Abstract
To assess environmental contamination, studies have been increasingly carried out using biomarkers to diagnose the effects of toxic metal exposure, such as metallothionein (MT). In this context, the aims of the present study were to determine Cd, Cu, Ni and Pb concentrations, by ICP-MS, in liver samples from Mugil cephalus from the municipality of Ilhéus, located in North-Southeastern Brazil, in the state of Bahia, which has been increasingly suffering from anthropogenic pressure, and to quantify total MT in liver and gills. Among essential metals, copper (199.2 μg g−1) presented concentrations approximately 7 times higher than allowed by the World Health Organization (30 μg g−1). Liver samples showed higher concentrations than gills. Metallothionein concentrations indicate differential contamination along the study area. The first sampling showed higher induction of MT synthesis overall. Differences between liver and gill MT concentration trends were relatively minor, which may indicate the adequacy of analyzing gills in an environmental monitoring context.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Environmental pollution by solid waste and agricultural activities is increasingly spreading through coastal municipalities, causing a series of adverse impacts to the ecosystem due to the dispersion of toxic metals, among others (Martins and Wanderley 2009). Humans continuously discharge contaminants to the aquatic environment, making these a pathway for pollutants (Kehrig et al. 2009). The exposure and bioaccumulation of high concentrations of potentially toxic metals is harmful to living organisms and are a major consequence of the introduction of these metals on aquatic systems (Yilmaz 2003). Fish, in particular have an important role in aquatic ecosystems, due to their high quality protein, high mobility and excellent digestibility (Naji et al. 2014). When contaminated, however, they represent a risk to the human population, as it is well established that food is one of the main routes of exposure to toxic substances (Carrola et al. 2014).
The effects of chemical agents may be diagnosed with simple and efficient methods, such as the incorporation of biomarker measurements in the assessment of pollutant exposure effects in environmental monitoring programs (Infante et al. 2006; Luo et al. 2014). Among these, metallothionein (MT) determinations are routinely used to evaluate toxicity and bioaccumulation of metals. These are cysteine-rich proteins, presenting thermal stability and metal binding capacity, participating in various functions in the organisms, among them metal- binding, sequestering of free radicals (Gagné et al. 2013) and functions against oxidative stress (Lavradas et al. 2014). Due to the formation of thiol-metal complexes, increased protein synthesis is induced by metal entry into the cells (Hauser-Davis et al. 2012). The presence of the thiol groups allows these proteins to bind to excess essential and non-essential metals, thus protecting organisms from metal toxicity. This allows for these metalloproteins to serve as biomarkers specific for metal contamination (Mao et al. 2012).
Environmental impacts to the municipality of Ilhéus, in the state of Bahia, have followed the trend of increased pollution described above. Increased discharges of industrial effluents, sewage, pesticides and local port construction have contributed to impacts along the coastal areas, which can cause negative impacts on local aquatic ecosystems (Mierzejewski et al. 2014). However, many fish, crustaceans and mollusks are still harvested in the Pontal Bay region of this municipality, providing basic food and income for coastal communities (Ourives et al. 2011), leading to increasing concern regarding contamination of these organisms. In particular, mullet (Mugil cephalus) are commonly consumed in the area. They are also considered adequate sentinels for evaluating pollution in aquatic systems (Carrola et al. 2014; Milinkovitch et al. 2013; Pereira et al. 2013), since they feed mainly on zooplankton and benthic organisms and are distributed worldwide in coastal waters, lakes, bays and estuaries (Shen et al. 2011). In this context, the aims of the present were to determine Cd, Cu, Ni and Pb concentrations in Mugil cephalus liver and gill samples; to quantify MT levels in these samples; and to compare metal and MT concentrations in fish from five sites in the immediate area of Ilhéus.
Materials and Methods
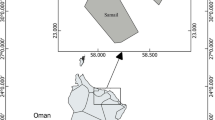
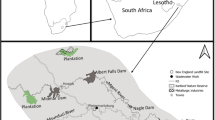
Three samplings (April, June and November) were carried out at five different sampling points at Pontal Bay, located in the municipality of Ilhéus, in the southern region of the state of Bahia, Brazil (Fig. 1; Table 1). The Cachoeira River basin is the largest of the basins in the East Atlantic hydrographic region, where it joins the Itacanoeira and Santana Rivers, forming an estuary at a location known as Coroa Grande, in the city of Ilhéus (Pinto and Boehs 2008). It has a large population, comprising the main municipalities of this region. It presents a striking diversity of agricultural areas distinguished by different natural characteristics and anthropic occupation dynamics. It has a drainage area of 4600 km², encompassing thirteen municipalities and an estimated population of 600 thousand inhabitants (Trindade et al. 2010).
The fish were sacrificed by spinal severing, and liver and gills were immediately removed and stored at −80°C. Samples were subsequently lyophilized.
For the inductively coupled plasma mass spectrometry (ICP-MS) analyses, approximately 100 mg of the samples were acid-digested with 0.5 mL double distilled nitric acid (Vetec, Rio de Janeiro, BR) and left to stand overnight (Balcerzak 2002). Sample mineralization was carried out in a water bath at 100°C for 5 h. After cooling, the volume of each sample was adjusted to 5 mL with ultrapure water. Appropriate dilutions were then prepared for metal assessment by ICP-MS (ELAN DRC II model, Perkin-Elmer Sciex, Norwalk, CT, USA). The ICP-MS operational conditions were as follows: RF power of 1300 W, plasma gas flow rate 15.0 L min−1, auxiliary gas flow rate 1.2 L min−1, carrier gas flow rate 0.42 L min−1, three replicate readings were taken with a reading time of 45 s. Metals were introduced into the instrument by a pneumatic nebulizer with a cyclonic spray chamber. Rhodium (103Rh) was used as internal standard at a concentration of 20 mg L−1 (Lavradas et al. 2014). The analyzed elements were Cd, Cu, Ni and Pb. The accuracy of the method was evaluated by using DORM-3 certified reference material (dogfish muscle, NRC, Ottawa, Ontario, CA).
Metallothionein extractions were carried out according to Erk and collaborators (Erk et al. 2002). Approximately 25 mg of each sample was weighed and homogenized at a 1:3 ratio (v/v) in a buffer containing 20 mM Tris-HCl, pH 8.6, 0.5 mM phenylmethylsulfonyl fluoride as a protease inhibitor, and 0.01 % β-mercaptoethanol as a reducing agent. Samples were then centrifuged at 20,000×g for 60 min at 4°C. The supernatants were carefully transferred to sterile 2 mL microtubes and heated at 70°C for 10 min, and centrifuged again under the same conditions for 30 min. Subsequently, the supernatants was collected and frozen at −80°C.
After the purification process, total protein content of the samples was determined by the Lowry method modified by Peterson (1979), using bovine serum albumin (BSA) as a standard, for later application in 1D SDS-gels. The samples were homogenized with a sample buffer containing 10 % sodium dodecyl sulfate (SDS) (w/v), 10 mM β-mercaptoethanol, 10 % glycerol (v/v), 0.2 M Tris-HCl, pH 6.8 % and 0.05 % bromophenol blue at a 1:1 ratio. Protein denaturation was carried out by heating the samples at 90°C for 5 min. Samples were then loaded onto 15 % gels, according to Laemmli (1970) and run at 10 mA for 15 min and then 35 mA until the end of the run. The gels were silver stained (Heukeshoven and Dernick 1985) and scanned via ImageScanner II (Amersham, Buckinghamshire, UK). The molecular weights of the visualized protein bands were determined by standards (Biorad Precision Plus Protein™, Hercules, CA, USA).
Metallothionein quantification was carried out using the spectrophotometric method described by Ellman (1959), in which 5,5′-dithio-bis-(2-nitrobenzoic acid) is used to quantify the thiol groups in the samples. The purified MT extracts were, thus, treated with 1 M HCl containing 4 mM EDTA, 2 M NaCl and 0.43 mM DTNB in 0.2 mM buffered sodium phosphate, pH 8.0, and incubated for 30 min in the dark. Sample absorbances were then measured at 412 nm in a microplate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA). MT concentrations were estimated using an analytical curve plotted with GSH as an external standard because, although this method measures all soluble acid thiols, glutathione represents more than 90 % of reactive thiol groups and is considered an adequate standard. MT content was estimated using 1 mol MT = 20 mol GSH, as described by Kagi (Kägi 1991).
A multivariate technique was applied to the data using the Statistica® software package, version 7.0 (Tulsa, OK, USA). A principal component analysis (PCA) was carried out to observe the separation of data points with the formation of groups.
Results and Discussion
Regarding the accuracy evaluation of the adopted analytical method, no statistically significant differences were observed between found and certified values for each element in the certified reference material, with a confidence level of 95 % using student’s t test, indicating the adequacy of the method for the selected elements using ICP-MS. The certified values for Cd, Cu, Ni and Pb (0.29 ± 0.02, 15.5 ± 0.63, 1.28 ± 0.24 and 0.39 ± 0.06 µg g−1), respectively, were in accordance to the observed values for Cd, Cu, Ni and Pb (0.25 ± 0.03, 15.0 ± 4.87, 1.22 ± 0.06 and 0.35 ± 0.03 µg g−1). Table 2 presents the concentrations for samplings 1 (S1-April) and 2 (S2-June) at the 5 sampling points. Unfortunately, the fish from the third sampling could not be analyzed by ICP-MS.
Copper concentrations were higher than the other determined metals at all sampling points, and also higher than levels recommended by FAO/WHO (1997), of 30 µg g−1. Copper is considered an essential metal, but can cause serious problems if present in high concentrations. In the present study, the highest concentrations were observed at point 3, Itacanoeira River (S1 = 199.2 µg g−1 d.w. and S2 = 123 µg g−1 d.w.), allowing for the inference of potential contamination by this element due to anthropic activities, as these levels are extremely high compared to other anthropogenically disturbed areas.
Cadmium, Pb and Ni, however, are toxic and do not show vital functions in the organism, as stated by Viana et al. (2012) who investigated these metals in muscle tissue. These were found in lower concentrations than those established by FAO/WHO, of 1.0, 2.0 and 5.0 µg g−1 (FAO/WHO 1997). Other studies, such as that by Viana et al. (2012), observed Cu concentrations in Mugil cephalus (159.5 µg g−1 d.w.) almost 6 times higher than the maximum permissible limit in muscle, while Atli and Canli (2008) also found extremely high Cu levels (169.2 ± 35.6 µg g−1) in the liver of another sentinel species, Oreochromis niloticus, although the author did not state whether this concentration was based on dry or wet weight. Another study, by Hauser-Davis et al. (2014), conducted in the extremely contaminated area of the Guanabara Bay (BR), reported Cu concentrations several orders of magnitude lower in both gills and liver than the levels observed in the present study. This seems to confirm significant Cu contamination in the study area of Pontal Bay, which is further corroborated by the fact that Mugil sp. is a planktivore fish that feeds on plankton and should not present high concentrations of inorganic species, since it feeds on the lowest trophic level (Sanches Filho et al. 2013). Thus, the high concentrations observed herein are probably indicative of exogenous sources of contamination.
Data from a full matrix were processed using a principal component analysis (PCA) to examine possible relationships between samples. The obtained PCA is displayed in Fig. 2a, b, indicating the loading and scores, respectively.
In Fig. 2a, a weight chart shows that it was possible to separate the variables Pb and Cd, with negative weights, and C u and Ni with positive weights in PC1. On PC2, it was possible to separate Cu, Ni, Pb and Cd, with negative weights. A graph generated by the analysis of the main components of fish samples and their respective codes allowed for the visualization of several groupings according to location. A graphical analysis of the scores allowed confirmation of sample separations into two groups. A comparison of Fig. 2a, b indicates that Cu and Ni were present in higher concentrations in both samples (S1 and S2) at each point, however sample 1 (S1-April) had higher concentrations, probably because months January to April are rainy periods in the Bay of Pontal/Ilheus, causing an increase in laminar erosion processes in that period, which enables the entrainment of these elements to water bodies. In sampling 2 (S2-June) higher Pb concentrations were recorded in Section 5 (S2P5-Bay of Pontal) that may be explained by PC1. The weights and scores of the graph analysis indicate that the two components explained 82.91 % of the data variance.
Metallothionein is used as a biomarker of exposure to metals, since synthesis is induced by the presence of increased metal concentrations. This metalloprotein can bind up to 7 metal ions, as it is composed of two domains: the alpha domain, which can bind to four metals, and the beta domain to three metals. Due to the presence of high amounts of thiol groups, that bind to metals, MT plays a central role in essential trace element homoeostasis and detoxification of toxic metals (Hauser-Davis et al. 2014).
The SDS-PAGE analyses were conducted as a first-step to verify the adequacy of the MT purification procedure (Fig. 3). After analysis of the gels, the thermal purification procedure was indeed deemed adequate, since very few protein bands appeared, while the appearance of 14 kDa bands was observed, characteristic of MT, which can present itself in the form of a dimer in fish (Hauser-Davis et al. 2014). Hauser-Davis (2012), when analyzing tilapia, also observed the appearance of 14 kDa MT bands, while further confirming their identify by other analytical techniques. Thus, MT identification was confirmed, the purification was considered adequate and the MT samples were then determined by UV–Vis spectrophotometry.
Figure 4a presents the MT concentrations in gills from the 5 sampling sites at Pontal Bay. Samples from P2-Cachoeira River and P3-Itacanoeira River were higher during the first sampling. Figure 4b presents MT concentrations in livers from the 5 sites. Points P1-Santana River, P2-Cachoeira River and P3-Itacanoeira River presented higher MT concentrations compared to points P4 and P5.
MT has been increasingly used as a biomarker to assess the biological effects of exposure to metals (Hauser-Davis et al. 2014) in situations of environmental contamination, because of the direct relationship between metal levels in the environment and metal concentrations found in aquatic animals tissues. Herein, MT concentrations in liver were higher than in the gills, as expected since the liver is a storage organ responsible for detoxification of toxic metals in the body (Labarrère et al. 2012). This corroborates other studies, in which MT concentrations were higher in the liver compared to gills in Mozambique tilapia (Oreochromis mossambicus) (Naji et al. 2014). The analysis of gills, however, is also interesting, since they are in direct contact with pollutants that are present in the water. In this case, differences were observed when comparing both tissues. Liver and P4 showed higher MT concentrations in liver at S1, whereas the opposite occurred in gills. This may denote higher susceptibility to induced MT synthesis in liver. However, this difference was relatively minor, which may indicate the adequacy of analyzing gills in environmental monitoring regarding metal exposure. In addition, gill MT concentrations in the first sampling at P2 and P3 where significantly higher than the second sampling, compared to liver, indicating induced MT synthesis in these cases. This may be due to the fact that Cu concentrations were significantly higher during the first sampling, since Cu has been shown to significantly induce MT synthesis in fish. The first sampling showed higher induction of MT synthesis, probably related to the higher metal concentrations observed in most of the sampling locations.
High Cu concentrations in liver were observed, with Point 3, Itacanoeira River (sampling 1-S1P3) showing the highest concentration of this metal (199.2 μg g−1), approximately 7 times higher than the concentration of 30 µg g−1 allowed by the WHO. Cadmium, Ni and Pb concentrations, on the other hand, were below the maximum permissible WHO levels. Groupings were observed by the PCA analysis, with sampling 1 explained by PC1 and sampling 2 by PC2. One sampling point showed different data results compared to the others (S2P5-Pontal Bay), being separated by PC1. This may be related to an increase of domestic sewage discharge, indicated by high Pb concentrations.
MT concentrations were higher in liver, as expected, since this is the main storage and detoxification organ for metals. MT showed differential expression in both samplings and all 5 locations, in which gill MT levels in the first sampling at P2 and P3 were significantly higher than the second sampling, compared to liver. This was possibly due to the fact that Cu concentrations were significantly higher in the first sampling. The first sampling also showed higher induction of MT synthesis overall, probably related to the higher metal concentrations observed at most of the sampling locations. Differences between liver and gill MT concentration trends were relatively minor, which may indicate the adequacy of analyzing gills in an environmental monitoring context.
References
Atli G, Canli M (2008) Responses of metallothionein and reduced glutathione in a freshwater fish Oreochromis niloticus following metal exposures. Environ Toxicol Phar 25:33–38
Balcerzak M (2002) Sample digestion methods for the determination of traces of precious metals by spectrometric techniques. Anal Sci 18:737–750
Carrola J, Santos N, Rocha MJ, Fontainhas-Fernandes A, Pardal MA, Monteiro RAF, Rocha E (2014) Frequency of micronuclei and of other nuclear abnormalities in erythrocytes of the grey mullet from the Mondego, Douro and Ave estuaries—Portugal. Environ Sci Pollut Res 21:6057–6068
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Erk M, Ivanković D, Raspor B, Pavičić J (2002) Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 57:1211–1218
FAO/WHO (1997) Joint FAO/WHO food standards programme Codex Committee on contaminants in foods 1–89
Gagné F, Smyth S, André C, Douville M, Gélinas M, Barclay K (2013) Stress-related gene expression changes in rainbow trout hepatocytes exposed to various municipal wastewater treatment influents and effluents. Environ Sci Pollut Res 20:1706–1718
Hauser-Davis RA, Gonçalves RA, Ziolli RL, de Campos RC (2012) A novel report of metallothioneins in fish bile: SDS–PAGE analysis, spectrophotometry quantification and metal speciation characterization by liquid chromatography coupled to ICP-MS. Aquat Toxicol 116:54–60
Hauser-Davis RA, Bastos FF, Tuton B, Rocha RC, Saint’Pierre T, Ziolli RL, Arruda MA (2014) Bile and liver metallothionein behavior in copper-exposed fish. J Trace Elem Med Biol 28:70–74
Heukeshoven J, Dernick R (1985) Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis 6:103–112
Infante HG, Van Campenhout K, Blust R, Adams FC (2006) Anion-exchange high performance liquid chromatography hyphenated to inductively coupled plasma-isotope dilution-time-of-flight mass spectrometry for speciation analysis of metal complexes with metallothionein isoforms in gibel carp (Carassius auratus gibelio) exposed to environmental metal pollution. J Chromatogr A 1121:184–190
Kägi JH (1991) Overview of metallothionein. Methods Enzymol 205:613–626
Kehrig HA, Fernandes KW, Malm O, Seixas TG, Di Beneditto APM, Souza C (2009) Transferência trófica de mercúrio e selênio na costa norte do Rio de Janeiro. Quim Nova 32:1822–1828
Labarrère CR, Menezes BD, Melo MM (2012) Avaliação dos teores de zinco em brânquias, carcaça, fígado e musculatura de diferentes espécies de peixes capturados no Rio São Francisco (MG, Brasil). Revista Geonomos 20
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lavradas RT, Hauser-Davis RA, Lavandier RC, Rocha RCC, Saint’Pierre TD, Seixas T, Kehrig HA, Moreira I (2014) Metal, metallothionein and glutathione levels in blue crab (Callinectes sp.) specimens from southeastern Brazil. Ecotox Environ Safe 107:55–60
Luo LZ, Ke CH, Guo XY, Shi B, Huang MQ (2014) Metal accumulation and differentially expressed proteins in gill of oyster (Crassostrea hongkongensis) exposed to long-term heavy metal-contaminated estuary. Fish Shellfish Immunol 38:318–329
Mao H, Wang D-H, Yang W-X (2012) The involvement of metallothionein in the development of aquatic invertebrate. Aquat Toxicol 110:208–213
Martins PTDA, Wanderley LDL (2009) Dinâmica de ocupação espacial de áreas contíguas (período 1987–2008) e sua relação com tensores de origem antrópica no manguezal do Rio Cachoeira, Ilhéus, Bahia. Sociedade Natureza 21:77–89
Mierzejewski J, Haney DC, van den Hurk P (2014) Biomarker responses in sunfish species and largemouth bass from the Saluda River, South Carolina. Ecotox Environ Safe 110:8–15
Milinkovitch T, Imbert N, Sanchez W, Le Floch S, Thomas-Guyon H (2013) Toxicological effects of crude oil and oil dispersant: Biomarkers in the heart of the juvenile golden grey mullet (Liza aurata). Ecotox Environ Safe 88:1–8
Naji A, Ismail A, Kamrani E, Sohrabi T (2014) Correlation of MT levels in livers and gills with heavy metals in wild tilapia (Oreochromis mossambicus) from the Klang River, Malaysia. B Environ Contam Tox 92:674–679
Ourives TM, Rizzo AE, Boehs G (2011) Composition and spatial distribution of the benthic macrofauna in the Cachoeira River estuary, Ilhéus, Bahia, Brazil. Rev Biol Mar Oceanogr 46:17–25
Pereira P, Raimundo J, Caná Rio J, Almeida A, Pacheco M (2013) Looking at the aquatic contamination through fish eyes: a faithful picture based on metals burden. Marine Poll Bull 77:375–379
Pinto TR, Boehs G (2008) Nematopsis sp.(Apicomplexa: Eugregarinida) em Mytella guyanensis (Lamarck, 1819)(Bivalvia: Mytilidae) da Região Estuarina do Rio Cachoeira, Ilhéus, Bahia, Brasil. Braz J Vet Res Anim Sci 45:95–100
Sanches Filho PJ, Fonseca VK, Holbig L (2013) Avaliação de metais em pescado da região do Pontal da Barra, Laguna dos Patos, Pelotas-RS. Ecotoxicol Environ Contam 8:105–111
Shen K-N, Jamandre BW, Hsu C-C, Tzeng W-N, Durand J-D (2011) Plio-Pleistocene sea level and temperature fluctuations in the northwestern Pacific promoted speciation in the globally-distributed flathead mullet Mugil cephalus. BMC Evol Biol 11:83
Trindade MEDJ, Cetra M, Jucá-Chagas R (2010) Ichthyofauna of the Ribeirão Limoeiro, Cachoeira River Basin, BA. Biota Neotrop 10:111–117
Viana ZCV, da Silva E, Fernandes GB, Korn DMGA, Santos VLCS (2012) Estudo da absorção de elementos químicos no músculo de peixes do litoral da Bahia. Rev Cien Med Bio 11:27–34
Yilmaz AB (2003) Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay, Turkey. Environ Res 92:277–281
Acknowledgments
The funding was provided by Fundação de Amparo à Pesquisa do Estado da Bahia, Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacheco, C.S.V., da Silva, E.G.P., Hauser-Davis, R.A. et al. Determination and Evaluation of Metallothionein and Metals in Mugil cephalus (Mullet) from Pontal Bay, Brazil. Bull Environ Contam Toxicol 98, 84–90 (2017). https://doi.org/10.1007/s00128-016-1959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1959-4