Abstract
Sulfamethazine (SMZ) is one of the most commonly used sulfonamide compounds in fish farming, and its physiological effects on fish are unknown. SMZ was administered to juvenile Nile tilapia (Oreochromis niloticus) at a dose level of 422 mg kg−1 body weight, for a period of 11 days, via medicated feed. Fish were divided into two groups, the control group (CG) and the group fed with SMZ in feed. The administration of SMZ did not alter the erythrograms and leukograms of the Nile tilapia. The SMZ-fed group showed the same hepatic lipid peroxidation (LPO) concentration as the CG. Nonetheless, the oral administration of SMZ raised the hepatic catalase (CAT) and glutathione S-transferase (GST) activities, the increase probably being sufficient to prevent hepatic LPO production. The oral administration of SMZ affects the hepatic GST and CAT activities of Nile tilapia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
According to FAO (2012), aquaculture has shown very rapid growth in recent decades, establishing itself as an industry of great economic importance. Parallel to the development and intensification of aquaculture, disease outbreaks and undesirable diseases in local production have also increased with the development of intensive production systems (Ferreira et al. 2012). Bacterial diseases stand out as important factors limiting productivity, and can cause stunted growth and a high mortality rate, becoming a major problem in intensive fish production (Carraschi et al. 2011). In an attempt to reduce these problems, antibiotics are administered in the diet to prevent or treat fish diseases (Ferreira et al. 2012).
A wide range of antimicrobials are available for use in animal production. Sulfonamides have been widely used because of their low cost, effectiveness in tackling some bacterial infections, and their ability to improve animal performance (Nonaka et al. 2012). Sulfonamides, mainly represented by sulfamethazine (SMZ), are sulfonamide-derived synthetic compounds. The combination of sulfadiazine and trimethoprim in a ratio of 5:1 is one of the most widely used treatments in aquaculture. The kinetics of sulfonamides after oral administration has been studied in a fish species, the gilthead sea bream (Sparus auratus) (Rigos et al. 2013). The most common way of administering antimicrobials to fish is by a mixture of the antimicrobial with specially formulated feed (Malvisi et al. 1997; Paschoal et al. 2012, 2013). Wood et al. (1957) suggested a therapeutic doses of 220–440 mg kg−1 bw for 10 days, and cited a daily prophylactic dose of 44 mg kg−1 bw. Wood (1968) indicated that sulfamethazine was also effective when administered at concentrations from 220 to 440 mg kg−1 bw per day in starter diets and at concentrations of 110 mg kg−1 bw in pelleted feed. Scott (1993) recommended a dosage of 25 mg kg−1 bw, during 5–10 days. In the USA, for example, sulfadimethoxine/ormetoprim antimicrobials have been authorized for use in aquaculture. In Brazil, even though their use has not been legalized and regulated, the use of sulfonamide compounds in aquaculture is common. The large range of recommended dosage and period, and the absent of rules and control processes can lead to an abusive use of SMZ in fish farms.
SMZ was first evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) at its 34th meeting (WHO 1990), when no data were available in relation to acute toxicity (LD50 values). Nevertheless, a temporary Acceptable Daily Intake (ADI) of 0–4 μg kg−1 bw day−1 was established. Latter, at its 42nd meeting (WHO 1994), the committee established an ADI of 0–50 μg kg−1 bw day−1. According to Commission Regulation (EU) N 37/2010 (EC 2010), all substances belonging to the sulfonamide group, including SMZ, are allowed to be used in all food-producing species (including fish). Nonetheless, the combined total residues of all substances within the group should not exceed the Maximum Residue Level (MRL) value of 100 μg kg−1. For fin fish the muscle MRL relates to muscle and skin in natural proportions. In Brazil, the Ministry of Agriculture, Livestock and Supply (MAPA 2014) established a reference limit in fish that should not exceed 100 μg kg−1 for the single or combined total residues of sulfamethazine, sulfathiazole and sulfadimethoxine.
According to Amend et al. (1967), sulfur drugs are routinely used to control bacterial infections in fish. The administration of sulfonamide in fish farming can be topical, in the water, or in the feed (Carraschi et al. 2011). Outbreaks of disease in aquaculture are usually treated via mass therapy by way of drugs incorporated in the feed (Varó et al. 2013). In Asia–Pacific and Japan, the use of sulfonamides against diseases and bacteria in aquaculture is common (Samanidou and Evaggelopoulou 2007; Harikrishnan et al. 2011; Defroirdt et al. 2011) and sulfur drugs represent 6 % of the antimicrobials marketed in China (Xu et al. 2007). Of the sulfur drugs, SMZ is widely used as an antimicrobial in animal treatment (Poirier et al. 1999).
Although most antimicrobials, including sulfur drugs, have been shown to be effective and of great benefit to farmers, they have been associated with certain effects such as a decrease in filet palatability and increase in kidney damage and infertility (McCarthy et al. 1974). Saglam and Yonar (2009) observed that the oral administration of sulfamerazin at concentrations of 100, 200 and 400 mg kg−1, reduced the immunoglobulin levels and damaged the B-lymphocytes in rainbow trout (Oncorhynchus mykiss). Yildiz and Altunay (2011) reported the effect of sulfamethoxazole and trimethoprim on gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax), observing disruptions in physiological and immunological response in both species.
Despite the benefit of most antibiotics in fish farming, some problems can be associated with their use. Several hazards and side effects are linked to the excessive use of antibacterial drugs in fish, such as immunosuppression, growth retardation, the development of resistant bacterial strains and environmental problems such as drug residues (Saglam and Yonar 2009). There is little or no information about the side effects of the oral administration of SMZ on the fish antioxidant defenses and hematological parameters. The efficacy of the oral administration of veterinary drugs to fish is still under discussion, and hence data that assist in understanding the possible effects of administering SMZ on the fish physiology may provide support for their use and information on their effectiveness. Thus, the present research studied the hematological parameters and oxidative stress biomarkers to evaluate the effect of the oral administration of SMZ to Nile tilapia (Oreochromis niloticus).
Materials and Methods
Juvenile Nile tilapia were obtained from the Rio Doce fish farm, São João da Boa Vista, São Paulo, Brazil, and acclimatized at the Ecotoxicology and Biosafety Laboratory, Embrapa Environment, Jaguariúna-SP, Brazil. The water quality variables during the acclimatization period were measured daily for each tank with a multiparameter probe, Horiba U10 (New Jersey, USA). The following averages (±standard deviation; SD) were obtained for the variables of water quality measured during the acclimatization period: pH 6.50 ± 0.22, dissolved oxygen 7.12 ± 0.33 (mg L−1), temperature 26 ± 2 (°C). Fish (total 108) weighing 30 ± 5 g were allocated in 250 L tank capacity (200 L water) with biological filters, artificial oxygenation and a temperature control system. Fish were divided into 2 groups and placed in 6 tanks (n = 18 fish/tank), 5 of which were fed with SMZ in feed and 1 with feed without SMZ (control group; CG). The water quality was measured daily for pH, conductivity (mS cm−1), turbidity (NTU), dissolved oxygen (mg L−1), temperature (°C) and salinity (%), using a Horiba U10 multiparameter probe. The tanks were cleaned and the water changed at least twice a week to remove waste and any leftover feed. The organic matter retention system of the filter was washed on a periodic basis.
To confirm the SMZ concentration in the medicated feed, it was analyzed by high performance liquid chromatography (HPLC) using a HPLC Waters system: Model 600 instrument with a Model 717 Plus autosampler, and a Diode Array Detector model 996 (Waters, Milford, MA, USA). Also, in order to verify SMZ uptake by the fish, the muscle tissue of two fish from each tank was analyzed. The muscle samples analyzed were from fish slaughtered 6 h after the discontinuation of the medication. The muscle sample analysis was performed using an Acquity Ultra Performance Waters System (UPLC) coupled to a hybrid quadrupole orthogonal time of flight (Q-Tof) mass spectrometer (UPLC-QTof MS), Synapt, Waters. Electrospray Ionization (ESI) in the positive mode was used as the ionizing source and the software of acquisition control and data treatment was the MassLynx, version 4.1 (Waters). The developed analytical methods were validated according to the recommendations of the European Community (EC 2002) and the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA 2011).
The fish were fed twice daily at the rate of approximately 2 % of the body weight with extruded food for fish (Nutripiscis, Nutrisamal N S Alimentos Animais), previously analyzed to verify the absence of SMZ. They were maintained in a room with photoperiod (16 h light: 8 h dark) and temperature (26 ± 2°C) controlled, at constant water aeration. At the end of the period of acclimation (10 days), the control group (CG) received the SMZ-free commercial feed and the exposed group received feed containing SMZ (analytical Fluka, Sigma Aldrich, USA, 99 % minimum), at a nominal dose of 400 mg kg−1 body weight, for 11 consecutive days. Feed orts were removed from the tanks 3 h after each feeding.
For the preparation of the medicated feed, SMZ was dissolved in ethanol with the support of an ultrasonic bath. The solution obtained was added to the ration by mixing and drying, using a hair dryer until total ethanol elimination. Then, the medicated feed was sprayed with soya oil, at a minimum amount, with the aim to avoid the SMZ leaching from the ration when in contact with water.
At the end of the experimental period, ten fish per group were anesthetized with benzocaine (65 mg L−1) and 1–2 mL of blood was collected from the caudal vessel of each fish using a heparinized syringe needle (22 G, 1½ inch). Blood smears were made immediately after collection and blood was placed in microtubes for further analytical procedures. With the exception of the differential white blood cells (WBC) count, the other procedures were carried out immediately after collection.
Hematocrit (Ht) was determined using the microhematocrit centrifugation technique and the hemoglobin concentration by the cyanmethemoglobin method, using a commercial Labtest Diagnóstica kit (Lagoa Santa, MG, Brazil). The red blood cells (RBC), white blood cells (WBC) and thrombocytes were determined optically in a Neubauer chamber using (Natt and Herrick 1952) solution as the diluent. The mean cell volume (MCV) and the mean cell hemoglobin concentration (MCHC) were calculated from the Ht, Hb and RBC values.
The differential WBC count was carried out by means of a panoptic blood smear in homogeneous areas, counting 200 leukocytes in each smear according to Natt and Herrick (1952). The total number of leukocytes was obtained by subtracting the percentage of thrombocytes from the total of leukocytes plus thrombocytes counted in a Neubauer chamber. From the total number of leukocytes (WBC; /μL) and the relative number of leukocytes (%), the absolute number (/μL) of neutrophils (NØ; /μL), lymphocytes (LØ; /μL), monocytes (MØ; /μL), eosinophils (EØ; /μL), basophils (BØ; /μL), special granulocytic cells (SGC; /μL) and thrombocytes (Trb; /μL) in the blood smear samples could be calculated, as described by Hrubec and Smith (2010).
The fish were sacrificed by spinal cord transection immediately after blood sampling. The livers were collected and washed with saline solution (0.9 % NaCl), dried with filter paper, identified and stored at −80°C for further biochemical analysis. In order to assess the levels of lipid peroxidation (LPO), catalase activity (CAT) and glutathione S-transferase (GST), the sampled tissues were weighed and homogenized at 12,000 rpm in phosphate buffer for 30 min. Centrifugation was carried out in a Hermle-Z323K (Hermle LaborTechnik, Wehingen, DE) refrigerated centrifuge and the supernatant used as the enzyme source. The procedures were approved by the Ethics Committee on Animal Experiments of Embrapa Environment (Registration no. 001/2013).
Liver protein was determined according to the Bradford (1976) method, adapted for the use of a Dynex MRXTC 250 microplate reader (model MRXTC 250, Dynex Technologies, Inc., West Sussex, UK) as described by Kruger (1994). Lipid peroxidation was quantified using the ferrous oxidation-xylenol orange (FOX) method as described by Jiang et al. (1992), and CAT activity according to Aebi (1983) by the continuous evaluation of the decrease in hydrogen peroxide (H2O2) concentration at λ = 240 nm. The GST activity was measured according to Habig et al. (1974), using l-chloro-2.4-dinitrobenzene (CDNB) as the substrate. Spectrophotometric readings were made in a Spectronic Genesys 5 (Milton Roy Co., Ivyland, PA, USA) spectrophotometer, and the microplate readings using a Dynex MRXTC 250 reader.
In order to confirm the assumption of equal SMZ uptake between fishes from treated tanks, a PROC GLM (General Linear Models) analysis was performed, followed by Tukey’s test, to verify differences in SMZ fish muscle concentration from the SMZ group, with a significance level of α = 0.05 using the SAS software (v.9.3, Cary, NC, USA). For the comparison of means from the control and SMZ groups, a Student t test was performed, with a significance level of α = 0.05 using the SAS software (v.8.2). The results are presented as mean ± S.D.
Results and Discussion
The water quality parameters were maintained constant and within acceptable levels for fish culture (Boyd 1990). The general health conditions of the fish were normal throughout the experiment, with no mortality in any experimental group during the 11-day exposure period.
Know the concentration of SMZ in the feed is important to ensure that the dose intended for the medication treatment is delivered correctly. To verify the SMZ concentration in the feed an HPLC–DAD analytical method was validated, and for the muscle an UPLC-QTof MS method. The method for the determination of SMZ in the feed and muscle showed the following validation parameters, respectively: linearity of the analytical curve (r2) 0.999 and 0.992; intra-day precision (CV%) lower than 9.6 and 15.0; inter-day precision (CV%) lower than 5.4 and 19.4, accuracy (%) between 78.0 to 86.2 and 59.1 to 87.7; limit of determination (LOD ng g−1) of 1.4 and 1.0 and limit of quantification (LOQ ng g−1) of 2.7 and 5.0, indicating that both methods met the adopted validation guidelines (EC 2002; MAPA 2011).
Regarding the medicated feed, the concentration of SMZ in the feed produced was 24.3 mg g−1, and the real SMZ dose administrated to the fish was 422 mg kg−1 body weight. SMZ residues were not detected in fish muscle from the control group. SMZ residues ranged from 947 to 2097 ng g−1 in fish from the SMZ group (Table 1). Otherwise, there was no difference in SMZ muscle concentration from fish of each SMZ tank. Thus, despite the fact that fish were kept in different tanks, the SMZ uptake from feed were similar. The maximum residue level (MRL) of 100 µg kg−1 for sulfonamides in fish muscle, defined by the EU Commission Regulation (EC 2010), seems to be the basis for permissible fish residues around the word. In addition, the Codex Alimentarius Commission recognizes the extreme difficulty in verifying hypersensitive human reactions from consuming tissues containing sulfonamide residues and therefore recommends that the MRL should be set as low as practically possible (CODEX 2016). Notwithstanding, the present residues of SMZ in the muscle were approximately 10 times higher than the MRL.
The hematological variables are useful in clinical diagnosis and are often used to detect physiological changes following different stress conditions. Thus hematology can be an essential index to the general health status in a number of fish species (Wedemeyer and Yasutake 1977). In this study, there was no difference in the RBC, Ht, Hb, MCHC and MCV between the control and SMZ groups (Table 2). A comparison of the present data with those of authors who studied the hematological profile of Nile tilapia under the same laboratory conditions mainly showed erythogram ranges similar to those deemed normal for the species.
Rijkers et al. (1981) affirmed that the oral administration of antibacterials can significantly decrease the RBC, resulting in anemia. Conversely, in the present study, the feeding of SMZ to the Nile tilapia did not interfere with the RBC counts. The present data also differed from those observed by Saglam and Yonar (2009), who analyzed the effect of the oral administration of 100, 200 and 400 mg kg−1 of sulfamerazine in rainbow trout for 3, 7, 14 and 21 days. They observed that fish fed with 200 mg kg−1 had increases in their Ht and MCV after 7 and 14 days.
Hematological parameters are also of importance in the understanding of the normal and pathological conditions of fish, assisting in the identification of adverse conditions (Tavares Dias and Moraes 2003). Lundèn and Bylund (2002) studied the effects of sulfadiazine and trimethoprim on the immune response of rainbow trout medicated orally with doses of 30 mg kg−1, and found no difference between the control and treated fish groups in the circulating lymphocyte levels.
In the present study, there was no difference between the control and SMZ groups in the WBC, MØ, NØ, LØ, EØ, BØ, SGC and Trb levels (Table 3). However, Saglam and Yonar (2009) observed a decrease in WBC in rainbow trout fed 400 mg kg−1 of sulfamerazine for 7 and 14 days, when compared with untreated fish. Caipang et al. (2009) affirmed that the immune response of fish to antibiotics depended on the drug, the procedure adopted, and the fish species. Lundèn and Bylund (2002) observed no effect of the oral administration of 30 mg kg−1 of fish of sulfadiazine associated with trimethoprim on the immune response of rainbow trout, and concluded that such antimicrobials could be used to prevent fish diseases.
Little or nothing is known about the effects of orally administered SMZ on the hematological parameters of fish, but one can affirm that the addition of 400 mg kg−1 of SMZ in the feed for a period of 11 days does not influence the erythrogram and leukogram results of Nile tilapia.
Reactive oxygen species (ROS) are produced in all aerobic organisms and normally occur in cells in equilibrium with an antioxidant defense system. Oxidative stress occurs when this critical balance is disrupted due to depletion of antioxidants or excess accumulation of ROS, or both. An indicator of oxidative stress is the induction of antioxidant defenses and/or increases in endogenous ROS levels. The formation of ROS can be accelerated as a consequence of various environmental stress conditions, including exposure to herbicides, metals and xenobiotics (Scandalios 2005). The primary effect of a diet supplement with a metal can be the increased production of ROS in tissues where this metal is accumulated. Previous studies have shown that combined sulfur drugs are rapidly absorbed and distributed throughout the fish body after oral administration, and can accumulate in liver tissue, for example (Bergsjo et al. 1979; Hormazabal and Rogstad 1992). Varó et al. (2013) affirmed that sulfur drugs are metabolized primarily in the liver. Liver tissue was investigated as the principal target of toxicity due to the liver’s role in the energetic and xenobiotic metabolism of drugs.
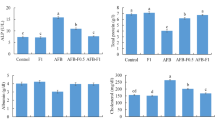
Lipid peroxidation (LPO) has been proposed as the main agent causing cellular damage in response to increases in ROS (Halliwell and Gutteridge 1989). In this study, the evaluation of liver damage related to oxidative stress induced by SMZ was tested, as determined by the measurement of LPO and the activities of CAT and GST. Our results showed that the hepatic LPO level from fish fed with SMZ did not differ from the control group (Fig. 1).
Enzyme activity levels are important biochemical parameters for evaluation in animals under toxicant stress. When an organ is diseased due to the effect of a toxicant, enzyme activity may be either increased or inhibited. The increase or decrease in their level may be sufficient to provide information of diagnostic value (Valarmathi and Azariah 2003). CAT, an important antioxidant defense enzyme, is a major primary antioxidant defense component working primarily to catalyze the decomposition of H2O2 to H2O.
In the present study, the oral administration of SMZ increased liver CAT activity (Fig. 2). CAT can be considered as a major antioxidant defense enzyme, acting against lipid peroxidation and preventing oxidative stress (Halliwell and Gutteridge 1989). Antioxidant defenses, including CAT, are inducible by conditions that increase the ROS flux such as O2, H2O2 and OH (Di Giulio et al. 1993). Increased liver CAT activity was observed in fish species from polluted environments (Livingstone et al. 1995; Wilhelm Filho et al. 2001). CAT decomposes H2O2 formed extensively during oxidative stress (Halliwell and Gutteridge 1989). In chemically stressed animals, the increase or decrease of CAT activity is possible, depending on the chemical nature, time and dose of exposure (Jemec et al. 2008). CAT activity is considered a sensitive biomarker of oxidative stress of harmful effects in fish (Gül et al. 2004; Sanchez et al. 2005).
An increase in hepatic GST activity can be observed in the SMZ fish group when compared with the control group (Fig. 3). GST molecules are proteins involved in the cell detoxification process, and act against the effects of xenobiotic drugs, herbicides and other environmental pollutants (Li et al. 2010). GST can catalyze endogenous or exogenous pollutant compounds, making them less toxic and ready for elimination (Cogo et al. 2009). According to Caipaing et al. (2009), the glutathione level can be used as a potential biomarker to verify the effect of orally administered antimicrobials. However, more studies are necessary to understand the effect of antimicrobials in fish oxidative metabolisms.
In the present study, the fish were treated with SMZ at a dosage of 422 mg kg−1 (body weight). Other authors used similar doses to control parasitosis in fish farming (Ankeli 1971). According to the present data, Nile tilapia fed with SMZ for 11 days at that dosage did not alter their hepatic LPO concentrations. Nevertheless, the hepatic activities of CAT and GST showed an increase in the group fed with SMZ. This fact was also observed for other antimicrobials. For instance, Nunes et al. (2014) observed that tetracycline increased the enzymatic activity of the hepatic CAT and GST in fish, indicating that this compound could exert pro-oxidative activity. Oxytetracycline and amoxicillin induced GST activity in zebrafish, but inhibited CAT activity (Oliveira et al. 2013).
The steady hepatic LPO level observed in the present study could be correlated with the increased activities of the antioxidant defenses, preventing hydroperoxide formation. This process can be explained as an equilibrium in oxidative metabolism. Altinordulu and Eraslan (2009) considered CAT and GST as the organisms’ primary defense instruments associated with ROS formation. The increase in CAT and GST activities can justify the hepatic hydroperoxide equilibrium presented by the medicated fish group, given that the antioxidant enzymes intermediated the oxidative process, avoiding peroxidation and hepatic oxidative stress.
Observing the antioxidant status of the liver, with the purpose of correlating the ROS production and the antioxidant defenses, the present study shows that fish fed with SMZ presented higher values of hepatic antioxidant defense activities. Since the LPO values are statistically equal in control and SMZ group, it can be suggested that the period and dose administered to fish was enough to combat the LPO production, but caused a disruption in antioxidant defense system characteristic of an oxidative stress condition.
The present data showed evidence that the oral administration of SMZ at a dose level of 422 mg kg−1 bw, for a period of 11 days, leads to SMZ residual levels in the muscle 10 times higher than the MRL. Nevertheless, the treatment did not influence the hematological parameters of the Nile tilapia. It did, however, induce significant disturbances in hepatic CAT and GST activity showing evidences of an oxidative stress condition. The use of the oral administration of SMZ in fish culture, mainly for therapeutic purposes, may disrupt the physiological antioxidant defenses system of the fish leading to an oxidative stress condition that can negatively influence fish development. Also, It is important to emphasize the need for further research to comprehend the kinetics of SMZ, as well as the depletion of SMZ residues in the muscle of fish fed a ration containing SMZ. It is essential to obtain a high quality fish product in terms of organoleptic and nutritional characteristics, free of any risks to human health. The present study provides research information for the use of SMZ in Brazilian fish farms, and may also contribute to the implementation of effective regulations regarding its use.
References
Aebi H (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn. Academic Press, London, pp 273–286
Altinordulu S, Eraslan G (2009) Effects of some quinolone antibiotics on malondialdehyde levels and catalase activity in chicks. Food Chem Toxicol 4:2821–2823
Amend DF, Fryer JL, Pilcher KS (1967) Comparison of oregon pellet and fish-meat diets for administration of sulfamethazine to chinook salmon. Res Briefs 13:20–25
Ankeli GO (1971) Furunculosis in the redspot salmon (Oncorhynchus rhodurus) and other salmonids in holding tanks. Can Vet J 12:136–138
Bergsjo T, Nafstad I, Ingebrigtsen K (1979) The distribution of 35S-sulfadiazine and 14C-trimethoprim in rainbow trout Salmo gairdneri. Acta Vet Scand 20:25–37
Boyd CE (1990) Water quality in ponds for aquaculture. Auburn University, Auburn
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caipang CMA, Berg I, Brinchmann MF, Kiron V (2009) Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 295:110–115
Carraschi SP, Cruz C, Machado Neto MP, Castro NL, Gírio ACF (2011) Eficácia do florfenicol e da oxitetraciclina no controle de Aeromonas hydrophila em pacu (Piaractus mesopotamicus). Arq Bras Med Vet Zootec 63:579–583
CODEX (2016) Codex Alimentarius. Veterinary drug residues in food. http://www.fao.org/fao-who-codexalimentarius/standards/vetdrugs/veterinary-drug-detail/en/?d_id=57. Accessed 08 Mar 2016
Cogo AJD, Siqueira AF, Ramos AC, Cruz ZMA, Silva AG (2009) Utilização de enzimas do estresse oxidativo como biomarcadoras de impactos ambientais. Nat Online 7:37–42
Defroirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258
Di Giulio RT, Habig C, Gallagher EP (1993) Effects of black rock harbour sediments on indices of biotransformation, oxidative stress, and DNA integrity in channel catfish. Aquat Toxicol 26:1–22
EC (2002) European Community. Commission Decision 2002/657/EC. Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun 221:8–36
European Commission (2010) Commission Regulation (EU) n 37/2010, 22 December 2009, on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. http://ec.europa.eu/health/files/eudralex/vol5/reg_2010_37/reg_2010_37_en.pdf. Accessed 10 Sep 2015
FAO (2012) The state of world fisheries and aquaculture. http://www.fao.org/fishery/sofia/en. Acessed 23 Mar 2015
Ferreira AHC, Araripe MNBA, Monteiro CAB, Lopes JB, Hararipe HGA (2012) Uso de probióticos na aquicultura: revisão. Rev Electron Nutr 9:1965–1980
Gül S, Belge-Kurutas E, Yildiz E, Sahan A, Doran F (2004) Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environ Int 30:605–609
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Oxford Claredon, New York
Harikrishnan R, Balasundaram C, Heo M (2011) Fish health aspects in grouper aquaculture. Aquaculture 320:1–21
Hormazabal V, Rogstad A (1992) Simultaneous determination of sulphadiazine and trimethoprim in plasma and tissues of cultured fish for residual and pharmacokinetic studies. J Chromatogr 583:201–207
Hrubec TC, Smith SA (2010) Hematology of fishes. In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology. Blackwell, Iowa, pp 994–1003
Jemec A, Tišler T, Drobne D, Sepčić K, Jamnik P, Roš M (2008) Biochemical biomarkers in chronically metal-stressed daphnids. Comp Biochem Physiol 147(C):61–68
Jiang ZY, Hunt JV, Wolf SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Kruger NJ (1994) The Bradford method for protein quantification. Met Mol Biol 32:9–15
Li Q, Peng S, Sheng Z, Wang Y (2010) Ofloxacin induces oxidative damage to joint chondrocytes of juvenile rabbits: excessive production of reactive oxygen species, lipid peroxidation and DNA damage. Eur J Pharmacol 626:146–153
Livingstone DR, Lemaire P, Matthews A, Peters LD, Porte C, Fitzpatrick PJ, Forlin L, Nasci C, Fossato V, Wootton N, Goldfarb P (1995) Assessment of the impact of organic pollutants on goby (Zostersessor ophiocephalus) and mussel (Mytilus galloprovincialis) from the Venice Lagoon, Italy, biochemical studies. Mar Environ Res 39:235–240
Lundèn T, Bylund G (2002) Effect of sulphadiazine and trimethoprim on the immune response of rainbow trout (Oncorhynchus mykiss). Vet Immunol Immunopathol 85:99–108
Malvisi J, Della Rocca G, Anfossi P, Giorgetti G (1997) Tissue distribution and depletion of flumequine after in-feed administration in sea bream (Sparus aurata). Aquaculture 157:97–204
MAPA (2011) Ministério da Agricultura, Pecuária e Abastecimento do Brasil. Guia de validação e controle de qualidade analítica: fármacos em produtos para alimentação e medicamentos veterinários. http://www.agricultura.gov.br/arq_editor/file/Laboratorio/Guia-de-validacao-controle-de-qualidade-analitica.pdf
MAPA—Ministério da Agricultura, Pecuária e Abastecimento (2014) Plano Nacional de Controle de Resíduos, Instrução normativa SDA no 11, de 07 de maio de 2014. http://www.agricultura.gov.br/arq_editor/file/CRC/IN%2011%20-%20PNCRB%202014.pdf. Accessed 10 Sep 2015
McCarthy DH, Stevenson JP, Salsbury AW (1974) Combined in vitro of trimethoprim and sulphonamides on fish-pathogenic bacteria. Aquaculture 3:87–91
Natt MP, Herrick CA (1952) A new blood diluent for counting erythrocytes and leucocytes of the chicken. Poultry Sci 31:735–738
Nonaka CKV, Oliveira AMG, Paiva CR, Almeida MP, Resende CP, Moraes CGO, Botelho BG, Souza LF, Dias PG (2012) Ocurrence of antimicrobial residues in Brazilian food animals in 2008 and 2009. Food Addit Contam 29:526–534
Nunes B, Antunes SC, Gomes R, Campos JC, Braga MR, Ramos AS, Correia AT (2014) Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: antioxidant effects, neurotoxicity and histological alterations. Arch Environ Contam Toxicol 68:371–381
Oliveira R, McDonough S, Ladewig JCL, Soares AMVM, Nogueira AJA, Domingues I (2013) Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ Toxicol Pharmacol 36:903–912
Paschoal JAR, Bicudo ÃJA, Cyrino JEP, Reyes FGR, Rath S (2012) Depletion study and estimation of the withdrawal period for oxytetracycline in tilapia cultured in Brazil. J Vet Pharmacol Ther 35(1):90–96
Paschoal JAR, Quesada SP, Gonçalves LU, Cyrino JEP, Reyes FGR (2013) Depletion study and estimation of the withdrawal period for enrofloxacin in pacu (Piaractus mesopotamicus). J Vet Pharmacol Ther 36:594–602
Poirier LA, Doerge DR, Gaylor DW, Miller MA, Lorentzen RJ, Carciano DA, Kadlubar FF, Schwetz BA (1999) An FDA review of sulfamethazine toxicity. Regul Toxicol Pharmacol 30:217–222
Rigos G, Zonaras V, Nikoloudaki X, Cotou E, Henry M, Varo I, Alexis M (2013) Distribution and depletion of sulfadiazine after a multiple per os dosing in gilthead sea bream (Sparus aurata) fed two different diets. Medit Mar Sci 14(2):377–383
Rijkers GT, Van Oosteron R, Van Muiswinkel WB (1981) The immune system of cyprinid fish. Oxytetracycline and the regulation of the humoral immunity in carp. Vet Immunol Immunopathol 2:281–290
Saglam N, Yonar ME (2009) Effects of sulfamerazine on selected haematological and immunological parameters in rainbow trout (Onchorhynchus mykiss, Walbaum, 1792). Aquacul Res 40:395–404
Samanidou VF, Evaggelopoulou EN (2007) Analytical strategies to determine antibiotic residues in fish. J Sep Sci 30:2549–2569
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher JM, Ait-Aissa S (2005) Copper-induced oxidative stress in three-spined stickleback, relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183
Scandalios JG (2005) Oxidative stress, molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38:995–1014
Scott P (1993) Therapy in aquaculture. In: Brown L (ed) Aquaculture for veterinarians. Pergamon Press, Oxford, pp 131–153
Tavares-Dias M, Moraes FR (2003) Características hematológicas da Tilápia rendalli Boulenger, 1896 (Osteichthyes: cichlidae) capturada em “pesque pague” de Franca, São Paulo, Brasil. Biociência J 19:107–114
Valarmathi S, Azariah J (2003) Effect of copper chloride on the enzyme activities of the crab Sesarma quadratum (Fabricius). Turk J Zool 27:253–256
Varó I, Navarro JC, Rigos G, Del Ramo J, Calduch-Giner J, Hernández A, Pertusa J, Torreblanca A (2013) Proteomic evaluation of potentiated sulfa treatment on gilthead sea bream (Sparus aurata L.) liver. Aquaculture 376:36–44
Wedemeyer G, Yasutake WT (1977) Clinical methods for the assessment of the effects of environmental stress on fish health. US Fish Wildl Serv 89:18
WHO (1990) Toxicological evaluation of certain veterinary drugs residues in foods. WHO Food Additives Series 25. World Health Organization, Geneva, pp 79–93. http://www.inchem.org/documents/jecfa/jecmono/v25je01.htm. Accessed 10 Sep 2015
WHO (1994) Toxicological evaluation of certain veterinary drugs residues in foods. WHO Food Additives Series 33. World Health Organization, Geneva, pp 91–103. http://www.inchem.org/documents/jecfa/jecmono/v33je07.htm. Accessed 10 Sep 2015
Wilhelm Filho D, Torres MA, Tribess TB, Pedrosa RC, Soares CHL (2001) Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz J Med Biol Res 34:719–726
Wood JW (1968) Diseases of Pacific salmon, their prevention and treatment. Washington Department of Fisheries, Olympia
Wood EM, Yasutake WT, Johnson HE (1957) Acute sulfamethazine toxicity in young salmon. The Prog Fish Cultur 19(2):64–67
Xu W, Zhang G, Zou S, Li X, Liu Y (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut 145:672–679
Yildiz HY, Altunay S (2011) Physiological stress and innate immune response in gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) exposed to combination of trimethoprim and sulfamethoxazole (TMP-SMX). Fish Physiol Biochem 37:401–409
Acknowledgments
Funding for this study was provided by São Paulo Research Foundation (FAPESP; Grant # 2013/50452-5) and by the Brazilian National Council for Scientific and Technological Development (CNPq; Process No. 305390/2013-9). The authors sincerely thank the reviewers whose comments and suggestions helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sampaio, F.G., Carra, M.L., Jonsson, C.M. et al. Effects of Dietary Exposure to Sulfamethazine on the Hematological Parameters and Hepatic Oxidative Stress Biomarkers in Nile Tilapia (Oreochromis niloticus). Bull Environ Contam Toxicol 97, 528–535 (2016). https://doi.org/10.1007/s00128-016-1837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1837-0