Abstract
Cholinesterase (ChE) activity was measured in Astyanax bifasciatus maintained in controlled conditions. Muscle ChE activity of individuals collected in field conditions in two seasons was compared among specimens collected in seven streams (forest and rural) of the lower Iguaçu river basin in association with physical, chemical, pesticides and biological factors. Significant differences in muscle ChE activity between control fish and fish collected in streams in both seasons were found, with higher activity in natural conditions. This the first time that differences in muscle ChE activity have been found among fish collected from different streams, suggesting synergism among multiple factors (e.g. temperature, pH, animal weight) and ecological attributes (richness and abundance) as influencing the variation in biomarkers. It is necessary to evaluate the quality of aquatic environments for a more accurate biomonitoring approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The increased use of pesticides in monocultures is a major cause of contamination in aquatic environments (Ahmad et al. 2006), affecting non-target organisms and consequently the whole ecosystem. Some authors have highlighted aquatic environments as being the most vulnerable to pollution (Marchesan et al. 2010; Rebelo and Caldas 2014). According to the National Health Surveillance Agency (ANVISA), Brazil is the third largest consumer of pesticides in the world (Brasil 2010), and Paraná State is the second largest consumer in the country.
The use of fish as bioindicators for biomonitoring programs is well established in the scientific community (Lopes et al. 2014). Astyanax bifasciatus is a teleost that is geographically restricted to the watershed of the Iguaçu River, and has been proposed as a potential bioindicator (Bueno-Krawczyk et al. 2015). The Iguaçu River is located in Southern Brazil, and presents a course of 1320 km (SEMA 2010). It is essential for the water supply of several cities and rural areas of Paraná State. Studies conducted in 2008 considered the Iguaçu River as the second most polluted in Brazil (IBGE 2010). The pollution results from disposal of sewage from urban areas, and from intense agricultural activity, mainly in the western region of the state (Freire et al. 2015).
Among the main contaminants derived from agriculture, organophosphate and carbamate pesticides are commonly used to control insects and other invertebrates. The effects of these pesticides have been evaluated by cholinesterase activity (ChE), which is a well-known biomarker for the diagnosis of neurotoxic effects on non-target organisms (Costa-Silva et al. 2015; Freire et al. 2008).
Inhibition of muscle ChE activity can occur in the presence of organophosphates and carbamates, which leads to the accumulation of acetylcholine in the synapses and neuromuscular junctions of vertebrates (Tong et al. 2013). Several studies have sought to analyze the contamination of aquatic environments with xenobiotics through the evaluation of cholinesterase activity as a biomarker (Bueno-Krawczyk et al. 2015; Chiang et al. 2012; Jesus et al. 2013; Vieira et al. 2014). However, there is a knowledge gap for using cholinesterase as a biomarker in the lower Iguaçu region, due to a lack of knowledge about native species and the association of environmental variables on biomarker. In this context, we investigated the muscular cholinesterase response of A. bifasciatus in the laboratory through experimental testing, and in streams with different types of land use and occupation.
Since lower cholinesterase activity occurs in the muscles of fishes submitted to greater exposure to pollutants, such as in rural streams, we tested the hypothesis that changes in muscular cholinesterase activity occur in A. bifasciatus, under the influence of environmental variables, in minimally impacted and rural streams. The objectives of this study were to: (i) compare the muscle ChE activity of A. bifasciatus submitted to controlled conditions and sampled in a stream considered minimally impacted, in summer and fall; (ii) compare muscle ChE activity of A. bifasciatus collected among different streams (forest and rural); and (iii) evaluate the association of muscle ChE activity with physical, chemical and biological factors.
Materials and Methods
Sampling and the procedures performed in the laboratory were approved by the Ethics Committee for Animal Experimentation of the State University of Western Paraná, protocol no. 02011, and licensed under SISBio license no. 25039-1.
Fifteen specimens were collected at Arroio Pedregulho, which is an indirect tributary of the Iguaçu river (25°6′6.10"S and 53°18′41.26"W), and sent to the Zoology Laboratory of the State University of Western Paraná. These animals were kept in an aquarium (30 L, 0.5 fish/L), with constant aeration. The fishes were acclimated for nine days, with controlled pH (6.8 ± 0.04), ammonia concentration (5.0‰ ± 0.3), and water temperature (22°C ± 0.13), and a photoperiod of 12/12 h. Commercial feed with 45% protein was offered twice a day and siphoning of food debris and excreta, as well as 30% water renewal was performed every 3 days. After the acclimation period, the fishes were anesthetized with 6.25 mg of cetylpyridinium chloride/L of water and their weight and standard length were measured. A 1 cm2 portion of the axial muscle tissue of the animal was removed and stored at −20°C.
Specimens of A. bifasciatus were also collected at Arroio Pedregulho, in a site where riparian vegetation consisting of herbaceous and arboreal species of medium size, including lianas, allowed the stream to be partially shaded for much of the sampled portion. For this stream, 15 specimens were collected in the summer and 15 in the fall. These were anesthetized and biological samples were collected as described above (0.5 fish/L).
To compare the cholinesterase activity of A. bifasciatus under controlled and field conditions, in the summer and fall, a one-way ANOVA was performed after evaluation of the assumptions of normality (Shapiro–Wilk test) and homoscedasticity (Levene test). A multiple comparison of means was performed using Tukey-HSD post-hoc test, assuming α = 0.05.
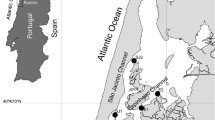
Samples were taken from indirect tributaries of the lower Iguaçu River, in the stretch between downstream of the Salto Caxias dam and the mouth of the Floriano River, Iguaçu National Park. The sampling sites were defined a priori using microbasin land use. The Google Earth Pro program was used for delimitation of the area of the microbasin (km²). By confirming the point where terrain elevation decayed, several points were marked for the definition of a polygon of the microbasin area, and categories were defined using the following criteria: area with vegetation (remaining forest and presence of riparian forest within the area of the microbasin) and rural area (presence of pasture area, plantation and property buildings). Each microbasin was classified according to the land use percentage as minimally impacted (more than 50% of area with vegetation) or rural (more than 50% of rural area) (Fig. 1).
During specimen sampling, a stretch of 50 m from the stream was delimited using blocking nets at either end to prevent fishes escaping, and then three successive passes of electrofishing were performed from downstream to upstream of the river. We characterized the fish community richness and abundance, and approximately ten specimens of A. bifasciatus were taken for the analysis of cholinesterase enzyme activity. The individuals were anesthetized and biological samples were collected as described above.
The temperature (Temp), pH, dissolved oxygen (DO) and water conductivity (Cond) were also measured using a multiparameter Horiba probe. We performed pesticide residual analysis in the stream soil, using multiresidue pesticide analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction (Fernandes et al. 2012). Validation was based on the SANCO reference system (SANCO 2011), and recoveries were determined by analyzing four blank soil samples spiked at four concentrations (10, 50, 100, and 300 g/kg corresponding respectively to low, medium, and high levels) and compared to four standard solutions at the same concentration. One sample with 1 kg of sediment at each site was aconditionated in a plastic bag and taken to the Agri-environmental Analysis Laboratory of the State University of Western Paraná. The organochorines and organophosphorous were expressed by parts per bilion (ppb).
The muscle ChE activity was measured by the method of Ellman et al. (1961), adapted for microplates by Herbert et al. (1995), which uses a colorimetric evaluation of the concentration of a conjugate of acetate with a color reagent, 5,5′-ditil-bis (2-nitrobenzoic) acid (DTNB). Acetylthiocholine (ATC) was used as substrate at a concentration of 9 mM and DTNB at a concentration of 0.5 mM. Microplate reading was performed at an absorbance of 415 nm. Protein concentrations were determined by the method of Bradford (1976). The muscle ChE activity was expressed in nmol/min/mg protein.
The condition factor (FC) was calculated based on the regression curve from the weight data (g) and standard length (cm), according to the formula FC = M × CPb, where b is the slope of the curve (Vazzoler 1996).
To test possible differences in cholinesterase activity of A. bifasciatus at the different sampling sites, a one-way ANOVA was used followed by a Tukey-HSD post-hoc test. The association of environmental variables with cholinesterase activity was evaluated by principal component analysis (PCA), according to McCune and Grace (2002). The axes selected for interpretation were chosen through the Broken-Stick criteria (Hammer et al. 2001), and the factor loadings were represented in an ordination diagram. Statistical analyses were performed in the programs STATISTICA 7.0 and Past 2.14.
Results and Discussion
The physical and chemical characterization data of the experiment, as well as those collected in the field are presented in Table 1.
The cholinesterase muscle activity of A. bifasciatus was significantly higher among the individuals collected in natural conditions when compared to controlled specimens. Intermediate means were recorded for the individuals collected in the field in summer, and higher values were found among the specimens collected in the fall (F2,21=21.295; p = 0.001) (Fig. 2), which suggests a relationship with environmental variables.
Cholinesterase muscle activity of Astyanax bifasciatus acclimatized in the laboratory and collected in the field in summer and fall in the Arroio Pedregulho. Data present the averages ± standard deviation of enzyme activity expressed in nmol/min mg/prot. Statistical differences are represented by different letters (p < 0.05) (n = 15)
The specimens collected in Arroio Pedregulho were characterized as minimally impacted. Higher values of temperature and pH, and lower values of dissolved oxygen were found in the summer, and the opposite conditions were observed in the fall (Table 1).
The lower values for muscle ChE activity observed in animals submitted to controlled conditions may be related to low variation in temperature (22°C ± 0.13), oxygen (4.2 ± 1 mg/L), pH (6.8 ± 0.04), ammonia levels (5.0‰ ± 0.3), and food supply and absence of predators, contributing to decreased metabolism and consequently decreased cholinesterase activity. When compared with individuals collected in the field in summer and fall, the physical, chemical and biological conditions were different and had high variation (Table 1). The field conditions promoted a significantly higher muscle ChE activity when compared to control specimens. Several factors directly or indirectly influence muscle ChE activity. Hochachka (1974) indicated that the hydrophobic contribution to binding at the anionic site of acetylcholinesterase is strongly disrupted at low temperatures and high pressures; Assis et al. (2010) demonstrated that when pH rises, there is an increase in AChE activity, and different fish species exhibit optimal enzymatic activity at different temperatures; Klemz and Silva de Assis (2005) showed that higher weight promotes lower cholinesterase activity. However, the relationship among these factors in the natural environment directly influences muscle ChE activity, which decreases the efficacy of this enzyme as the single biomarker in natural environments.
According to Chiang et al. (2012), seasonality promotes changes in biomarkers during biomonitoring studies. This was highlighted by Bueno-Krawczyk et al. (2015), who demonstrated that in A. bifasciatus, cholinesterase activity of both brain and muscle presented variations in different seasons. The seasons present different stressors on fish metabolism, which leads to fluctuations in cholinesterase activity (Menéndez-Helman et al. 2015). According to Hochachka (1974), animals living in different physical environments circumvent this problem by adjusting the enthalpic and entropic contributions to binding at the anionic site of acetylcholinesterase.
Given the differences in cholinesterase activity observed between the control and field conditions in different seasons, a discussion about the natural variation in this biomarker is relevant. This biomarker is widely used in biomonitoring, therefore it is necessary to evaluate its variation along with several covariates.
The physical and chemical variables of the sampling sites presented significant differences (p < 0.05), with the exception of dissolved oxygen (Table 1). The highest values of organophosphates in sediment were observed in Córrego Manoel Gomes (P1), followed by the Córrego Bom Retiro (P7) and Arroio Nene (P4), and lower values were found in Córrego Arquimedes, Córrego Tormenta and Rio da Paz. Organophosphates were not found in Córrego Jumelo; however, the presence of organochlorines was observed (Table 1).
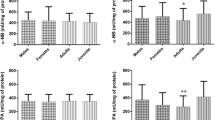
The cholinesterase activity evaluated in A. bifasciatus presented statistically significant differences among the environments evaluated (F6,54 = 7.8152; p < 0.001). The highest values were observed for individuals collected in the Córrego Bom Retiro (P7), followed by the Córrego Tormenta (P5) and Manoel Gomes (P1), and lower values were found in Rio da Paz (P6), Córrego Arquimedes (P2), Arroio Nene (P4) and Córrego Jumelo (P3) (Fig. 3).
Muscle Cholinesterase activity of Astyanax bifasciatus. P1 – Córrego Manoel Gomes (n = 14), P2 – Córrego Arquimedes (n = 11), P3 – Córrego Jumelo (n = 13), P4 – Arroio Nene (n = 7), P5 – Rio Tormenta (n = 6), P6 – Rio da Paz (n = 3) and P7 – Córrego Bom Retiro (n = 7). Data present the averages ± standard deviation of enzyme activity expressed in nmol/min mg/prot. Statistical differences are represented by different letters (p < 0.05)
Principal components analysis showed an accumulated variation of 61.73% in the two main components. The first component (CP1) explained 40.59% of the variation in the data and the second component (CP2), 21.15%. CP1 characterized the land use, and the positive scores showed a higher association of minimally impacted sites (Mim) (P1, P2, P3) with the biological variables, richness and abundance. Negative scores of this component were related to rural sites (Rur) (P4, P5, P6 and P7), where higher dissolved oxygen values (DO), and lower richness and abundance were observed (Fig. 4).
Principal component analysis (PCA) on cholinesterase activity of Astyanax bifasciatus at the different sites correlated with environmental variables. Arrangement of seven streams (polygons P1 – Córrego Manoel Gomes, P2 – Córrego Arquimedes, P3 – Córrego Jumelo, P4 – Arroio Nene, P5 – Córrego Tormenta, P6 – Rio da Paz and P7 – Córrego Bom Retiro), related to 11 physical (Temp temperature), chemical (pH, Cond conductivity and DO dissolved oxygen) and biological variables (richness and abundance)
This is the first study to report the possible influence of ecological attributes, such as richness and abundance, on the enzymatic activity of cholinesterase. Previous reports have shown the influence of other species on community behavior (Latini and Petrere 2004; Pelicice and Agostinho 2009), but these were not related to enzymatic activity.
CP2 showed association among enzyme activity and physical and chemical variables, demonstrating the influence of environmental variables on cholinesterase activity. Positive scores of CP2 represented the highest values of temperature (Temp) and conductivity (Cond) in the sampling sites (P3 and P7), and higher values of cholinesterase activity in A. bifasciatus in these sites. Negative scores showed a relationship with higher condition factor values (FC) and pH, as well as lower values of cholinesterase activity among the individuals collected in P1, P2, P4, P5 and P6 (Fig. 3).
The spatio-temporal variability of AChE was analyzed relative to water temperature and salinity as well as fish size by Durieux et al. (2011). These authors found that AChE activity was highly positively correlated with water temperature, and to a lesser extent, negatively with fish size, while no relationship was detected with salinity in striped bass (Morone saxatilis).
Variation in cholinesterase muscle activity in A. bifasciatus was observed at all sites, but this had no association with the presence of orgaphosphorous and organochlorines in stream sediment. Our results corroborate the data collected by Cazenave et al. (2009) and Vieira et al. (2014), who did not observe differences in cholinesterase activity among fishes from sites considered polluted and preserved. The influence of environmental variables on this enzyme may cause a bias in the interpretation of this biomarker, since it is difficult to define a standard high or low value of cholinesterase activity in variable conditions. Sanchez et al. (2008) observed this when they demonstrated the difficulty in making inferences about the presence of contaminants in environments in biomonitoring studies using cholinesterase activity as a biomarker.
However, there are some measures that can minimize such biases, such as performing sampling over a short period, collecting fishes of similar sizes, using a larger number of samples (Payne et al. 1996), and using a greater scope in the characterization of physical and chemical variables (Chiang et al. 2012; Menéndez-Helman et al. 2015). A wider consideration of these factors may allow differentiation between responses caused by exposure to pollutants and the possible influence of abiotic and biotic factors (Vidal et al. 2002). Nevertheless, in biomonitoring situations in the field, it is not always possible to follow these measures.
The possible influence of biotic and abiotic factors, combined with the complexity of polluting substances may point to the need to use other complementary biomarkers together with the cholinesterase enzyme, in order to present more conclusive results. In conclusion, the use of the ChE biomarker should always be associated with biotic (richness, abundance, biometric data) and abiotic (temperature, dissolved oxygen, etc.) variables. Furthermore, biomonitoring programs need to use a set of biomarkers to obtain a more precise evaluation of toxic products that affect the quality of aquatic environments.
References
Ahmad I, Pacheco M, Santos M (2006) Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere 65:952–962
Assis CRD, Castro PF, Amaral IPG et al (2010) Characterization of acetylcholinesterase from the brain of the Amazonian tambaqui (Colossoma macropomum) and in vitro effect of organophosphorus and carbamate pesticides. Environ Toxicol Chem 29:2243–2248
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Brasil (2010) Ministério da Saúde. Secretaria Nacional de Assistência à Saúde. Anvisa divulga resultado do monitoramento de agrotóxico em alimentos. Brasília
Bueno-Krawczyk ACD, Guiloski IC, Piancini LDS et al (2015) Multibiomarker in fish to evaluate a river used to water public supply. Chemosphere 135:247–264
Cazenave J, Bacchetta C, Parma MJ et al (2009) Multiple biomarkers responses in Prochilodus lineatus allowed assessing changes in the water quality of Salado River basin (Santa Fe, Argentina). Environ Pollut 157:3025–3033
Chiang G, Munkittrick KR, Urrutia R et al (2012) Liver ethoxyresorufin-O-deethylase and brain acetylcholinesterase in two freshwater fish species of South America; the effects of seasonal variability on study design for biomonitoring. Ecotoxicol Environ Saf 86:147–155
Costa-Silva DG, Nunes ME, Wallau GL et al (2015) Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environ Sci Pollut Res 22:15526–15535
Durieux EDH, Farver TB, Fitzgerald PS, Eder KJ, Ostrach DJ (2011) Natural factors to consider when using acetylcholinesterase activity as neurotoxicity biomarker in Young-Of-Year striped bass (Morone saxatilis). Fish Physiol Biochem 37:21–29
Ellman GL, Courtney KO, Andrres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fernandes VC, Domingues VF, Mateus N, Delerue-Matos C (2012) Multiresidue pesticides analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J Sep Sci 36:376–382
Freire MM, Santos VG, Ginuino ISF, Arias ARL (2008) Biomarcadores na avaliação da saúde ambiental dos ecossistemas aquáticos. Oecologia Brasiliensis 12:347–354
Freire CA, Souza-Bastos LR, Chiesse J et al (2015) A multibiomarker evaluation of urban, industrial, and agricultural exposure of small characins in a large freshwater basin in southern Brazil. Environ Sci Pollut Res 22:13263–13277
Hammer DA, Harper T, Ryan PD (2001) PAST: paleontological Statistics Software package for education and data analysis. Paleontol Electron 4:1–9
Herbert A, Guilhermino L, Silva de Assis HC, Hansen PD (1995) Acetylcholinesterase activity in aquatic organisms as pollution biomarker. Angew Zoo 3:1–5.
Hochachka PW (1974) Temperature and pressure adaptation of the binding site of acetylcholinesterase. Biochem J 143:535–539
IBGE (2010) Instituto Brasileiro de Geografia e Estatística. Indicadores de desenvolvimento sustentável (IDS). Brasília.http://www.ibge.gov.br/home/default.php. Accessed 21 Feb 2017
Jesus TB, Colombi JS, Ribeiro CAO et al (2013) Cholinestarase activity in methylmercury and Mercury chloride exposure fish. Ecotoxicol Environ Contam 8:147–148
Klemz C, Silva de Assis HC (2005) Effects of endosulfan on acetylcholinesterase activity of “cascudo” (Ancistrus multispinnis, fish, Teleostei). Animal 3:51–58
Latini AO, Petrere M (2004) Reduction of a native fish fauna by alien species: an example from Brazilian freshwater tropical lakes. Fish Manage Ecol 11:71–79
Lopes MR, Silva Filho MV, Salles JB et al (2014) Cholinesterase activity of muscle tissue from freshwater fishes: characterization and sensitivity analysis to the organophosphate methyl-paraoxon. Environ Toxicol Chem 33:1331–1336
Marchesan E, Sartori GMS, Avila LA et al (2010) Residues of pesticides in the water of the Depression Central rivers in the State of Rio Grande do Sul, Brazil. Ciência Rural 40:1053–1059.
McCune B, Grace JB (2002) Analysis of ecological communities. MjM software, Gleneden Beach, Oregon
Menéndez-Helman RJ, Ferreyroa GV, Afonso MS, Salibián A (2015) Circannual rhythms of acetylcholinesterase (AChE) activity in the freshwater fish Cnesterodon decemmaculatus. Ecotoxicol Environ Saf 111:236–241
Payne JF, Mathieu A, Melvin W, Fancey LL (1996) Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Pollut Bull 32:225–231
Pelicice FM, Agostinho AA (2009) Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biol Invasions 11:1789–1801
Rebelo RM, Caldas ED (2014) Avaliação de risco ambiental de ambientes aquáticos afetados pelo uso de agrotóxicos. Química Nova 37:1199–1208.
Sanchez W, Katsiadaki I, Piccini B et al (2008) Biomarker responses in wild three-spined stickleback (Gasterosteus aculeatus L.) as a useful tool for freshwater biomonitoring: a multiparametric approach. Environ Int 34:490–498
SANCO/12495 (2011) Method validation and quality control procedures for pesticide residues analysis in food and feed, eu reference laboratories for residues of pesticides. European Commission, Brussels
SEMA (2010) Bacias hidrográficas do Paraná – Série Histórica. Secretaria de Estado do Meio Ambiente e Recursos Hídricos. Curitiba
Tong F, Islam RM, Carlier PR et al (2013) Effects of anticholinesterases on catalysis and induced conformational change of the peripheral anionic site of murine acetylcholinesterase. Pestic Biochem Physiol 106:79–84
Vazzoler AEAM (1996) Biologia da reprodução de peixes teleósteos: teoria e prática. Nupelia, Maringá
Vidal ML, Basseres A, Narbonne JF (2002) Influence of temperature, pH, oxygenation, water-type and substrate on biomarker responses in the freshwater clam Corbicula flumínea (Müller). Comp Biochem Physiol Part C 132:93–104
Vieira CED, Almeida MS, Galindo BA et al (2014) Integrated biomarker response index using a Neotropical fish to assess the water quality in agricultural areas. Neotrop Ichthyol 12:153–164
Acknowledgements
We are grateful to the support of the Western Paraná State University. This research was supported by grants from the CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nimet, J., Guimarães, A.T.B. & Delariva, R.L. Use of Muscular Cholinesterase of Astyanax bifasciatus (Teleostei, Characidae) as a Biomarker in Biomonitoring of Rural Streams. Bull Environ Contam Toxicol 99, 232–238 (2017). https://doi.org/10.1007/s00128-017-2111-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2111-9