Abstract
This manuscript reports results of an integrated theoretical and experimental investigation of adsorption of two emerging contaminants (DNAN and FOX-7) and legacy compound TNT on cellulose surface. Cellulose was modeled as trimeric form of the linear chain of 1 → 4 linked of β-D-glucopyranos in 4C1 chair conformation. Geometries of modeled cellulose, munitions compounds and their complexes were optimized at the M06-2X functional level of Density Functional Theory using the 6-31G(d,p) basis set in gas phase and in water solution. The effect of water solution was modeled using the CPCM approach. Nature of potential energy surfaces was ascertained through harmonic vibrational frequency analysis. Interaction energies were corrected for basis set superposition error and the 6-311G(d,p) basis set was used. Molecular electrostatic potential mapping was performed to understand the reactivity of the investigated systems. It was predicted that adsorbates will be weakly adsorbed on the cellulose surface in water solution than in the gas phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polysaccharides are ubiquitous in nature and they exhibit broad range of variation in their molecular structures and properties. Cellulose is a natural polysaccharide and is a major component of plant cell walls. Cellulose is characterized by the linear chain of 1 → 4 linked polymer of β-D-glucopyranose in the 4C1 chair conformation. The hydroxyl groups on sugar rings form hydrogen bonds within the same chain and that with neighboring parallel chains. Moreover, due to biodegradable and renewable abilities cellulose is regarded as the greenest available renewable polymeric material on earth (Moon et al. 2011). Armed forces worldwide are developing and adopting insensitive munitions (IM) to avoid accidental and spontaneous detonation of traditional munitions due to shock and high temperature sensitivity at various stages of handling (Felt et al. 2013; Taylor et al. 2015). Obviously, production, storage, training and application of these compounds may lead to their release in the environment and thus may lead to contamination of soils and aquifers and can leach to ground water. Thus, insensitive munition compounds are regarded as emerging contaminants and therefore their fate and transport need to be rigorously investigated.
Therefore, it is logical to thoroughly investigate the sorption of these emerging contaminants on easily available cellulosic biomass. Such investigation may not only shed light on their fate and transport; they can also lead to development of remediation and sensing technologies. For example, cellulose fibers have long been served as raw material in the textile and paper industries or in composite material as filler (Bledzki and Gassan 1999) and have been a major part of composting in bioremediation processes (Thorn et al. 2002). In this manuscript, we have investigated the sorption of two emerging contaminants such as 2,4-dinitroanisole (DNAN), 1,1-diamino-2,2-dinitroethene (FOX-7) and legacy compound 2,4,6-trinitrotoluene (TNT) on cellulose surfaces using a combined Density Functional Theory (DFT) level theoretical and experimental approaches. In the theoretical investigation, cellulose was modeled as the trimeric form of the linear chain of 1 → 4 linked of β-D-glucopyranos in the 4C1 chair conformation. It was revealed that investigated contaminants will be weakly adsorbed on cellulose surface in the water solution.
Materials and Methods
Geometries of modeled cellulose in the form of trimer of 1 → 4 linked linear chain of β-D-glucopyranos, hereafter called modeled cellulose, and adsorbates (DNAN, FOX-7 and TNT) and modeled cellulose-adsorbate complexes were optimized using M06-2X meta-hybrid Density Functional Theory (DFT) functional and the 6-31G(d,p) basis set both in the gas phase and in the bulk water solution. Nature of potential energy surfaces (PESs) of optimized structures was ascertained through calculation of harmonic vibrational frequency analysis; all geometries were found to be minima at the respective PES. Effect of bulk water solvation was modeled using the CPCM approach (Cossi et al. 2003). Interaction energies between the adsorbate and adsorbent in the gas phase were computed at the same theoretical level and using the 6-311G(d,p) basis set and were corrected for basis set superposition error using counterpoise correction scheme (Boys and Bernardi 1970). The interaction energies of complexes in the bulk water solution (hereafter called BSSE corrected interaction energy in bulk water) were obtained using the formula:
where EAB(CPCM) represents the total energy of the complex in the bulk water, EA(CPCM) and EB(CPCM) represent the total energy of the monomer A and B respectively in the bulk water within the complex geometry optimized in water and BSSEgas represents the basis set superposition energy correction obtained from the gas phase calculation using the counterpoise correction scheme. All calculations were performed using Gaussian 09 program (Frisch et al. 2009).
An E4 (QCM-D, Q-Sense AB) was used to measure adsorption of DNAN, FOX-7 and TNT onto thin cellulose films. Cellulose sensors (QSX 334) and Silicon (QSX 301) were purchased directly from Biolin scientific and used as received. The cellulose coating on the QSX 334 sensors was based on work done by Päakkö et al. (2007) and the films consist of isolated cellulose fibers from softwood cellulose pulp (Domsjo ECO Bright; Domsjo Fabriker AB). The material specifications indicate that the cellulose film is made from nanofibular cellulose with both crystalline cellulose I and amorphous regions with a Poly(ethylene imine) adhesive layer that ranges in thickness between 5 and 6 nm between the cellulose fibers and silicon surface. The reported nanofibular cellulose thickness is 6 nm with some fibril aggregates sizing between 10 and 20 μm. Surfaces were imaged in tapping mode with a Dimension Icon Atomic force Microscope (Bruker) and height images were collected under ambient conditions with a silicon tip (TAP150A Part: MPP-1(2120-10). The reported root mean square (RMS) roughness were determined from 5 μm × 5 μm scan areas. The cellulose film was fibrous in nature as discussed in the “Results and Discussion” section. The RMS roughness for these films was 3.1 ± 0.5 nm.
Water Uptake Total water content of the saturated cellulose film was determined with a QCM-D and a H2O/D2O solvent exchange procedure (Fält et al. 2003). The water content of the thin films was determined using a previously published technique in which the scaled frequency shift caused by switching the solvent from deionized water (H2O) to deuterium oxide (D2O) is used to measure the water within the film (Craig and Plunkett 2003). For this comparison a bare uncoated silicon crystal was subjected to the identical procedure as the cellulose film sensor. The utilization of a bare crystal allowed for compensation of the density changes between D2O and H2O. For this method, (Δf/n) water can be calculated from the difference between (Δf/n) film and (Δf/n)bare:
where ρD2O and ρH2O are the densities of D2O and H2O, respectively at 25°C. The constant C is defined as the Sauerbrey constant (0.177 mg s m−2). Thus, (Δf/n)water from Eq. 1 can be used to calculate the [Γ water ] “surface concentration” of water in the film through a Sauerbrey relationship (1959 ):
Adsorption Data Deionized water was introduced into the flow cell at a rate of 0.150 mL min−1 at 25°C until a stable baseline was obtained. During this period of water introduction baseline stabilization corresponded to maximum water uptake associated with the cellulose films. After stabilization the deionized water was switched to D2O and allowed to equilibrate. After equilibration the D2O was exchanged for deionized water until baseline stabilization was re-acquired. Figure 4 shows representative plots of (Δf/n) versus time, t, for a silicon sensor and a silicon sensor coated with a 6.0 nm film of cellulose switched from H2O to D2O and back to H2O. Values of Γwater were calculated from QCM results (see “Results and Discussion” section) using Eqs. 1 and 2. After re-stabilization in deionized water, adsorbate solutions (50 ppm in deionized water) was introduced into the flow cell at the same rate and temperature as the deionized water and the change in frequency was recorded (Marx 2003).
Results and Discussion
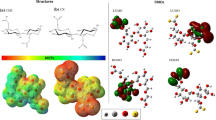
Optimized geometries of modeled cellulose and adsorbates (DNAN, FOX-7, and TNT) at the M06-2X/6-31G(d,p) level along with their numbering schemes and some selected geometrical parameters are shown in the Fig. 1. In the modeled cellulose, the trimeric form of β-D-glucopyranos in the 4C1 chair conformation in 1 → 4 linear chain linkage was considered. Moreover, the terminal –OH groups were replaced with –OCH3 group to avoid their interaction with adsorbates. Selection of such model was to avoid computational cost and moreover the size of the model cellulose system was sufficient considering the size of adsorbates under investigation. Selected computed dihedral angles of modeled cellulose are shown in the Table 1. The corresponding geometrical parameters of cellulose Iβ form obtained from the X-ray and neutron diffraction data (Nishiyama et al. 2002) are also shown in the Table 1. Computed geometrical parameters can be regarded in good agreement with the experimental data. The obtained disagreement (Table 1) is due to the fact that experimental data belong to the crystalline form of cellulose Iβ where confirmational movements are significantly restricted due to the presence of inter-chain hydrogen bonds. Theoretical calculation performed in this work predicted that modeled cellulose will have similar conformation both in the gas phase and in the bulk water solution.
Computed gas phase and in the bulk water solution bond lengths of adsorbates are shown in the Table 2. The experimentally determined bond lengths using X-ray crystallography are also shown in the same table. We would like to point out that for DNAN and TNT two different geometries with equal probability was predicted experimentally (Nyburg et al. 1987; Carper et al. 1982) and bond lengths for both (presented as A and B in the Table 2) are shown in the table. For FOX-7, the experimental bond lengths shown in Table 2 were obtained from the corresponding cif files (Bemm and Ostmark 1998). It is evident from the data shown in the Table 2 that noticeable change between computed bond length in the gas phase and in the bulk water solution is not revealed. Further, the computed bond lengths are generally in good agreement with the corresponding experimental data and small disagreement can be accounted to the crystal environment in the X-ray crystallographic measurement. Computed dipole moment of considered adsorbates in the gas phase and in the bulk water solution are shown in the Table 2. It is clear from this table that comparison to the gas phase, dipole moments in the bulk water solution are generally larger. Moreover, our calculation predicted that FOX-7 has the largest and TNT has the lowest dipole moment.
Molecular electrostatic potential (MEP) is an important property which is related to the reactivity of molecular system (Politzer et al. 1985). Computed MEP map plotted on electron density of modeled cellulose and adsorbates are shown in the Fig. 2. It is clear from this figure that negative potential regions are located at lone pair site of oxygen centers. And therefore, it is not unexpected that most negative potentials are predicted to be oxygen atoms of nitro groups and that of the hydroxyl groups. Moreover, among adsorbates, FOX-7 has been predicted to have the most negative potential while TNT has the least negative potential. Based upon the computed negative potential FOX-7 can be speculated to be adsorbed on cellulose surface stronger than other absorbates. However, adsorption is complicated process and number of bonding centers and type of interaction play prominent role in such complexation.
Molecular electrostatic potential (MEP) maps of modeled cellulose and adsorbates mapped on to electron density. The isosurface value is 0.006 and range for MEP for each system is next to their name in the unit of Hartree. Blue color shows the region of negative potential and red color shows the region of positive potential (Color figure online)
Geometries of optimized complexes obtained by adsorption of adsorbates on cellulose surface are shown in Fig. 3. Initial geometries of these complexes were obtained by putting adsorbates in proper orientation which can maximized the number of bonds between the surface and the adsorbates and then completely relaxing complex geometries at the M06-2X/6-31G(d,p) level. Moreover, molecules were adsorbed on both sides of the modeled cellulose and complex with higher interaction energy is considered. We would like to state that there are several arrangements for the interaction of adsorbates on cellulose surface, but only one orientation of adsorbates with respect to surface was considered for each of the munitions compounds. It is evident that these complexes are stabilized by the presence of several hydrogen bonds between hydrogen bond accepting and donating sites of adsorbates with the complementary sites of the surface. Moreover, these hydrogen bond distances were predicted to be in the range of 2.046–2.874 Å for DNAN, 1.924–2.920 Å for FOX-7, and 2.004–2.945 for TNT in the gas phase and 2.028–2.908 Å for DNAN, 1.902–2.886 Å for FOX-7, and 2.002–2.928 for TNT in the bulk water solution. Computed BSSE corrected interaction energies of investigated complexes are shown in the Table 2. It is clear from the data shown in the Table 2 that FOX-7 would form the strongest complex while the DNAN will form the weakest complex on the cellulose surface. Moreover, such binding was found to be significantly decreased in the bulk water solution compared to the gas phase. Further, it is evident from the Fig. 3 and data shown in the Table 2 that adsorption of considered energetic compounds on the cellulose surface is weak and such adsorption is dominated by the weak hydrogen bonding interactions. This conclusion is also supported from the QCM-D experiment which suggested low adsorption of investigated energetic molecules. The QCM-D water exchange experiments as represented in Fig. 4 showed that there was a significant amount of water adsorption in the cellulose films with a total surface concentration of water for the cellulose films found to be 4.12 ± 0.7 mg/m2. The frequency change observed for the sorbent solutions in the QCM-D experiment which is related to the adsorbed mass was measured. The measured amount of surface adsorption from the sorbent solutions was significantly lower with the mass uptake for TNT measuring 0.108 mg/m2, DNAN measuring 0.0242 mg/m2 and FOX 7 measuring 0.121 mg/m2 and the results are depicted in Fig. 5. Different frequency responses were observed for each explosive solution. The highest adsorption response was obtained from FOX7 with a frequency change reaching equilibrium quickly. TNT showed a similar amount of adsorption as compared to FOX-7 but never reached equilibrium during the time of contact. Such phenomena may be related to the relatively lower solubility of TNT in water. Minor sorption of DNAN was observed on the cellulose surface.
(Right) Change in resonance frequency due to the adsorption of water on cellulose surface and bare silicon surface using solvent exchange QCM-D experiment. Representative 3rd overtone for bare silica (black/solid) and silicon sensor (red/dashed). (Left) AFM image of cellulose sensor surface 15 μm × 15 μm (Color figure online)
We have investigated the adsorption of emerging contaminants (DNAN and FOX-7) and legacy compound TNT on cellulose surface using DFT level of theoretical and QCM-D experimental methods. It was found that such adsorption on cellulose surface would be significantly decreased in the bulk water solution compared to that in the gas phase. Moreover, all adsorptions were characterized by the presence of weak hydrogen bonds between adsorbates and adsorbent surface and thus adsorption was predicted to be weak. Among the investigated compounds, the FOX-7 was found to have slightly stronger binding on the cellulose surface. The cellulose films showed much higher water uptake as compared to the adsorbate solutions.
References
Bemm U, Ostmark H (1998) 1,1-Diamino-2,2-dinitroethylene: a novel energetic material with infinite layers in two dimensions. Acta Cryst C54:1997–1999
Bledzki AK, Gassan J (1999) Composites reinforced with cellulose based fibers. Prog Polym Sci 24:221–274
Boys SF, Bernardi F (1970) Calculation of small molecular interactions by differences of separate total energies—some procedures with reduced errors. Mol Phys 19:553–566
Carper WR, Davis LP, Extine MW (1982) Molecular structure of 2,4,6-trinitrotoluene. J Phys Chem 86:459–462
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comp Chem 24:669–681
Craig VSJ, Plunkett MJ (2003) Determination of coupled solvent mass in quartz crystal microbalance measurements using deuterated solvents. J Colloid Interface Sci 262:126–129
Fält S, Wågberg L, Vesterlind E-L (2003) Swelling of model films of cellulose having different charge densities and comparison to the swelling behavior of corresponding fibers. Langmuir 19:7895–7903
Felt DR, Johnson JL, Larson S, Hubbard B, Henry K, Nestler C, Ballard JH (2013) Evaluation of treatment technologies for wastewater from insensitive munitions production phase I: technology down-selection. U.S. Army Engineer Research and Development Center, ERDC/EL TR-13-20, Vicksburg
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, IzmaylovAF Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian Inc., Wallingford
Marx KA (2003) Quartz crystal microbalance: a useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromol 4:1099–1120
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082
Nyburg SC, Faerman CH, Prasad L, Palleros D, Nudelman N (1987) Structures of 2,4-dinitroanisole and 2,6-dinitroanisole. Acta Cryst C43:686–689
Päakkö M, Ankerfors M, Kosonen H, Nykanen A, Ahola S, Osterberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindstrom T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 6:1934–1941
Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Health Perspect 61:191–202
Sauerbrey G (1959) Verwendung von Schwingquarzenzur Wagungdunner Schichten und zur Mikrowagung. Zeitschrift für Physik 155:206–222
Taylor S, Park E, Bullion K, Dontsova K (2015) Dissolution of three insensitive munitions formulations. Chemosphere 119:342–348
Thorn KA, Pennington JC, Hyes CA (2002) 15N NMR investigation of the reduction and binding of TNT in an aerobic bench scale reactor simulating windrow composting. Environ Sci Technol 36:3797–3805
Acknowledgments
The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the U.S. Government. The tests described and the resulting data presented herein, unless otherwise noted, were obtained from research conducted under the Environmental Quality Technology Program of the United States Army Corps of Engineers and the Environmental Security Technology Certification Program of the Department of Defense by the USAERDC. Permission was granted by the Chief of Engineers to publish this information. The findings of this report are not to be construed as an official Department of the Army position unless so designated by other authorized documents. The authors thank Dr. Guilherme Lotufo, and Dr. Chris Griggs of USACE for their editorial comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, M.K., Poda, A. Adsorption of Emerging Munitions Contaminants on Cellulose Surface: A Combined Theoretical and Experimental Investigation. Bull Environ Contam Toxicol 96, 784–790 (2016). https://doi.org/10.1007/s00128-016-1791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1791-x