Abstract
A microcystin-LR (MC-LR)-degrading bacterium was isolated from Lake Chaohu, a eutrophic freshwater lake containing toxic cyanobacterial blooms. Based on the analysis of 16S rDNA gene sequence and physiobiochemical characteristics, the isolated strain, most likely belongs to the genus Bacillus with the highest sequence similarity value with Bacillus nanhaiencis strain K-W39 (JQ799091.1), was named B. nanhaiencis strain JZ-2013. The strain JZ-2013 could grow on mineral salt medium supplied with MC-LR as sole carbon and nitrogen sources. The optimal temperature and pH for strain JZ-2013 growth and MC-LR biodegradation were 30°C and 8.0, respectively. The MC-LR with the initial concentration of 15 mg/L could be consumed 80 % by strain JZ-2013 within 9 days. The existence of exogenous carbon and nitrogen sources could significantly increase the removal efficiency of MC-LR. The strain JZ-2013 can efficiently removed MC-LR of low concentration in real water sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
With the nitrogen and phosphorus enriching wastewater continuously discharged into natural water bodies, harmful cyanobacterial blooms frequently occur in eutrophic lakes, reservoirs, ponds, canals, and slow-flowing rivers throughout China. The cyanobacterial blooms not only destroy the ecological environment of natural water bodies, but also produce many types of cyanobacterial toxins including the most dangerous microcystins (MCs). MCs are a group of toxic monocyclic heptapeptides produced by multiple genera of cyanobacteria such as, Microcystis, Anabaena, Oscillatoria, Planktothrix, Chroococcus, Nostoc, Hapalosiphon, Anabaenopsis, Snowella and Woronichinia (Merel et al. 2010; Pearson et al. 2010; Valério et al. 2010). MCs share the general structure of cyclo (-d-Ala-l-X-d-MeAsp-l-Y-Adda-d-Glu-Mdha-), in which Adda is 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid which is crucial for biological activities of MCs (Harada et al. 2004), Mdha is N-methyldehydroalanine, and X and Y are variable amino acids. MC-LR has leucine (L) and arginine (R) at these positions and is one of the most common forms. To date, over 90 isoforms of MCs have been identified (Pearson et al. 2010). MCs are produced and retained within healthy cyanobacterial cells but released into the surrounding water bodies when cells die and break down. MCs inhibit protein phosphatase 1 and 2A, induce oxidative stress (Campos and Vasconcelos 2010), and promote liver tumor growth (Falconer 1991; Nishikawa-Matsushima et al. 1992; Ueno et al. 1996). Because of potential chronic toxicity from MCs, the World Health Organization recommends a level of less than 1 µg/L of the toxin in drinking water to be safe for humans (Gągała and Mankiewicz-Boczek 2012).
Under natural conditions, the cyclic structure of MCs is relatively stable against physicochemical and biological factors, such as pH, temperature, sunlight, and typical proteases (Tsuji et al. 1994; Gągała and Mankiewicz-Boczek 2012). The traditional water treatment processes, including chemical coagulation, flocculation, and sand filtration are unreliable for removal of MCs. Biodegradation is thought to be a promising method for effective removal of MCs in the water treatment process (Bourne et al. 2006). The degradation of MCs by microorganisms requires rather specific enzymes. The first MCs-degrading bacterium isolated and identified was Sphingomonas sp. ACM-3962, which was found to possess an enzymatic pathway and a gene cluster for MCs-degrading (Bourne et al. 1996, 2001). Four genes are sequentially located on the cluster as mlrC, mlrA, mlrD and mlrB. These genes encode a putative transporter protein (MlrD) allowing MCs uptaked into the cell and three enzymes, MlrA, MlrB and MlrC, are involved in the degradation of MCs. In the degradation pathway, microcystinase (MlrA), a metalloprotease, is the first enzyme to hydrolyze cyclic MCs into linear MCs. Because the toxicity of linear intermediate significantly decreases, MlrA has been regarded as a crucial enzyme for removal of MCs (Bourne et al. 1996). Therefore, detection of this mlrA gene is important for monitoring MCs-degrading bacteria in natural environment. There are a growing number of isolated MCs-degrading bacteria, such as Pseudomonas aeruginosa (Takenaka and Watanabe 1997), Ralstonia solanacearum (Yan et al. 2004), Paucibacter toxinivorans (Rapala et al. 2005), Sphingosinicella microcystinivorans (Maruyama et al. 2006), Burkholderia sp. (Lemes et al. 2008), Methylobacillus sp. (Hu et al. 2009), Arthrobacter spp. (Manage et al. 2009), Brevibacterium sp. (Manage et al. 2009), Rhodococcus sp. (Manage et al. 2009), Morganella morganii (Eleuterio and Batista 2010), Sphingopyxis sp. (Zhang et al. 2010), Stenotrophomonas sp. (Chen et al. 2010), Novosphingobium sp. (Jiang et al. 2011), Bacillus flexus (Alamri 2012), Microbacterium sp. (Ramani et al. 2012), and Rhizobium gallicum (Ramani et al. 2012).
Lake Chaohu, located in the middle of Anhui Province, is the fifth largest freshwater lake in China. Because of eutrophication, Lake Chaohu frequently outbreaks toxic cyanobacterial blooms during summer and autumn in the last decade. Being frequently exposed to such toxic cyanobacterial bloom scenarios in Lake Chaohu, maybe there are indigenous bacteria which adapt to high toxin levels and exhibit toxin degradation abilities. The aim of this study was to screen MCs biodegrading bacteria or bacterial consortium from Lake Chaohu.
Materials and Methods
Toxins and Other Reagent
MC-LR was purchased from Sigma-Aldrich (purity > 95 % by HPLC). M9 broth medium was acquired from Sangon Biotech Co., Ltd., Shanghai, China. All reagents used were of analytical or HPLC grade.
Isolation of MC-LR-Degrading Bacterial Strain
Water samples were collected during September 2013 from Lake Chaohu which was experiencing toxic cyanobacterial blooms. After delivered to the laboratory, the water samples were filtered, diluted, and inoculated onto M9 broth medium agar plates containing MC-LR (1 mg/L). The single bacterial colonies from these plates were picked up and inoculated onto the plates containing MC-LR (5, 10 mg/L), sequentially. Lastly, the single bacterial colonies from the plates containing MC-LR (10 mg/L) were picked up and inoculated onto into liquid M9 broth medium containing MC-LR (15 mg/L) at 30°C with a shake rate of 120 rpm for isolating MC-LR biodegrading bacterial strains. The remaining concentrations of MC-LR in the medium were measured by enzyme-linked immunosorbent assay (ELISA) using the MCs plate kit (Beacon Analytical Systems Inc., Saco, Maine, USA) according to the kit instructions. Among the tested bacteria grown on the plates, only one strain showed high MC-LR-degrading activity and was then identified by 16S rDNA sequence analysis.
The bacterial cells were sedimented by centrifugation and the supernatant was discarded. The cells were fixed with 2.5 % glutaraldehyde for 2 h. And then the cells were sedimented again and the supernatant was discarded. The cells were resuspended in distilled water and washed for three times. Finally the bacterial suspension was dropped on copper grid covered with cover slip and dried under an infrared lamp for SEM observation.
Biodegradation of MC-LR
The ability of the isolated bacterial strain to degrade MC-LR was examined by inoculating same amount of isolated bacterial cells into sterile 100-mL Erlenmeyer flasks with 50 mL M9 medium containing MC-LR (15 mg/L) and incubating the flasks on a shaking incubator for 9 days at different temperature (20–37°C) and pH values (6.0–9.0) in the dark. Flasks with M9 medium containing 15 mg/L MC-LR without bacterial cells were used as control. At the predetermined time intervals, aliquot samples were taken and centrifuged at 12,000 rpm for 10 min. The remaining concentrations of MC-LR in the supernatants were measured.
The bacterial growth was monitored by measuring the optical density of the medium at 600 nm (OD600).
Identification of Bacterial Strain
The MC-LR-degrading bacterium was performed by Gram staining and physiobiochemical experiments (including: anaerobic growth, acids resistance, gelatin liquefaction, parasporal crystal, H2S test, and phenylalanine deaminase test).
The total genomic DNA of the MC-LR-degrading bacterium was extracted using EZ-10 Spin Column Bacterial Genomic DNA Isolation Kit (Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. The bacterial gene encoding 16S rDNA was amplified by PCR reaction using universal primers (Sense: 5′-AGAGTTTGATCATGGCTCAG-3′; antisense: 5′-ACGGTTACCTTGTTACGACTT-3′) provided by Sangon Biotech Co., Ltd., Shanghai, China. The PCR reaction was performed as follows: one hotstart at 94°C for 5 min, 38 amplification cycles of denaturation at 94°C for 30 s, primer annealing at 54.5°C for 30 s, extension at 72°C for 45 s, and a final extension step at 72°C for 10 min. The size of amplified PCR product was detected by agarose gel electrophoresis. The amplified nucleotide product was sequenced in Sangon Biotech Co., Ltd., Shanghai, China. Similar sequences were identified using online BLAST in NCBI neulecotide database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). A multiple alignment and a phylogenetic tree were obtained using CLUSTAL X 2.0 software (Larkin et al. 2007) and MEGA 5 software (Kumar et al. 2008).
Biodegradation Validation
Water sample collected from Lake Chaohu was filtered by filter paper (pore-size: 80–120 μm) for removing sediment and other suspended solids, and its pH value was adjusted to 8. The validation experiment was carried out by inoculating same amount of isolated bacterial cells with biodegradation experiment into sterile 100-mL Erlenmeyer flasks with 50 mL water sample containing MC-LR (500 µg/L) and incubating the flasks on a shaking incubator for 9 days at 30°C in the dark. Flask with water sample containing 500 µg/L MC-LR without bacterial cells was used as control. At the predetermined time intervals, aliquot samples were taken and centrifuged at 12,000 rpm for 10 min. The remaining concentrations of MC-LR in the supernatants were measured.
All biodegradation experiments were carried out in triplicate.
Results and Discussion
Isolation of MC-LR-Degrading Bacterial Strain
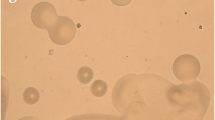
In order to obtain native bacteria with high MCs-degradation efficiency, water samples was collected from the Lake Chaohu occurring cyanobacterial blooms and screened according to the method described above. Fifteen isolated colonies were inoculated into the aqueous M9 medium containing MC-LR. Among the test strains, only one strain showed the ability to degrade MC-LR. This strain was facultative anaerobic, gram-positive and rod-shaped. The photo of scanning electron microscopy (Fig. 1) showed that the cells were about 3 μm in length and 1 μm in width.
Identification of MC-LR-Degrading Bacterial Strain
The strain was found to be gram-positive bacteria by microscopy observation after Gram staining. The physiobiochemical experiments confirmed that the strain was facultative anaerobes.
The size of amplified PCR product was about 1450 bp measured by agarose gel electrophoresis. The analysis results of 16S rDNA sequence showed that isolated strain appeared to be closely related to Bacillus nanhaiencis strain K-W39 (JQ799091.1) with the highest sequence similarity. Based on the phylogenetic analysis of 16S rDNA sequences, the isolated bacterial strain was identified and named as B. nanhaiencis strain JZ-2013. The complete 1454 bp sequences of 16S rDNA of strain JZ-2013 had been deposited in the GeneBank database under accession number KF841622.
Effect of Temperature and pH on MC-LR Degradation
The MC-LR biodegradation by strain JZ-2013 under the different initial temperatures and pHs was investigated.
The biodegradation curves of MC-LR at various incubation temperatures showed that removal of MC-LR was temperature dependent, with the highest removal observed at 30°C. After 9 days the MC-LR concentrations were reduced to about 20 % at 30°C, but the MC-LR concentrations only were reduced to 72 % and 91 % at 20 and 37°C, respectively. The concentration of MC-LR stopped decreasing after 9 days (Fig. 2).
The MC-LR degradation activity of strain JZ-2013 varied significantly with change of the pH of medium. It was found (Fig. 3) that the degradation rate increased with increase of pH from 6 to 8, and then decreased with the increase of pH from 8 to 9. The strain showed the highest MC-LR degradation activity at pH 8.0. After incubation for 9 days, the strain degraded 80 % MC-LR (initial concentration: 15 mg/L) in medium at pH 8.0. In contrast, the removal percentage of MC-LR in medium by the strain only was 5.5 %, 41.0 %, and 30.3 % at pH 6.0, 7.0 and 9.0, respectively.
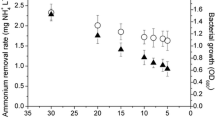
Then the degradation of MC-LR in medium by strain JZ-2013 was carried out under the optimal conditions of at 30°C and pH 8.0. It was found (Fig. 4) that the decrease in the concentration of MC-LR was accompanied by the increase in bacterial growth. When 10 mg/L of glucose and ammonium chloride was added as exogenous carbon and nitrogen sources, the removal percentage of MC-LR in medium (15 mg/L) increased from about 80 % to about 90 %. At the same time, the bacterial growth also improved.
Validation Experiment
Validation experiment presented similar MC-LR degradation trend with biodegradation experiment. It could be found (Fig. 5) that MC-LR in real water sample had been completely degraded within 8 days, which suggested strain JZ-2013 can efficiently degrade MC-LR in real water sample.
The kinetic study of MC-LR degradation was modeled with Michaelis–Menten equation. The Michaelis–Menten equation was rearranged into Eadie–Hofstee equation (v = Vmax−Km·v/[MC-LR]). The simulation result using Eadie–Hofstee plot indicated the MC-LR degradation in real water sample by strain JZ-2013 followed Michaelis–Menten kinetics.
Conclusions
A new MC-LR-degrading bacterial strain of the genus Bacillus was isolated from a eutrophic freshwater lake in China and identified as B. nanhaiencis strain JZ-2013. The optimal temperature and pH for strain JZ-2013 growth and MC-LR biodegradation were 30°C and 8.0, respectively. Strain JZ-2013 could degrade 80 % MC-LR with the initial concentration of 15 mg/L in 9 days and did not show any lag period during the degradation process. The exogenous carbon and nitrogen sources could improve both the degradation of MC-LR and bacterial growth. The MC-LR of low concentration in real water sample can be efficiently degraded by strain JZ-2013. This research result showed strain JZ-2013 can detoxify MCs and possesses significant potential for use in bioremediation of water bodies contaminated by MCs.
References
Alamri SA (2012) Biodegradation of microcystin-RR by Bacillus flexus isolated from a Saudi freshwater lake. Saudi J Biol Sci 19:435–440
Bourne DG, Jones GJ, Blakeley RL, Jones A, Negri AP, Riddles P (1996) Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl Environ Microbiol 62:4086–4094
Bourne DG, Riddles P, Jones GJ, Smith W, Blakeley RL (2001) Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environ Toxicol 16:523–534
Bourne DG, Blakeley RL, Riddles P, Jones GJ (2006) Biodegradation of the cyanobacterial toxin microcystin LR in natural water and biologically active slow sand filters. Water Res 40:1294–1302
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11:268–287
Chen J, Hu LB, Zhou W, Yan SH, Yang JD, Xue YF, Shi ZQ (2010) Degradation of microcystin-LR and RR by a Stenotrophomonas sp. strain EMS isolated from Lake Taihu, China. Int J Mol Sci 11:896–911
Eleuterio L, Batista JR (2010) Biodegradation studies and sequencing of microcystin-LR degrading bacteria isolated from a drinking water biofilter and a fresh water lake. Toxicon 55:1434–1442
Falconer IR (1991) Tumor promotion and liver injury caused by oral consumption of cyanobacteria. Environ Toxicol 6:177–184
Gągała I, Mankiewicz-Boczek J (2012) The natural degradation of microcystins (Cyanobacterial Hepatotoxins) in fresh water-the future of modern treatment systems and water quality improvement. Pol J Environ Stud 21:1125–1139
Harada K, Imanishi S, Kato H, Mizuno M, Ito E, Tsuji K (2004) Isolation of Adda from microcystin-LR by microbial degradation. Toxicon 44:107–109
Hu LB, Yang JD, Zhou W, Yin YF, Chen J, Shi ZQ (2009) Isolation of a Methylobacillus sp. that degrades microcystin toxins associated with cyanobacteria. New Biotechnol 26:205–211
Jiang Y, Shao J, Wu X, Xu Y, Li R (2011) Active and silent members in the mlr gene cluster of a microcystin-degrading bacterium isolated from Lake Taihu, China. FEMS Microbiol Lett 322:108–114
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lemes GA, Kersanach R, Pinto LS, Dellagostin OA, Yunes JS, Matthiensen A (2008) Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicol Environ Saf 69:358–365
Manage PM, Edwards C, Singh BK, Lawton LA (2009) Isolation and identification of novel microcystin-degrading bacteria. Appl Environ Microb 75:6924–6928
Maruyama T, Park HD, Ozawa K, Tanaka Y, Sumino T, Hamana K, Hiraishi A, Kato K (2006) Sphingosinicella microcystinivorans gen. nov., sp. nov., a microcystin-degrading bacterium. Int J Syst Evol Microbiol 56:85–89
Merel S, Clément M, Thomas O (2010) State of the art on cyanotoxins in water and their behaviour towards chlorine. Toxicon 55:677–691
Nishikawa-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H (1992) Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin Oncol 118:420–424
Pearson L, Mihali T, Moffitt M, Kellmann R, Neilan B (2010) On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar Drugs 8:1650–1680
Ramani A, Rein K, Shetty KG, Jayachandran K (2012) Microbial degradation of microcystin in Florida’s freshwaters. Biodegradation 23:35–45
Rapala J, Berg KA, Lyra C, Niemi RM, Manz W, Suomalainen S, Paulin L, Lahti K (2005) Paucibacter toxinivorans gen. nov., sp. nov., a bacterium that degrades cyclic cyanobacterial hepatotoxins microcystins and nodularin. Int J Syst Evol Microbiol 55:1563–1568
Takenaka S, Watanabe MF (1997) Microcystin LR degradation by Pseudomonas aeruginosa alkaline protease. Chemosphere 34:749–757
Tsuji K, Naito S, Kondo F, Ishikawa N, Watanabe MF, Suzuki M, Harada K (1994) Stability of microcystins from cyanobacteria: effect of light on decomposition and isomerization. Environ Sci Technol 28:173–177
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park H-D, Chen G-C, Chen G, Yu S-Z (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17:1317–1321
Valério E, Chaves S, Tenreiro R (2010) Diversity and impact of prokaryotic toxins on aquatic environments: a review. Toxins 2:2359–2410
Yan H, Pan G, Zou H, Li X, Chen H (2004) Effective removal of microcystins using carbon nanotubes embedded with bacteria. Chin Sci Bull 49:1694–1698
Zhang M, Pan G, Yan H (2010) Microbial biodegradation of microcystin-RR by bacterium Sphingopyxis sp. USTB-05. J Environ Sci 22:168–175
Acknowledgments
This work was financially supported by the Specialized Research Fund for Doctoral Program of Higher Education from Chinese Ministry of Education (No. 20123424110004), the Anhui Provincial Natural Science Foundation (No. 1208085MC42), the Key Laboratory of Bioresource Protection and Utilization of Anhui Province, the Key Laboratory of Biotic Environment and Ecological Safety of Anhui Province, and the Program for Innovative Research Team at Anhui Normal University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J., Shi, H., Liu, A. et al. Identification of a New Microcystin-Degrading Bacterium Isolated from Lake Chaohu, China. Bull Environ Contam Toxicol 94, 661–666 (2015). https://doi.org/10.1007/s00128-015-1531-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1531-7