Abstract
To investigate whether phytoremediation can remove arsenic from the contaminated area, a study was conducted for three consecutive years to determine the efficiency of Pteris vittata, Adiantum capillus veneris, Christella dentata and Phragmites karka, on arsenic removal from the arsenic contaminated soil. Arsenic concentrations in the soil samples were analysed after harvesting in 2009, 2010 and 2011 at an interval of 6 months. Frond arsenic concentrations were also estimated in all the successive harvests. Fronds resulted in the greatest amount of arsenic removal. Root arsenic concentrations were analysed in the last harvest. Approximately 70 % of arsenic was removed by P. vittata which was recorded as the highest among the four plant species. However, 60 % of arsenic was removed by A. capillus veneris, 55.1 % by C. dentata and 56.1 % by P. karka of arsenic was removed from the contaminated soil in 3 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Arsenic is a food chain contaminant and class I carcinogen Zhao et al. (2010). Due to its carcinogenic behaviour it poses risks to human and animal health. The major risk of arsenic soil contamination is through leaching into the groundwater, thus causing threat to plant and human life. Although many physical and chemical technologies have been proposed for the remediation of arsenic contaminated sites. However phytoremediation has received much attention due its cost effectiveness, economical and ecofriendly nature. Phytoremediation of As-contaminated soil and water by As hyper accumulators, such as Pteris vittata (Ma et al. 2001), Pityrogramma calomelanos (Visoottiviseth et al. 2002) and Pteris cretica (Zhao et al. 2002), has proven to be effective in practice. To date, twelve As hyperaccumulators have been well documented (Srivastava et al. 2006; Wang et al. 2007; Zhao et al. 2009; Singh et al. 2010). P. vittata is an efficient accumulator of arsenic because it accumulates large amount of arsenic (2.3 %) in its aboveground biomass (Zhao et al. 2009). When grown in arsenic contaminated sites it accumulates 10 times the concentration in soil (Kertulis-Tartar et al. 2006; Shelmerdine et al. 2009). Kertulis-Tartar et al. (2006) reported that arsenic concentration decreased from 190 to 140 mg kg−1 after three successive harvests of P. vittata. With this focus, the present study highlights the use of accumulator plants as a remediation strategy for arsenic contaminated sites. The present study aims at (i) use of accumulator plants (P.vittata, A. capillus veneris, C. dentata and P. karka) for potential use in phytoextraction/phytostabilization of contaminated soil; (ii) Execution of a long term (3 year) study to investigate amount of arsenic removal in the given time period; and (iii) comparison of arsenic removal potential by the plant species (P.vittata, A. capillus veneris, C. dentata and P. karka) in a given time period.
Materials and Methods
Pots of 8-inches were taken and filled with 4 kg of soil. Sodium arsenate was mixed with soil to provide 50 and 100 mg As kg−1 soil/pot. Garden soil, without As amendment, was taken as the control. Plants were raised under greenhouse conditions during the experiment with the ambient temperature ranging between 30 and 40°C and a natural light regime. Around 4 month old ferns–Pteris vittata L, Christella dentata Forssk, Adiantum capillus veneris L, were taken from National Botanical research Institute (NBRI) fernery. Phragmites karka (Cav.) Trin. Ex. Steud slips were taken from NBRI farm. The plants were analysed for arsenic exposure. Only fronds were harvested during harvesting and were dried in at 65°C in oven for 48 h for digestion and analysis. Arsenic was determined by ICP-MS (Agilent technologies 7500cx). After 1 week of arsenic treatment soil was collected from each treatment pot and was analysed for arsenic. After buffering in the soil it was determined that in 50 mg kg−1 arsenic treatment, the measured As concentration was 47.56 mg kg−1 and in the 100 mg kg−1 arsenic treatment the measured As concentration was 96.56 mg kg−1.
Sampling of soil and fronds was performed by the following during the study:
-
i.
Plant Harvesting in First year Two harvests were performed in 2009. Senescing fronds (mostly brown with little green) were removed at ground level by hand. In June and December 2009, all fronds were harvested.
-
ii.
Plant Harvesting in Second year Two harvests were performed in 2010. Senescing fronds (mostly brown with little green) were removed at ground level by hand. In June and December 2010, all fronds were harvested.
-
iii.
Plant Harvesting in Third year Two harvests were performed in 2011. Senescing fronds (mostly brown with little green) were removed at ground level by hand. In June 2011, all fronds were harvested. Later in December 2011 complete harvesting of the plants were done and the plants were separated into shoot and root portions and were kept on oven for drying for the estimation of arsenic.
-
iv.
Soil Sampling Soil samples were collected, air dried and sieved (1 mm mesh) for analysis. Soil samples were collected simultaneously after each harvesting of the experiment.
The standard reference materials of metals (E-Merck, Germany) were used for the calibration and quality assurance for each analytical batch. Analytical data quality of metal was ensured with repeated analysis (n = 3) of quality control samples, and the results were found within (±2.82) the certified values. As, rice flour NIST 1568a was used as a reference material with known spiked samples, and recovery of total As were 85.3 % (±2.8; n = 5) and 89.5 % (±3.1; n = 5), respectively. The detection limit for As was 1 μg l−1.
Statistical analysis of variance for treatment effects were performed using SPSS 17.0 software. Data were summarized as Mean ± SD. Groups were compared by two way factor (treatments and plants) analysis of variance (ANOVA) and the significance of mean difference within and between the groups was done by Tukey test at a probability level of p ≤ 0.05.
Results and Discussion
Plant biomass and regrowth capacity are important factors in the phytoextraction of arsenic from contaminated soils using perennial plants since multiple harvests are necessary to reduce soil arsenic to acceptable levels (Fayiga and Ma 2006). Despite the difference in arsenic concentrations in the soil, ferns grew well in the arsenic treated soil. Out of six harvests biomass increased in the first two harvests as shown in Table 1. In the first two harvests higher amounts of biomass were removed in all the four plant species in the 50 and 100 mg kg−1 arsenic treatments. Biomass decreased in later harvest due to the successive harvesting of fronds within the regular time period. A significant reduction in biomass was observed after the second harvesting. This was primarily due to successive harvesting of the plant. All the fronds were harvested and it became difficult for the plant to regenerate. Due to successive harvesting, the biomass of each plant species was decreased. Similar results were reported by Gonzaga et al. (2008) in which P. vittata biomass was decreased after each subsequent harvest. Similar results were also observed by McGrath et al. (2002), Kertulis-Tartar et al. (2006) in P. vittata which showed that the amount of biomass harvested decreased after two or three successive harvestings.
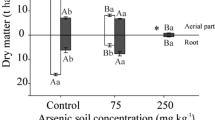
The concentrations of arsenic in the fronds of P. vittata, A. capillus veneris, C. dentata and P. karka after six harvestings are shown in the Fig. 1 and measured soil arsenic concentration are presented in Table 2. There was a significant (p < 0.05) increase in arsenic accumulation in the fronds collected during the 1st and 2nd harvesting in all the plant species. As compared with all six consecutive harvests, As in the fronds was highest in the 1st and 2nd harvest. In the 50 mg kg−1 As treatment, As accumulation was significantly increased in each plant species. In June 2009, measured As accumulation by PV fronds was 9.79 mg, by ACV it was 8.29, by CD As accumulation was 6.33 mg and by PK it was 7.28 mg. In Dec 2009, 2nd harvest As accumulation was increased (PV, ACV, CD and PK increased 10.62, 9.27, 7.12 and 8.25 mg, respectively). In June 2010, 3rd harvest As accumulation was decreased where As measured concentrations in PV, ACV, CD and PK fronds were 8.82, 7.34, 7.32 and 7.01 mg, respectively. Due to successive harvesting, the decrease in As accumulation was most likely related to the measured decrease in biomass (Table 1). In Dec 2010, during 4th frond harvest, As accumulation was further decreased (PV, ACV, CD and PK As measured concentrations were 1.34, 1.16, 1.23 and 1.19 mg, respectively). In June 2011, 5th harvest As frond concentrations were similar to what was recorded during the 4th harvest. However, in 6th and final harvest of the 50 mg kg−1 As treatment group, the complete harvesting of the plant (root and shoot) was carried out. In PV, 1 mg of As was measured in fronds, and 1.26 mg of As was measured in roots. In ACV As accumulation in fronds was 1 mg and in roots was 1.13 mg. While in CD fronds, As accumulation was 0.99 mg and root As accumulation was 1.1 mg. Finally, PK fronds accumulated 0.92 mg of As while roots accumulated 1.14 mg of As.
Similar results were observed in the 100 mg kg−1. As treatment group were measured As concentration in fronds increased between the 1st and 2nd harvests. Similarly, due to a decrease in biomass (Table 1), As accumulation was decreased in all four fern species. By the 4th frond harvest, a significant decrease in As accumulation was observed (PV, ACV, CD and PK As measured concentrations were 7.78, 7.27, 6.83 and 6.91 mg, respectively). Similar concentrations of As in fronds collected during the 5th (June 2011) sampling period were observed relative to the levels recorded during the 4th sampling period. However, in the 6th and final harvest of the 100 mg kg−1 As treatment group, the complete harvesting of the plant (root and shoot), as in the 50 mg kg−1 As treatment group, was carried out. In PV As accumulation was recorded higher as compare to other three plant species, As accumulation in fronds was 4.55 mg and in roots was 2.55 mg. ACV accumulated 2.89 mg of As in fronds and 1.65 mg As in roots. While in CD fronds, As accumulated 3.09 mg and roots accumulated 1.78 mg. At last in PK at 100 mg kg−1 arsenic treatment fronds accumulated 2.96 mg of As and roots accumulated 1.85 mg of As.
Compared with the control (47.56 mg in 50 and 96.56 mg in 100 mg kg−1 As treatment) soil As concentrations were decreased. The total removal of As by the plant varieties is presented in the Fig. 2. The total removal of arsenic by P. vittata after 3 years of harvesting at an interval of 6 months was, 71.38 % in 50, and 70.29 % in 100 mg kg−1 As treatment. In A. capillus veneris % arsenic removal was 61.52 % in 50 and 60.75 % at 100 mg kg−1 As treatment. In C. dentata As removal was 53.22 % in 50 and 54.17 % in 100 kg−1 arsenic treatment. However in P. karka % of arsenic removed from 50 and 100 kg−1 of arsenic treated soil was 56.11 and 50.97 %.
Arsenic accumulation was recorded high in the field trials which show that these plant species (P. vittata, C. dentata and P. karka) were well equipped in combating arsenic stress. Similar results were also observed by Ye et al. (2011). Arsenic concentration decreased in the fronds after two harvesting. The lower frond arsenic concentrations from third harvesting compared with the first and second harvesting were due to decrease in biomass. Although not much significant difference in removal of arsenic was seen in plant species. But high amount of arsenic was removed from the soil by the plants. There was no significant difference in plant arsenic removal during the 3rd, 4th, 5th and 6th harvesting periods. Similar results were observed by Gonzaga et al. (2008); Kertulis-Tartar et al. (2006); and Salido et al. (2003) in P. vittata. Regrowth of ferns and comman reed is relatively quick, allowing for a harvest every month or two. High amount of arsenic was accumulated by the root and translocated to the shoot and during harvesting of fronds more of arsenic was removed from the soil through plants.
Based on our study it was observed that these plant species were capable of arsenic removal from arsenic contaminated site. Regular harvesting of mature fronds before they senesce will ensure maximum arsenic removal from the site. Our data also suggest that better agronomic practices, such as fertilization, were needed to enhance plant growth and arsenic uptake to obtain maximum soil arsenic removal.
References
Fayiga AO, Ma LQ (2006) Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ 359:17–25
Gonzaga MIS, Santos JAG, Ma LQ (2008) Phytoextraction by arsenic hyperaccumulator Pteris vittata L. from six arsenic-contaminated soils: repeated harvests and arsenic redistribution. Environ Pollut 154:212–218
Kertulis-Tartar G, Ma LQ, Tu C, Chirenje T (2006) Phytoremediation of an arsenic-contaminated site using Pteris vittata L.: a two-year study. Int J Phytorem 8:311–322
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
McGrath SP, Zhao FJ, Lombi E (2002) Phytoremediation of metals, metalloids and radionuclides. Adv Agron 75:1–56
Salido A, Hasty KL, Lim J, Butcher DJ (2003) Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int J Phytoremed 5:89–103
Shelmerdine PA, Black CR, McGrath SP, Young SD (2009) Modelling phytoremediation by the hyperaccumulating fern, Pteris vittata, of soils historically contaminated with arsenic. Environ Pollut 157:1589–1705
Singh N, Raj A, Khare PB, Tripathi RD, Jamil S (2010) Arsenic accumulation pattern in 12 Indian ferns and assessing the potential of Adiantum capillus-veneris, in comparison to Pteris vittata, as arsenic hyperaccumulator. Bioresour Technol 101:8960–8968
Srivastava M, Ma LQ, Santos JAG (2006) Three new arsenic hyperaccumulating ferns. Sci Total Environ 364:24–31
Visoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118:453–461
Wang HB, Wong MH, Lan CY, Baker AJM, Qin YR, Shu WS, Chen GZ, Ye ZH (2007) Uptake and accumulation of arsenic by 11 Pteris taxa from southern China. Environ Pollut 145:225–233
Ye WL, Khan MA, McGrath SP, Zhao FJ (2011) Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ Pollut 159:3739–3743
Zhao FJ, Dunham SJ, McGrath SP (2002) Arsenic hyperaccumulation by different fern species. New Phytol 156:27–31
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgments
The authors are grateful to the Director, CSIR-NBRI for keen interest and continuous encouragement to conduct the experiments. AR thankfully acknowledges CSIR for awarding SRF grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj, A., Singh, N. Phytoremediation of Arsenic Contaminated Soil by Arsenic Accumulators: A Three Year Study. Bull Environ Contam Toxicol 94, 308–313 (2015). https://doi.org/10.1007/s00128-015-1486-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1486-8