of 780 mm and a mean annual air temperature of 3.4°C (Sebestyen et al. 2011).
Sampling for this study took place during summer 2011. Nine randomly distributed samples of surface peat were removed by gloved hand across the study area, transported on ice in clean polyethylene containers back to the field station, and stored frozen until being later freeze-dried and homogenized. Peatland invertebrates were collected using 24 pitfall traps (64 cm2 opening), ¼ filled with deionized water, and placed with rims at moss surface level across the 1200 m2 ecotone area (Fig. 1). For replication, the interface trapping area was partitioned into three approximately equal adjacent areas and invertebrate samples from eight pitfall traps in each of these areas were composited to obtain enough biomass for analyses. Pitfall traps were examined daily for 4 days and contents sorted immediately in the lab. Accidental catches of 16 juvenile S. cinereus were saved for additional examination of MeHg uptake. Each S. cinereus was analyzed for Hg content separately.
Invertebrate taxa were sorted to the lowest taxonomic level that still allowed for enough biomass for MeHg determination, enumerated, transferred to clean polyethylene vials and frozen until being later freeze-dried and homogenized. Insufficient biomass did not allow us to analyze total Hg concentrations in the invertebrate samples, thus we opted to analyze the more bioaccumulation-relevant MeHg. The mass and body length of each shrew were recorded. Teeth were inspected to estimate age using the method of Rudd (1955), which is based on tooth transparency and wear, and has an uncertainty of approximately ± 1 month. Shrews were dissected and samples of brain, kidney, liver and muscle were stored frozen in clean polyethylene vials until Hg analysis. Since shrew samples were accidental catches, replication beyond two individual shrews and their tissues in all age classes was not possible.
Methylmercury was extracted from fauna samples in hot 25% KOH–methanol solution (Bloom 1989) to which a known quantity of enriched Me199Hg was added. A small amount of the extract was diluted with deionized water, ethylated by addition of sodium tetraethylborate and purged with Hg-free nitrogen while a glass trap filled with Tenax was used to trap MeHg. The Tenax trap was thermally desorbed in a stream of Hg-free argon, mercury species were separated on a gas chromatography column, and Hg detection was completed via hyphenation with an ICP-MS. Isotope dilution calculations, according to Hintelmann and Ogrinc (2003) were used to determine MeHg concentration. Methylmercury determination in peat samples was similarly conducted except that samples were first distilled in a KCl–H2SO4–CuSO4 solution with a known quantity of enriched Me199Hg, prior to analysis as above. If sufficient biomass was available, samples were also analyzed for total Hg content by first digesting in boiling concentrated nitric acid and oxidizing with BrCl. Oxidized samples were then analyzed by cold-vapour atomic fluorescence spectroscopy using a Tekran 2600 automated total Hg analysis system, according to US Environmental Protection Agency method 1631 (USEPA 2002). Quality assurance and control measures included the analysis of certified reference materials (DORM-3), duplicates analyses on approximately 10% of samples and blanks. The recovery (mean ± standard deviation) of MeHg and THg in DORM-3 was 89% ± 9% and 94% ± 1% of the mean certified value, respectively. The relative standard deviation of duplicate analyses was 4% ± 2% and 4 % ± 3% for MeHg and THg, respectively. Method detection limits for MeHg and THg were 0.04 and 0.35 ng g−1 dw, respectively.
Statistical analyses were carried out using R Studio (RStudio Team 2016). One-way analysis of variance followed by Tukey’s HSD post-hoc test was used to examine differences in MeHg concentrations among biotic samples. α = 0.05 in all analyses.
Results and Discussion
This study builds on previous hypotheses by Mitchell et al. (2008) to examine whether Hg methylation “hot spots” at the upland–peatland interface translate into important areas of biotic MeHg accumulation. Little data exists for MeHg bioaccumulation in small terrestrial mammals or peatland invertebrates, particularly in areas where Hg inputs are derived entirely by atmospheric deposition. Peat collected from the upland–peatland interface had total Hg concentrations of 183 ± 42 ng g−1 and MeHg concentrations of 6.3 ± 3.7 ng g−1 (3.5% total Hg as MeHg). This relatively high proportion of total Hg found as MeHg in peat at the study site suggests MeHg is efficiently produced and/or accumulated at the site, presumably via the activity of anaerobic microbes, as has been observed in an adjacent watershed (Strickman et al. 2016).
A variety of invertebrate taxa were collected in the pit fall traps: Lumbriculidae (oligochaete worms, n = 103), Lycosidae (wolf spiders, n = 64), Araneae (mixed spiders excluding Lycosidae and Pholcidae, n = 34), Carabidae (ground beetles, n = 33), Polydesmus spp. (millipedes, n = 9), and Pholcidae (cellar spiders, n = 8). All 16 S. cinereus collected were juveniles (< 30 weeks).
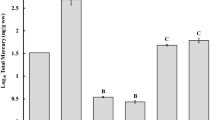
Invertebrate MeHg concentrations followed a trend most likely related to diet with predaceous Lycosidae (mean ± standard deviation: 141 ± 59 ng g−1) and mixed spiders excluding Lycosidae and Pholcidae (mean: 70 ± 19 ng g−1) having the highest concentrations, followed by Carabidae (mean: 42 ± 16 ng g−1), Pholcidae (mean: 39 ± 6 ng g−1), and the detritivorous taxa Polydesmus (29 ± 6 ng g−1) and Lumbriculidae (mean: 11 ± 5 ng g−1) (Fig. 2). Invertebrate MeHg concentrations at our peatland site were similar to those reported at non-point source contaminated sites for Lumbriculidae (mean: 14 ± 4 ng g−1 dw) in forest soil (Rieder et al. 2011), and Lycosidae (range: 40–127 ng g−1 dw) and Phalangiidae (range: 12–45 ng g−1 dw) in Sphagnum spp. dominated wetlands and heathland ecosystems of northern Iceland (Bartrons et al. 2015). Bartrons et al. (2015) refer to a similar range in spider concentrations as being relatively high for a non-point source contaminated area, particularly in comparison to spiders in a remote forest of New Hampshire with concentrations of 16 ± 4 ng g−1 dw (Wyman et al. 2011). Whereas Bartrons et al. (2015) attributed their relatively high MeHg concentrations to Hg emissions from volcanic eruptions or nearby geothermal power production, we believe our similarly elevated spider MeHg concentrations are most likely attributable to a greater Hg methylation capacity in the peatland habitat and bioaccumulation up the peatland food web. Similar to other terrestrial studies (e.g. Rimmer et al. 2010, Wyman et al. 2011; Bartrons et al. 2015), we observed higher MeHg concentrations in peatland invertebrates at higher trophic positions.
Amongst all samples, shrew muscle had moderate MeHg concentrations (mean: 71 ± 41 ng g−1) and statistically significantly greater concentrations than Lumbriculidae. Half of the 16 shrew samples were also analyzed for total Hg concentrations. On average, 99% of the total Hg in shrew muscle was MeHg. Bellocq et al. (1994) found juvenile and adult S. cinereus have different diets, with adult males consuming more spiders. Whitaker and Schmeltz (1973), reported S. cinereus in Minnesota consume significant amounts of Gryllidae (crickets), Lepidoptera larvae (butterfly and moths), Phalangiidae (harvestmen), Cicadellidae (leafhoppers), Rhagionidae (snipe flies), Araneae (spiders) and Oligochaeta (worms). Ryan (1986), whose study took place in a peatland setting most similar to the one in our study, found that S. cinereus consumed primarily ants, spiders, and Lepidoptera larvae. Some of these prey would not have been collected in pitfall traps, however few of these invertebrates were observed at the time of our sampling. Lycosidae, with their high visual acuity, and the feeding behavior of shrews make wolf spiders unlikely prey items for shrews (Blossom 1932; Edgar 1969). S. cinereus has been observed to pull earthworms from the ground and consume earthworms both preferentially and in the absence of other food (Blossom 1932). Thus, the consumption of a higher proportion of abundant but low MeHg content prey such as Lumbriculidae, may contribute to the juvenile S. cinereus’ low MeHg levels. Methylmercury concentrations in shrew organs increased with age of the juvenile shrews (Fig. 3). A lack of sample replication in some age classes precludes a statistical analysis of differences among organs, but the organ MeHg concentrations generally followed the trend muscle (21 ± 15 ng g−1) ≈ kidneys (20 ± 12 ng g−1) > liver (12 ± 5 ng g−1) > brain (7 ± 7 ng g−1). Our findings of greatest MeHg (and THg) concentrations in muscle and kidney contrast some previous work involving non-shrew small mammals wherein THg concentrations are generally greatest in the liver (Wren et al. 1980). Important to our work is the focus on MeHg instead of total Hg, as is measured in most other studies, because MeHg is the principal bioaccumulative form. The differences among organs and studies are most likely a function of varying bioaccumulation factors of inorganic and organic Hg species in various organs.
Contrary to our predictions and despite their voracious appetites, MeHg concentrations in shrews at this upland–peatland interface were similar to concentrations in top invertebrate predators. Despite the fact that shrews have high metabolism and food consumption rates, we suggest that MeHg concentrations were not substantially higher than top invertebrate predators due to the diet and the age of the juvenile shrews. While we had no adult shrews in our analyses, Sánchez-Chardi et al. (2007) demonstrated that total Hg accumulation in the greater white-toothed shrew Crocidura russula was age-dependent, with adult shrews having higher concentrations than juveniles. Also, since shrews live up to 64 weeks of age (Rudd 1955), MeHg concentrations would likely have been greater for older individuals in the population at our site.
Invertebrates and shrews are often the most numerous small animals in forested peatland sites and are successful in a variety of environmental conditions (Nordquist 1992; Batzer et al. 2016). This might make them an important prey item for predators. However, in comparison to areas with point source Hg contamination, the MeHg concentrations in invertebrates and shrews in our study are relatively low and are well below levels likely to induce deleterious impacts either in themselves or their predators (CCME 2000; Gerstenberger et al. 2006; Scheuhammer et al. 2007; Talmage and Walton 1991). While spiders and other invertebrates have previously been implicated as an important link between terrestrial and avian food chains (Cristol et al. 2008), our results suggest that MeHg transfer from terrestrial or wetland systems via invertebrates or shrews is unlikely to be an important Hg pathway in peatland systems without Hg point sources or nearby large emissions.
References
Bartrons M, Gratton C, Spiesman BJ, Vander Zanden MJ (2015) Taking the trophic bypass: aquatic-terrestrial linkage reduces methylmercury in a terrestrial food web. Ecol Appl 25:151–159
Batzer DP, Wu H, Wheeler T, Eggert SL (2016) Peatland invertebrates. In: Batzer DP, Boix D (eds) Invertebrates in freshwater wetlands: an international perspective on their ecology. Springer, New York, pp 219–250
Bellocq MI, Bendell JF, Duncan GLI (1994) Diet of Sorex-cinereus, the masked shrew, in relation to the abundance of lepidoptera larvae in northern Ontario. Am Midl Nat 132:68–73. doi:10.2307/2426201
Bloom N (1989) Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas-chromatography with cold vapor atomic fluorescence detection. Can J Fish Aquat Sci 46:1131–1140. doi:10.1139/f89-147
Blossom PM (1932) A pair of long-tailed shrews (Sorex cinereus cinereus) in captivity. J Mammal 13:136–143. doi:10.2307/1374050
Branfireun BA, Heyes A, Roulet NT (1996) The hydrology and methylmercury dynamics of a Precambrian Shield headwater peatland. Water Resour Res 32:1785–1794. doi:10.1029/96WR00790
CCME (2000) Canadian tissue residue guidelines for the protection of wildlife consumers of aquatic biota—Methylmercury. In: Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg
Coleman Wasik JK, Mitchell CPJ, Engstrom DR, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Eggert SL, Kolka RK, Almendinger JE (2012) Methylmercury declines in a boreal peatland when experimental sulfate deposition decreases. Environ Sci Technol 46:6663–6671. doi:10.1021/es300865f
Coleman Wasik JK, Engstrom DR, Mitchell CPJ, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Kolka RK, Almendinger JE (2015) The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J Geophys Res Biogeosci 120:1697–1715. doi:10.1002/2015JG002993
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335–335. doi:10.1126/science.1154082
Eagles-Smith CA, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck JA, Aiken GR, Lepak JM, Jackson AK, Webster JP, Stewart AR, Davis JA, Alpers CN, Ackerman JT (2016) Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci Total Environ 568:1213–1226. doi:10.1016/j.scitotenv.2016.05.094
Edgar WD (1969) Prey and predators of the wolf spider Lycosa lugubris. J Zool 159:405–411
Gann GL, Powell CH, Chumchal MM, Drenner RW (2015) Hg-contaminated terrestrial spiders pose a potential risk to songbirds at Caddo Lake (Texas/Louisiana, USA). Environ Toxicol Chem 34:303–306. doi:10.1111/j.1469-7998.1969.tb03897.x
Gerstenberger SL, Cross CL, Divine DD, Gulmatico DD, Rothweiler AM (2006) Assessment of mercury concentrations in small mammals collected near Las Vegas, Nevada, USA. Environ Toxicol 21:583–589. doi:10.1002/tox.20221
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA Jr, Bailey KL, Elias DA (2013) Mercury methylation by novel microorganisms from new environments. Environ Sci Technol 47:11810–11820. doi:10.1021/es403075t
Haynes KM, Mitchell CPJ (2012) Inter-annual and spatial variability in hillslope runoff and mercury flux during spring snowmelt. J Environ Monit 14:2083–2091. doi:10.1039/c2em30267e
Hintelmann H, Ogrinc N (2003) Determination of stable mercury isotopes by ICP/MS and their application in environmental studies. ACS Symp Ser 835:321–338
Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA (2013) Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Technol 47:2441–2456. doi:10.1021/es304370g
Kolka RK, Mitchell CPJ, Jeremiason JD, Hines NA, Grigal DF, Engstrom DR, Coleman-Wasik JK, Nater EA, Swain EB, Monson BA, Fleck JA, Johnson B, Almendinger JE, Branfireun BA, Brezonik PL, Cotner JB (2011) Mercury cycling in peatland watersheds. In: Kolka RK, Sebestyen SD, Verry ES, Brooks KN (eds) Peatland biogeochemistry and watershed hydrology at the Marcell Experimental Forest. CRC Press, Boca Raton, pp 349–370
Kwon SY, Blum JD, Nadelhoffer KJ, Dvonch JT, Tsui MTK (2015) Isotopic study of mercury sources and transfer between a freshwater lake and adjacent forest food web. Environ Sci Technol 532:220–229
McCay TS, Storm GL (1997) Masked shrew (Sorex cinereus) abundance, diet and prey selection in an irrigated forest. Am Midl Nat 138:268–275. doi:10.2307/2426820
Mitchell CPJ, Branfireun BA, Kolka RK (2008) Spatial characteristics of net methylmercury production hot spots in peatlands. Environ Sci Technol 42:1010–1016. doi:10.1021/es0704986
Nordquist GE (1992) Small mammals. In: Wright HE Jr, Coffin BA, Aaseng NE (eds) The patterned peatlands of minnesota. University of Minnesota Press, Minneapolis, pp 85–110
Oswald CJ, Heyes A, Branfireun BA (2014) Fate and transport of ambient mercury and applied mercury isotope in terrestrial upland soils: insights from the METAALICUS watershed. Environ Sci Technol 48:1023–1031. doi:10.1021/es404260f
Petkovsek SA, Kopusar N, Krystufek B (2014) Small mammals as biomonitors of metal pollution: a case study in Slovenia. Environ Monit Assess 186:4261–4274. doi:10.1007/s10661-014-3696-7
Rieder SR, Brunner I, Horvat M, Jacobs A, Frey B (2011) Accumulation of mercury and methylmercury by mushrooms and earthworms from forest soils. Environ Pollut 159:2861–2869
Rimmer CC, Miller EK, McFarland KP, Taylor RJ, Faccio SD (2010) Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology 19:697–709
RStudio Team (2016) RStudio: integrated development for R. RStudio, Inc., Boston. http://www.rstudio.com
Rudd R (1955) Age, sex, and weight comparisons in three species of shrews. J Mammal 36:323–339. doi:10.2307/1375674
Ryan JM (1986) Dietary overlap in sympatric populations of Pygmy shrews, Sorex hoyi, and Masked shrews, Sorex cinereus, in Michigan. Can Field-Nat 100:225–228
Sánchez-Chardi A, Lopez-Fuster MJ, Nadal J (2007) Bioaccumulation of lead, mercury, and cadmium in the greater white-toothed shrew, Crocidura russula, from the Ebro Delta (NE Spain): sex-and age-dependent variation. Environ Pollut 145:7–14. doi:10.1016/j.envpol.2006.02.033
Sánchez-Chardi A, Ribeiro CAO, Nadal J (2009) Metals in liver and kidneys and the effects of chronic exposure to pyrite mine pollution in the shrew Crocidura russula inhabiting the protected wetland of Donana. Chemosphere 76:387–394. doi:10.1016/j.chemosphere.2009.03.036
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18
Schroeder WH, Munthe J (1998) Atmospheric mercury—an overview. Atmos Environ 32:809–822. doi:10.1016/S1352-2310(97)00293-8
Sebestyen SD, Dorrance C, Olson DM, Verry ES, Kolka RK, Elling AE, Kyllander R (2011) Long-term monitoring sites and trends at the Marcell Experimental Forest. In: Kolka RK, Sebestyen SD, Verry ES, Brooks KN (eds) Peatland biogeochemistry and watershed hydrology at the Marcell Experimental Forest. CRC Press, Boca Raton, pp 15–71
Speir SL, Chumchal MM, Drenner RW, Cocke WG, Lewis ME, Whitt HJ (2014) Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ Toxicol Chem 33:2506–2509
Strickman RJS, Fulthorpe RR, Wasik JKC, Engstrom DR, Mitchell CPJ (2016) Experimental sulfate amendment alters peatland bacterial community structure. Sci Total Environ 566:1289–1296. doi:10.1016/j.scitotenv.2016.05.189
Talmage SS, Walton BT (1991) Small mammals as monitors of environmental contaminants. Rev Environ Contam Toxicol 119:47–108. doi:10.1007/978-1-4612-3078-6_2
Tjerngren I, Karlsson T, Bjorn E, Skyllberg U (2012) Potential Hg methylation and MeHg demethylation rates related to the nutrient status of different boreal wetlands. Biogeochemistry 108:335–350. doi:10.1007/s10533-011-9603-1
US Environmental Protection Agency (2002) Method 1631, Revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. US Environmental Protection Agency Office of Water, Washington
Whitaker JO (2004) Sorex cinereus. Mamm Species 743:1–9
Whitaker JO, Schmeltz LL (1973) Food and external parasites of Sorex-palustris and food of Sorex cinereus from St-Louis County, Minnesota. J Mammal 54:283–285. doi:10.2307/1378897
Wren C, MacCrimmon H, Frank R, Suda P (1980) Total and methylmercury levels in wild mammals from the Precambrian Shield area of south central Ontario, Canada. Bull Environ Contam Toxicol 25:100–105. doi:10.1007/BF01985495
Wyman KE, Rodenhouse NL, Bank MS (2011) Mercury bioaccumulation, speciation, and influence on web structure in orb-weaving spiders from a forested watershed. Environ Toxicol Chem 30:1873–1878
Acknowledgements
We thank Planck Huang for his help with lab analysis and staff at the Marcell Experimental Forest Research Station and the USFS Northern Research Station. Funding was provided by a NSERC Discovery grant and a Great Lakes Air Deposition Program grant to CPJM. Rachel Strickman and Planck Huang provided useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures and protocols were approved by the University of Toronto-Scarborough’s Animal Care Committee.
Rights and permissions
About this article
Cite this article
Tavshunsky, I., Eggert, S.L. & Mitchell, C.P.J. Accumulation of Methylmercury in Invertebrates and Masked Shrews (Sorex cinereus) at an Upland Forest–Peatland Interface in Northern Minnesota, USA. Bull Environ Contam Toxicol 99, 673–678 (2017). https://doi.org/10.1007/s00128-017-2198-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2198-z