Abstract
Bench-scale soil washing experiments were conducted to remove fluoride from contaminated soils. Five washing solutions including hydrochloric acid (HCl), nitric acid (HNO3), sodium hydroxide (NaOH), sulfuric acid (H2SO4) and tartaric acid (C4H6O6) were tested. The concentration of the washing solutions used ranged from 0.1 to 3 M with a liquid to solid ratio of 10. The soil washing results showed that the most effective washing solution for the removal of fluoride from contaminated soils was HCl. The highest fluoride removal results of approximately 97 % from the contaminated soil were obtained using 3 M HCl. The fluoride removal efficiency of the washing solution increases in the following order: C4H6O6 < NaOH < H2SO4 < HNO3 < HCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fluorine may occur in the ecosphere (soil, water, air and vegetation) as a natural contaminant (Jha et al. 2009; D’Alessandro et al. 2012). Occurrence, adverse effects, exposure routes, and risk assessment of ionic fluorine (fluoride) have been well documented in the literature (WHO 2002; ATSDR 2003). Chronic intake of excessive fluorine can cause severe permanent bone and joint deformations of skeletal fluorosis (Wang and Huang 1995; Camargo 2003). Human populations in many countries around the globe (China, India, Iran, etc.) suffer from endemic fluorosis caused by excess intake of fluoride (Wang et al. 2012). Fluorides may leach from soils and contaminate surface and ground drinking water supplies thus posing a danger to the human health (Zhu et al. 2009).
In the Republic of Korea, fluorine contamination has received a great deal of attention recently (September 2012) caused by a hydrogen fluoride leak accident that occurred at a local manufacturing facility that uses the chemical for the production of displays. As a result of this accident, five people died while more than 2,000 residents exposed suffered from a variety of adverse dermal, ocular, and respiratory effects. Moreover, in the Republic of Korea, fluorine is used in the steel manufacturing industry in the form of fluorite and in the electronics industry in the form of hydrofluoric acid for cleaning and washing (Kim et al. 2009). Consequently, soils in the vicinity of these industrial complexes may potentially become contaminated with fluorine. In the Republic of Korea, the Ministry of the Environment has issued a warning standard regulating fluoride levels in residential area soils at 400 mg/kg.
In this study, the soil washing process was selected as a remediation technique for fluorine contaminated soils. Soil washing is the most widely applied treatment technology for heavy metal contaminated field soil in the Republic of Korea. The process can extract a variety of organic or inorganic contaminants adsorbed or precipitated onto the surface of the solid particles. Selection of the appropriate extraction solution is critical for improving the extraction effectiveness. The type of washing solution depends on the target contaminants, the bonding/chelating strength of the extraction solution, and the soil characteristics (Mulligan et al. 2001). Several researchers have investigated a variety washing solutions (e.g., inorganic salts, inorganic acids, organic acids and alkaline agents, etc.) for heavy metal and metalloid removal from soil. However, soil washing research regarding fluorine contaminated soil is limited. One electrokinetic remediation study reported a low fluorine removal efficiency (22 %) using 1 M NaOH (Kim et al. 2009). The major remediation technology options for fluorine contaminated soil used in the field are replacement and solidification/stabilization (Luther et al. 1996). Soil washing could be a viable alternative technology for the remediation of soils contaminated with fluoride. Although the majority of fluorine in soils tends to be in the form of fluoride insoluble or strongly bound to the particulate phase, acidic and alkaline conditions may result in fluoride mobilization. More specifically, acidic conditions are known to enhance fluoride mobility most likely in the form of Al or Fe complexes (e.g., AlF2+, AlF2 +, AlF 03 , AlF4 −, FeF2+, FeF2 +, FeF 03 ) rather than in the free ionic form (F−) (Barrow and Ellis 1986; Skjelkvaale 1994). Similarly, under alkaline conditions retention of F on the soil decreases and the F− concentration in soil solution increases. This may be due to unfavorable electrostatic potential or displacement of adsorbed F− by the increased concentration of hydroxyl (OH–) in the soil solution at the higher pH (Larsen and Widdowson 1971). Thus, investigating acid and alkaline extraction solutions and their effect on fluoride removal from the contaminated soil is warranted. The effectiveness of fluoride removal from contaminated soil was tested and evaluated using five different washing solutions encompassing three strong acids (HCl, HNO3·H2SO4), a weak organic acid (C4H6O6), and a strong base (NaOH). These washing solutions are widely used for the removal of heavy metals and metalloids (Ko et al. 2005; Ke et al. 2006; Jang et al. 2007; Yang et al. 2009; Wuana et al. 2010; Zhou et al. 2012). In addition, these washing solutions have been successfully used for the removal of Zn in contaminated soil (Moon et al. 2012).
The purpose of this study was to investigate whether soil washing is a viable remediation technology for fluoride contaminated soil. The effectiveness of the washing process was evaluated by measuring the residual fluoride concentrations on the soil after the washing process. The residual fluoride concentrations were compared to the Korean warning standard of 400 mg/kg for residential areas (1 area).
Materials and Methods
Fluoride contaminated soil was collected from a chemical company in Changwon-si, Gyeongsangnam-do, Republic of Korea. The contaminated soil was classified using a particle size analysis system (Sedigraph 5100, USA). Physicochemical characterization information of fluorine contaminated soil is presented in Table 1. The bulk chemistry of the fluoride contaminated soil was analyzed using X-ray fluorescence (XRF, ZSX100e, Rigaku, Japan) and is presented in Table 2.
The total organic content (TOC) was obtained using the TOC-SSM-5000A (Shimadzu, Kyoto, Japan) analyzer and it was determined at 4.31 % and the total fluoride concentration was measured at 740 mg/kg (Table 1). The pH value of the contaminated soil was measured at 3.7 and the fluoride contaminated soil was classified as sandy soil according to the United States Department of Agriculture (USDA). The soil was composed of approximately 89 % sand, 9.8 % silt and 1.3 % clay (Table 1). In order to remove the large particles and obtain a homogeneous soil size, the fluoride contaminated soil was air-dried and sieved using the #10 mesh (2 mm).
As explained previously, acid and alkaline solutions are capable of mobilizing fluoride from contaminated soils. In this study three mineral acids, one organic acid and a strong base were investigated. Reagent grade hydrochloric acid (HCl), nitric acid (HNO3), sodium hydroxide (NaOH), sulfuric acid (H2SO4, SA) and tartaric acid (C4H6O6, TA) were used as extraction agents. Deionized water (DI) was used as a blank washing solution for benchmarking purposes. The concentration of the washing solutions was varied in the ranges of 0.1–3 M. The washing experiments were performed with 5 g of soil mixed with 50 mL of washing solution in a 125 mL plastic bottle. The suspensions were agitated in a shaking incubator (LabTech, Daihan, South Korea) at 200 RPM, 20°C for 1 h. Following agitation, the suspended solids were separated by filtration through a 0.45-µm micropore filter and air-dried. Upon completion of the washing process, the fluoride concentration in the soil was measured according to the Korean Standard Test (KST) methods described in the following section and the results were compared to the Korean warning standard.
The soil pH was measured in accordance with the KST method (MOE 2010) with a liquid to solid ratio of 5:1. The total fluoride concentration in the soil was measured in accordance with the KST method (MOE 2010). Specifically, 1 gram of soil with a particle size less than 0.075 mm and 5 g of CaO were put into a 50 mL Ni crucible and mixed thoroughly. Then, the crucible was placed in a muffle furnace for 5 h at 500°C. Next, the temperature was slowly raised to 800°C for 2 h and then cooled at room temperature. The sample was then washed with 25 mL DI as well as, 50 mL of 70 wt% HClO4 and transferred into a 300 mL three neck flask. Then, 10 drops of 17 % perchloric acid silver solution and 8–10 boiling stones were added into the reaction flask. 600 mL of purified water was heated in a distillation flask and connected to the reaction flask with a U-shaped glass-rod. The distillation temperature was kept at 135°C ± 2. 500 mL of distillate was collected in a volumetric flask with an effluent velocity of 5–6 mL/min, where 1 drop of 50 % NaOH and 1 drop of nitro-phenol were added to the flask. Thereafter, 50 mL of distillate and an equal volume of TISAB (pH 5.2) were mixed thoroughly into a 200 mL beaker. The fluoride concentration in the solution was measured using a selective ion electrode (Orion 4starTM, Thermo Sci., USA). All sample analyses were conducted in triplicate and the averaged values were reported.
Results and Discussion
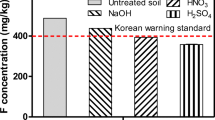
The soil washing results using five different solutions are presented in Figs. 1, 2, 3, 4 and 5. The soil washing process using DI water alone as an extraction agent was not effective for the removal of fluoride from the contaminated soil. The remaining fluoride concentration on the soil after washing with DI water as the blank extractant exceeded the Korean warning standard. This indicates that fluoride extraction is very limited using DI water. The washing results, for all the extracting solutions used in this study, showed that fluoride removal increased with increasing washing solution concentration. In all of the samples, the highest removal results were obtained in the samples treated with each extraction solution at a concentration of 3 M, except for NaOH. HCl was the most effective solution for the removal of fluoride from the contaminated soils. The lowest residual fluoride level of approximately 25 mg/kg (approximately 97 % removal) was attained in the sample treated with the 3 M HCl extraction solution. This behavior agrees in principal with reported literature that fluoride is strongly adsorbed onto soils in the pH range from 5.5 to 6.5 and that fluoride solubility increases at pH values less than 5.5 as well as at pH values higher than 6.5 (Wenzel and Blum 1992). The increased mobility of fluoride under acidic conditions has been associated with increased aluminum solubility and the possible formation of aluminum-fluoride complexes (Davison 1983). The residual fluoride concentrations in all of the samples after washing with the HCl extraction solution were less than the Korean warning standard. This indicated that the HCl washing solution with a concentration of 0.1 M was strong enough to reduce the fluoride concentration in the contaminated soil to 97 mg/kg. The second most effective washing solution for the removal of fluoride was HNO3. Similar to HCl extraction, the fluoride concentration remaining in the soil decreased with increasing HNO3 concentration. The fluoride concentrations remaining in the soil for all samples after washing with the HNO3 extraction solution were less than the Korean warning standard. After 3 M HNO3 extraction, the lowest fluoride concentration remaining in the soil was 67 mg/kg.
The third most effective washing solution for the removal of fluoride was SA. Similar to the HCl and HNO3 soil washing results, the residual fluoride concentrations decreased with increasing SA concentrations. After 3 M SA extraction, the lowest fluoride concentration remaining in the soil was 90 mg/kg.
Soil washing using strong mineral acids may raise two concerns, namely, liberation of hydrogen fluoride fumes resulting in occupational exposure of personnel and corrosive behavior of the washing solution that may affect adversely soil washing equipment. In our case the best removal scenario (3 N HCl) producing a final fluoride residual contamination of 25 mg/kg (approximately 97 % removal), using a washing solution to solids ratio of 10 results in a washing solution concentration of 71.5 mg/l fluoride at a pH less than 3.2 (pKa of HF). Assuming that all HF is undissociated and a Henry’s constant of 0.104 atm·L/mol the resulting concentration is the off-gas is 3.32 μg/m3 which is higher than the ACGIH threshold limit value ceiling of 2.5 μg/m3 NIOSH recommended exposure limit for an 8- or 10-h time-weighted-average exposure (http://www.epa.gov/ttn/atw/hlthef/hydrogen.html). It should be noted that the above estimations represent worst case scenario as complexation reactions are neglected. Taking into account that under acidic conditions much of the fluoride ends up in Al and Fe soluble complexes the above concentration represents a severe overestimate. Nevertheless, the HF liberation concern can be easily remedied by enclosing the operation and capturing the off-gases. The corrosive behavior can be addressed by using weaker solutions of the mineral acids. It should be noted that even the 0.1 N washing solutions generated soils well below the Korean standard. In this case, stainless steel equipment should be able to resist the corrosivity of the washing solution.
Evidently, mineral acids are capable of mobilizing fluoride; however, what remains unclear is the mobilization mechanism. At the low pHs (often less than 1) prevailing during soil washing using strong mineral acids it is unlikely that fluoride exists in the ionic form. Much of the literature suggests that fluoride mobility under acidic pHs is due to the formation of Al and Fe complexes (Barrow and Ellis 1986; Skjelkvaale 1994). A more recent study of fluoride mobility in the vicinity of an aluminum smelting plant correlates fluoride mobility with labile Al due to formation of AlFx complexes, the most abundant being AlF2+ and AlF2 + (Gago et al. 2002). Although plausible, the mechanism of fluoride mobilization was not confirmed in the present study. The investigation of the chemical or mineralogical form of fluorine attempted using XRD analysis of the treated soil was inconclusive due to low fluorine concentrations. Advanced analytical method (extended X-ray absorption fine structure, EXAFS) could potentially shed some light into the chemical or mineralogical forms of fluorine in a subsequent phase of the study.
The fourth most effective washing solution was NaOH. It has been reported that under alkaline conditions, fluoride could be desorbed or dissolved from the soil minerals more than in acidic or neutral conditions (Kim et al. 2002). Kim et al. (2009) conducted fluoride extraction experiments using a NaOH solution. It was reported that Kim et al. (2009) obtained fluoride removal at 4.9 % and 22.8 % from contaminated soil with 0.5 and 1 M NaOH, respectively and a liquid to solid ratio of 10:1. Moreover, this previous study concluded that the NaOH solution was not effective in the removal of fluoride from the contaminated soil. However, in this study, approximately 62 % and 64 % fluoride removal was attained with 0.5 and 1 M NaOH, respectively. Moreover, the highest fluoride removal result of approximately 71 % was obtained with using the 2 M NaOH washing solution. This indicated that the effectiveness of fluoride removal from the contaminated soil using the NaOH solution could depend on the type of contaminated soil. It is expected that the sandy soil studied here should have a higher fluoride removal efficiency than clay soil types. Unlike complexation which is the main mechanism of fluoride mobilization in acidic conditions, in strong alkaline pHs the increased hydroxyl concentration in the washing solution is responsible for unfavorable electrostatic potential or displacement of adsorbed F- from the soil matrix (Larsen and Widdowson 1971).
The least effective washing solution was TA, which showed a high fluoride concentration of 270 mg/kg after washing with the 3 M TA washing solution. The residual fluoride concentration after 0.1 M TA extraction exceeded the Korean warning standard. Moreover, the residual fluoride concentrations upon 0.1, 0.5, 1 and 2 TA extraction were around 300 mg/kg, indicating no significant effect on fluoride removal with increases in the TA concentration. This is probably due to TA being a weak organic acid which is not strong enough to desorb or dissolve the fluoride compounds in the contaminated soil, as compared to the HCl and HNO3 washing solutions. Incidentally, TA was reported to perform poorly in the removal of multiple heavy metals from contaminated soil, in comparison to EDTA and citric acid (Wuana et al. 2010). Wuana et al. (2010) recommended that TA be used only to treat instances of moderate contamination. Overall, this study determined that the effectiveness of the fluoride removal treatments had the following order: HCl > HNO3 > SA > NaOH > TA with the most effective treatment listed first and the least effective treatment listed last.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2003) Toxicological profile for fluorides, hydrogen fluoride, and fluorine. US Department of Health and Human Services, Atlanta, Georgia
Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soil. J Soil Sci 15:84–92
Barrow NJ, Ellis AS (1986) Testing a mechanistic model. III. The effects of pH on fluoride retention by a soil. J Soil Sci 37:287–293
Camargo JA (2003) Fluoride toxicity to aquatic organisms: a review. Chemosphere 50:251–264
D’Alessandro W, Bellomo S, Parello F (2012) Fluorine adsorption by volcanic soils at Mt. Etna, Italy. Appl Geochem 27:1179–1188
Davison AW (1983) Uptake, transport and accumulation of soil and airborne fluorides by vegetation. In: Shupe JL, Peterson HB, Leone NC (eds) Fluorides effects on vegetation, animals and humans. Proceedings of an international symposium on fluorides at Utah State University, Salt Lake City, UT, 24–27 May 1982. Salt Lake City, UT, Paragon Press Inc. pp 61–82
FitzPatrick EA (1983) Soils: their formation, classification and distribution. Longman Science & Technical, London, p 353
Gago C, Fernández Marcos ML, Álvarez Lugo E (2002) Aqueous aluminium species in forest soils affected by fluoride emissions from an aluminium smelter in NW Spain. Fluoride 35(2):110–121
Jang M, Hwang JS, Choi SI (2007) Sequential soil washing techniques using hydrochloric acid and sodium hydroxide for remediating arsenic-contaminated soils in abandoned iron-ore mines. Chemosphere 66:8–17
Jha SK, Nayak AK, Sharma YK (2009) Fluorine toxicity effects in onion (Allium cepa L.) grown in contaminated soils. Chemosphere 76:353–356
Ke X, Li P, Zhou Q, Zhang Y, Sun T (2006) Removal of heavy metals from a contaminated soil using tartaric acid. J Environ Sci 18:727–733
Kim SO, Kim KW, Stüben D (2002) Evaluation of electrokinetic removal of heavy metals from tailing soils. J Environ Eng 128:705–715
Kim D-H, Jeon C-S, Baek K, Ko S-H, Yang J-S (2009) Electrokinetic remediation of fluorine-contaminated soil: conditioning of anolyte. J Hazard Mater 161:565–569
Ko I, Chang Y-Y, Lee C-H, Kim KW (2005) Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J Hazard Mater A127:1–13
Larsen S, Widdowson AE (1971) Soil fluorine. J Soil Sci 22:210–222
Luther SM, Poulsen L, Dudas MJ, Rutherford PM (1996) Fluoride sorption and mineral stability in an Alberta soil interacting with phosphogypsum leachate. Can J Soil Sci 76:83–91
Ministry of Environment (MOE) (2010) The Korean Standard Test (KST) methods for soils. Korean Ministry of Environment, Gwachun, Kyunggi, p. 225 (in Korean)
Moon DH, Lee J-R, Wazne M, Park J-H (2012) Assessment of soil washing for Zn contaminated soils using various washing solutions. J Ind Eng Chem 18:822–825
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Skjelkvaale BL (1994) Factors influencing fluoride concentrations in Norwegian lakes. Water Air Soil Poll 77:151–167
Wang LF, Huang JZ (1995) Outline of control practice of endemic fluorosis in China. Soc Sci Med 41:1191–1195
Wang C, Yang Z, Chen L, Yuan X, Liao Q, Ji J (2012) The transfer of fluorine in the soil-wheat system and the principal source of fluorine in wheat under actual field conditions. Field Crop Res 137:163–169
Wenzel WW, Blum WEH (1992) Fluorine speciation and mobility in F-contaminated soils. Soil Sci 153:357–364
World Health Organization (WHO) (2002) Environmental Health Criteria 227. Fluorides, Geneva
Wuana RA, Okieimen FE, Imborvungu JA (2010) Removal of heavy metals from a contaminated soil using organic chelating acids. Int J Environ Sci Technol 7(3):485–496
Yang J-S, Lee JY, Baek K, Kwon T-S, Choi J (2009) Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. J Hazard Mater 171:443–451
Zhou L, Wu Q, Gao G (2012) Feasibility of tartaric acid washing of soil contaminated by Pb and Zn. Adv Mater Res 550–553:2194–2197
Zhu S, Zhang J, Dong T (2009) Removal of fluorine from contaminated field soil by anolyte enhanced electrokinetic remediation. Environ Earth Sci 59:379–384
Acknowledgments
This study was supported by the Korea Ministry of Environment as the GAIA (Geo-Advanced Innovative Action) Project (No. 2014000540011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, D.H., Jo, R., Koutsospyros, A. et al. Soil Washing of Fluorine Contaminated Soil Using Various Washing Solutions. Bull Environ Contam Toxicol 94, 334–339 (2015). https://doi.org/10.1007/s00128-014-1449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1449-5