Abstract
Concentrations of seven metals (As, Cd, Cr, Cu, Pb, Ni, and Zn) were analyzed in 33 bone tissue samples of Antillean manatees (Trichechus manatus manatus) found dead in lagoons and rivers of Tabasco and Campeche in the Gulf of Mexico and Chetumal Bay in the Caribbean region. The concentrations of Cr, Cu, Pb, and Zn were significantly different between regions, with greater levels found in the Gulf of Mexico group than in the Mexican Caribbean group (p < 0.05). Pb concentrations differed significantly between adults and calves. No differences were observed between sexes. Metal concentrations detected in the manatee bones were higher than most of those reported for bones in other marine mammals around the world. Future studies are necessary to establish whether the metal concentrations represent a risk to the health of the species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
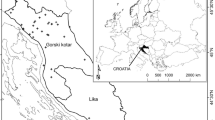
The Antillean manatee (Trichechus manatus manatus) is an endangered species according to the International Union for Conservation of Nature (Self-Sullivan and Mignucci-Giannoni 2008). Manatees are affected by diverse classes of persistent organic contaminants such as organochlorine pesticides, industrial compounds, and PCBs (Wetzel et al. 2012). Trace metals have been studied in soft tissues such as blood or skin (Stavros et al. 2008; Siegal-Willott et al. 2013; Anzolin et al. 2012) to explore baseline concentrations, but not in hard tissues such as bones, where long-term accumulation can occur. Mexico has an important Antillean manatee population, principally in Chetumal Bay off southern Quintana Roo (Morales-Vela et al. 2000), which is shared with Belize. Populations are also found in river and lagoon systems of the states of Tabasco, Veracruz, and Chiapas, and the coastal region of Campeche, which all border the Gulf of Mexico (SEMARNAT/CONANP 2010) (Fig. 1).
The aforementioned Chetumal Bay and Gulf of Mexico regions have long been exposed to contaminants associated with intensive livestock husbandry and the oil industry in Tabasco and Campeche, and with agricultural activities such as sugar cane production in southern Quintana Roo and northern Belize. Oil production is the principal industry in Mexico, with extensive activity in the states of Veracruz, Tabasco, and Campeche. However, the effects of oil and gas pollution on manatees have not been examined worldwide (Marsh et al. 2011). Oil production in Mexico may increase in the coming years because of recent energy reforms (SENER 2013).
Manatees in fluvial-lagoon systems such as in Tabasco and Campeche show seasonal differences in feeding habits, and movements are regulated by the rainy and dry seasons (Pablo-Rodriguez et al. 2015). In extensive droughts, the manatees are isolated in small lakes within ranches or other private lands. Such seasonality causes the species greater exposure to anthropogenic contaminants. In contrast, in Chetumal Bay, the manatees exhibit free coastal movement of a regular form throughout the year (Castelblanco-Martínez et al. 2013), with full access to food. However, those manatees are exposed to principally land-based pollution and contaminants in coastal water that arrive with the coastal current from the southern Caribbean (Kramer and Kramer 2002). However, in general, we expect to find minor metal concentrations in samples from Chetumal Bay.
Metal concentrations in marine mammals are measured in various tissues based on ease of access for sampling. Stranding events frequently provide sampling opportunities (Lavery et al. 2008). Cortical bone can reflect the concentration of certain metals accumulated over many years, unlike soft tissues such as blood, which reflects recent exposure (Hu et al. 2007). On this basis, we used the cortical bone of manatee carcasses deposited in scientific collections. The aims of the present study were to determine metal concentrations in manatee bone tissue from the Chetumal Bay region of Mexico and coastal and riverine areas of the states of Tabasco and Campeche on the Gulf of Mexico. This yielded baseline information for the exploration of pollutants in the species, and for comparing concentrations between regions that are host to the largest populations of manatees in Mexico.
Methods and Materials
Thirty-three bone tissue samples were taken from manatees stranded in Chetumal Bay (Quintana Roo), Tabasco, and Campeche from 1990 to 2013 (n = 22, n = 7, and n = 4, respectively) under the research permits: SGPA/DGVS/09924/10, 11348/10, 08287/12, 05845/13, and 08666/14. The authorization for scientific collection was ECOSUR-CHETUMAL DGVS/DF-CC-277-13 and SGPA/DGVS/04237/13. Tissues were vouchered at the Zoological Museum of ECOSUR-Chetumal (ECOSUR-CH-MM), in the collection of the Biological Sciences Division of Universidad Juárez Autónoma de Tabasco (DACBIOL-UJAT) and in the collection of marine mammals of the Faculty of Natural Sciences (DACNAT) of the Universidad Autónoma del Carmen, Campeche, under registration No. CAMP-MAM-173-04-05. As described in Table 1, specimens were classified in two age classes, adults (270–325 cm long) and calves (128–194 cm long). There was a sample with 250-cm length (from Chetumal Bay) classified as adult.
Approximately 2 g of cortical bone was obtained from the shaft of the humerus or radius using a disc saw with a continuous rim diamond blade. Each sample was placed in a plastic bag at room temperature and analyzed at the Toxicology Laboratory, Faculty of Veterinary Medicine and Zootechny, Universidad Nacional Autónoma de México, on the main campus in Mexico City. Each sample was prepared according to Helrich (1990), with an acid digestion procedure. Then, samples were filtered and diluted to 14 mL with deionized water. Metal concentration was determined by atomic absorption spectrometry (Perkin-Elmer, model Analyst 3110 and 100) with a flame and hydride generator. Blank replicates and calibration curves were executed to read absorbance using known concentration and standard reference material, i.e., bone ash (SRM 1400) and single element standard solutions (SRM 3100 series: 3103a (arsenic), 3108 (cadmium), 3112a (chromium), 3114 (copper), 3128 (lead), 3136 (nickel), and 3168a (zinc)) from the National Institute of Standards and Technology. Standard conditions and procedures selected the elements arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn), to construct the standard curve. Statistical regression indexes (r2 = 0.99) were used to transform absorbance to concentration and, once the initial concentration was obtained, it was multiplied by the dilution factor of the final volume and divided by its weight. Final metal concentration was expressed in µg/g when measured in the flame only. Arsenic concentration measured by borohydride generator was expressed in ng/g. The standard curve for arsenic titration was built with 10 ng (absorbance 0.02), 25 ng (0.05), and 50 ng (0.09), and absorbance for these concentrations was the mean of at least three readings (r2 = 0.99).
Tests for data normality (Shapiro–Wilks) and homogeneity of variances (Brown–Forsythe) were performed. The Kruskal–Wallis test was used to detect differences between the three regions and for post hoc analysis of pairwise comparisons using the Tukey and Kramer (Nemenyi) test, with Tukey–Distance approximation performed in the R package PMCMR (see Sect. 1.1 of Pohlert 2015). The Student’s t test was used to analyze differences between specimen sex and age. Statistical analyses were done using GraphPad Prism Version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA).
Results and Discussion
Thirty-three samples of bone tissue were analyzed, 22 from the Mexican Caribbean (Chetumal Bay) and 11 from the Gulf of Mexico (seven and four from the states of Tabasco and Campeche, respectively; Table 1).
Concentrations in relation to sex and age. No significant differences were observed between the sexes. Regarding age, only the Pb concentration was higher in adults (n = 17; mean 13.5 ± 0.6) than in calves (n = 12; mean 10.4 ± 0.9), with a significant difference between them (t = 2.8, df = 27, p = 0.00).
Concentrations in relation to regions. Significant differences of metal concentration was observed for Cr (H = 10.2, df = 2, p = 0.01), Cu (H = 12.4, df = 2, p = 0.00), Pb (H = 6.3, df = 2, p = 0.04), and Zn (H = 6.4, df = 2, p = 0.04) between regions. Specifically, a post hoc procedure for these results show differences (p < 0.05) between Chetumal and Tabasco, except for Pb, for which all pairwise comparisons were non-significant.
Significant differences were found in Pb concentrations between the two age classes, with higher concentrations in adults. This is most likely attributable to the longer duration of exposure. Similar results were reported by Honda et al. (1986), who showed that Pb concentration increased with age in dolphins.
We also found differences in metal concentrations among regions. Cr, Cu, and Zn concentrations were higher in the group from Tabasco as compared with the group from Chetumal Bay. The use and production of fertilizers and pesticides, domestic effluents, and oil production in the Gulf region are extensive (Páez-Osuna 2005) and may be the main sources of these metals. Studies have also shown elevated concentrations of Pb in fish, shrimp, and crocodiles from the Gulf (Vázquez et al. 2001; Trillanes et al. 2014). No differences were found between the Tabasco and Campeche groups, probably because they represent the same freshwater ecosystem and are exposed to the same contaminants derived from livestock husbandry and the oil industry. However, the effect of fluvial-lagoon ecosystems on the coastal environment should be addressed in future studies.
In general, the metal concentrations in our study were greater than those detected in some marine mammals worldwide (Table 2) and baseline levels of certain metals proposed by Takeuchi (2012). However, our metal concentrations were low compared with the maximum tolerable levels of minerals for domestic animals reported by the National Research Council (1980). The level at which a mineral element has an adverse effect is uncertain and depends on many factors, such as the route of exposure and bioavailability of the compound. Additonally, toxicities and baseline levels of tolerance to heavy metals are not well documented for sirenians and other marine mammals. Pb is an element that significantly accumulates in the body with age, possibly posing a hazard. The Pb concentrations found in manatees are high compared with other marine mammals (Table 2). This may reflect differences in metabolic capacity in manatees for eliminating contaminants or regional differences of exposure to contaminants, as mentioned above.
This descriptive study established a baseline information on metal concentration in bone tissue of manatees for future monitoring in the Gulf of Mexico area. Regional differences were also shown, with values from Tabasco on the Gulf of México greater than those in Chetumal Bay. Long-term exposure to pollutants derived from oil production may be the principal factor, but further sampling should be addressed towards this assumption. With the new energy reforms announced by the Mexican Government in 2014, the activity of oil and gas industries will increase in coming years. Therefore, as a precautionary measure, prevention activities must be undertaken to avoid further exposure to potential damage in the manatee population of the Gulf of Mexico.
The examination of live manatees for signs of toxicosis and assessment of their general state of health, as well as the use of other tissue types and organs to estimate relationships between metal concentrations in the bone, blood, kidney, and liver, are valuable for establishing the risk of metal toxicity in this species. The present study does not establish the consequences of these toxic metals on manatee health.
Additionally, it is necessary to study the degree of metal tolerance in manatees to better manage and conserve the species, as Takeuchi (2012) proposed. Other important essential metals, such as Ca and Fe, should be studied in complementary analyses.
References
Anzolin DG, Sarkis JE, Diaz E, Soares DG et al (2012) Contaminant concentrations, biochemical and hematological biomarkers in blood of West Indian manatees Trichechus manatus from Brazil. Mar Pollut Bull 64:1402–1408
Castelblanco-Martínez DN, Padilla-Saldívar J, Hernández-Arana HA et al (2013) Movement patterns of Antillean manatees in Chetumal Bay (Mexico) and coastal Belize: a challenge for regional conservation. Mar Mamm Sci 29:E166–E182
Fujise Y, Honda K, Tatsukawa R, Mishima S (1988) Tissue distribution of heavy metals in Dall’s porpoise in the northwestern Pacific. Mar Pollut Bull 19:226–230
Goldblatt CJ, Anthony RG (1983) Heavy metals in northern fur seals (Callorhinus ursinus) from the Pribilof Islands, Alaska. J Environ Qual 12:478–482
Helrich K (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists Inc, USA
Honda K, Fujise Y, Tatsukawa R, Itano K, Miyazaki N (1986) Age-related accumulation of heavy metals in bone of the striped dolphin, Stenella coeruleoalba. Mar Environ Res 20:143–160
Hu H, Shih R, Rothenberg S, Schwartz BS (2007) The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodological issues. Environ Health Perspect 115:455
Kramer, PA, Kramer PR (2002) Ecoregional conservation planning for the Mesoamerican Caribbean Reff. World Wildlife Fund, May 2002
Lavery TJ, Butterfield N, Kemper CM et al (2008) Metals and selenium in the liver and bone of three dolphin species from South Australia, 1988–2004. Sci Total Environ 390:77–85
Marsh H, O’Shea TJ, Reynolds JE III (2011) Ecology and conservation of the Sirenia: dugongs and manatees. Cambridge University Press, UK
Morales-Vela B, Olivera-Gómez LD, Reynolds JE III, Rathbun GB (2000) Distribution and habitat use by manatees (Trichechus manatus manatus) in Belize and Chetumal Bay, Mexico. Biol Conserv 95:67–75
National Research Council (1980) Mineral tolerance of domestic animals, 1st edn. National Academy of Sciences, Washington
Pablo-Rodriguez N, Olivera-Gómez LD, Aurioles-Gamboa D, Vega-Cendejas ME (2015) Seasonal differences in the feeding habits of the Antillean manatee population (Trichechus manatus manatus) in the fluvial-lagoon system of Tabasco, Mexico. Mar Mamm Sci. doi:10.1111/mm.12245
Páez-Osuna F (2005) Fuentes de Metales en la Zona Costera Marina. In: Botello AV, Rendón-Von Osten J, Gold-Bouchot G, Agraz-Hernández C (eds) Golf. México Contam. E Impacto Ambient. Diagnóstico y Tendencias, 2nd edn. Univ. Autón. De Campeche, Univ. Nal. Autón. De México, Instituto Nacional de Ecología, Mexico, pp 329–342
Pohlert T (2015). R package “PMCMR”: calculate pairwise multiple comparisons of mean rank sums, vers. 1.2. https://cran.r-project.org/web/packages/PMCMR/index.html. Accesed 23 Sept 2015
Rojas-Mingüer A, Morales-Vela B (2002) Metales en hueso y sangre de manatíes de (Trichechus manatus manatus) de la Bahía de Cheturnal, Quintana Roo, México. In: Rosado-May FJ, Romero Mayo R, De Jesús Navarrete A (eds) Contribuciones de la ciencia al manejo costero integrado de la Bahía de Chetumal y su área de influencia. Universidad de Quintana Roo, Mexico, pp 133–142
Self-Sullivan C, Mignucci-Giannoni A (2008) Trichechus manatus ssp. Manatus. IUCN red list of threatened species 2008: e T22105A9359161. Downloaded on 10 Sept 2015
SEMARNAT/CONANP (2010) Programa de Acción para la Conservación de la Especie: Manatí (Trichechus manatus manatus). México, D.F. http://procer.conanp.gob.mx/pdf/pace_manati.pd. Accessed 13 Oct 2014
SENER (2013) Prospectiva de Petróleo y Petrolíferos 2013-2027. http://sener.gob.mx/res/PE_y_DT/pub/2013/Prospectiva_de_Petroleo_y_Petroliferos_2013-2027.pdf. Accessed 13 Oct 2014
Siegal-Willott JL, Harr KE, Hall JO et al (2013) Blood mineral concentrations in manatees (Trichechus manatus latirostris and Trichechus manatus manatus). J Zoo Wildl Med 44:285–294
Stavros HC, Bonde RK, Fair PA (2008) Concentrations of trace elements in blood and skin of Florida manatees (Trichechus manatus latirostris). Mar Pollut Bull 56:1221–1225
Szteren D, Aurioles-Gamboa D (2013) Trace elements in bone of Zalophus californianus from the Gulf of California: a comparative assessment of potentially polluted areas. Cienc Mar 39:303–315
Takeuchi NY (2012) Trace metal concentrations and the physiological role of zinc in the West Indian manatee (Trichechus manatus). Dissertation, University of Florida
Trillanes CE, Pérez-Jiménez JC, Rosíles-Marínez R, González-Jáuregui M (2014) Metals in the caudal scutes of Morelet’s crocodile (Crocodylus moreletii) from the Southern Gulf of Mexico. Bull Environ Contam Toxicol 93:423–428. doi:10.1007/s00128-014-1349-8
Vázquez FG, Sharma VK, Mendoza QA, Hernandez R (2001) Metals in fish and shrimp of the Campeche sound, Gulf of Mexico. Bull Environ Contam Toxicol 67:756–762
Watanabe I, Ichihashi H, Tanabe S, Amano M, Miyazaki N, Petrov EA, Tatsukawa R (1996) Trace element accumulation in Baikal seal (Phoca sibirica) from the Lake Baikal. Environ Pollut 94:169–179
Wetzel DL, Pulster E, Reynolds JE III (2012) Organic contaminants and sirenians. In: Hines E, Reynolds J, Mignucci-Giannoni A, Aragones L, Marmontel M (eds) Sirenian conservation: issues and strategies in developing countries. University Press of Florida, Gainesville, pp 196–203
Yamamoto Y, Honda K, Hidaka H, Tatsukawa R (1987) Tissue distribution of heavy metals in Weddell seals (Leptonychotes weddellii). Mar Pollut Bull 18:164–169
Acknowledgments
This study was supported by scholarship no. 355408 granted by CONACYT-México. We thank the Collection of Marine Mammals of ECOSUR-Chetumal, Universidad Autónoma del Carmen, Región Naval 3 of Ciudad del Carmen, Campeche, and Universidad Juárez Autónoma de Tabasco for the bone samples. We thank Dr. José Rogelio Cedeño Vázquez for all his comments and help with the manuscript. We also thank Nicolás Martínez for the sampling assistance of ECOSUR-Chetumal, the Toxicology Laboratory of the Universidad Nacional Autónoma de México for help with the laboratory analyses, and Daniel Díaz Espinosa de los Monteros for his help with the statistical analyses and GraphPad Prism software. We thank Carlos Trillanes-Flores for his immeasurable support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero-Calderón, A.G., Morales-Vela, B., Rosíles-Martínez, R. et al. Metals in Bone Tissue of Antillean Manatees from the Gulf of Mexico and Chetumal Bay, Mexico. Bull Environ Contam Toxicol 96, 9–14 (2016). https://doi.org/10.1007/s00128-015-1674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1674-6