Abstract
This study evaluated the effects of exposure medium and culture age on intracellular reactive oxygen species (ROS) development and cytotoxicity in fish hepatocytes following exposure to copper (Cu). ROS was quantified using the fluorescent probes DHR 123 and CM-H2DCFDA following exposure to Cu in Leibovitz’ medium (L-15) or Tris-buffered saline (TBS). Similarly, culture age effects were investigated using 1-, 2- and 4-day-old cultured hepatocytes by exposing them to Cu in TBS. The exposure in L-15 resulted in significantly higher ROS compared to TBS using CM-H2DCFDA, but not DHR 123. The age of the primary cultures significantly affected the development of ROS for both probes. None of the exposures caused cytotoxicity in the hepatocytes. The results showed that both factors may affect responses to stressors, and suggested that the use of a simple medium such as TBS may be preferable for some applications. It is also preferable to use 1-day-old primary hepatocyte cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endogenously produced reactive oxygen species (ROS) are believed to be essential for the physiological health of organisms at low concentrations in a normal cellular environment. For example, ROS act as signaling molecules and play a role in the cellular response to anoxia (Sorg 2004). Oxidative stress may occur following ROS overproduction or exhaustion of cellular protective mechanisms, thereby potentially causing harmful effects (Gomes et al. 2005). In addition to being associated with external stressors such as chemical stress or radiation, oxidative stress has been shown to be relevant to ageing processes and the development of diseases such as atherosclerosis and cancer (Heyne et al. 2007). A range of responses has been observed in aquatic organisms following oxidative stress (Winston and Di Giulio 1991), including toxicant-induced inflammation and DNA oxidative damage (Nishimoto et al. 1991; Otto and Moon 1996; Pedrajas et al. 1995). It is therefore important to increase our understanding of how ROS affect biological systems. Most ROS have a short half-life within cells and are generally quickly removed by cellular oxidant scavengers or through the activity of antioxidant enzymes, making it challenging to detect their presence (Gomes et al. 2005; Tarpey et al. 2004). Fluorescent probes have been widely used due to the non-invasive character of their use, high sensitivity, high resolution for microscopy applications and simplicity in data collection (Gomes et al. 2005; Heyne et al. 2007). Probes such as 2′, 7′-dichlorodihydrofluorescein diacetate (DCDHF-DA) and dihydrorhodamine 123 (DHR 123) have therefore been frequently used to quantify the production of ROS in cells from fish, rodents and humans.
The aquatic environment is the major sink for many pollutants with a potential to induce oxidative stress (Winston and Di Giulio 1991). The results of Rau et al. (2004) suggest that fish hepatocytes may be as sensitive or more sensitive than rat cells in oxidative stress response. Primary hepatocyte cultures from fish may therefore be useful tools to study the effects of oxidative stress, both with regard to toxicity and cellular adaptive responses.
The choice and composition of the culture medium is vital for the culture and exposure of cells (Bopp et al. 2008; Pourahmad and O’Brien 2000; Radice et al. 2004). Some cell culture medium components have been shown to have the potential to generate ROS (Halliwell 2003) or interact with xenobiotics (Weglarz and Bartosz 1991). The above studies notwithstanding, few studies have been carried out to investigate the influence of exposure medium on oxidative stress (Grzelak et al. 2001; Wright et al. 2000), particularly with regard to conditions during exposure to toxicants. Freshly made hepatocyte cultures have been suggested to remain viable for at least 1 week under appropriate culture conditions (Pesonen and Andersson 1997), but their properties may change even after a few days, which may subsequently affect the results in oxidative stress studies (Ruch et al. 1989).
To induce oxidative stress in cultured cells, increasing the radical load or inhibiting the antioxidant defenses may be followed to disturb the prooxidant–antioxidant balance as an approach (Gille and Joenje 1992). Copper (Cu) has previously been used as a model chemical to induce intracellular ROS in hepatocytes (Farmen et al. 2010; Nawaz et al. 2005; Pourahmad and O’Brien 2000). Metals like Cu form ROS by the redox cycling Haber–Weiss reaction in which cuprous ions (Cu+) react with H2O2. In addition, they can catalyze the production of ROS by the Fenton reaction. Consequently, ROS causes the peroxidation of membrane lipids, membrane disruption and molecular damage (Britton 1996; Halliwell and Gutteridge 2007).
This study aimed to evaluate the influence of cell culture medium on ROS development in 1-day-old primary hepatocyte cultures from rainbow trout. In addition, ROS development was quantified after longer time in culture, i.e. 2 and 4 days. Rainbow trout hepatocytes were exposed to different concentrations of Cu, and membrane integrity and glutathione (GSH) content were quantified for all treatments to quantify cytotoxicity and change in antioxidant status, respectively.
Materials and Methods
CuSO4·5H2O, NaHCO3, Na2HPO4, Trizma hydrochloride, Trizma base (tris[hydroxymethyl]aminomethane), trypan blue, penicillin, streptomycin and amphotericin (PSA), l glutamine and serum-free Leibovitz’L-15 medium were from Lonza Walkersville, Inc (Walkersville, MD, USA). NaCl, KCl and NaH2PO4 were from Merck (Darmstadt, Germany). Collagenase (Type IV), bovine serum albumin (BSA), CaCl2, dimethyl sulfoxide (DMSO), MgSO4, NaHCO3 solution for cell culture and N,N,N′,N′-tetra acetic acid (EGTA) were purchased from Sigma Norway (Oslo, Norway). Fluorescence probes CM-H2DCFDA, DHR 123, CFDA-AM and mBCl were obtained from Molecular Probes Invitrogen (Paisley, UK). All chemicals were of the highest commercial grade available.
Juvenile rainbow trout (Oncorhynchus mykiss) were obtained from a local hatchery and were acclimated to holding conditions for at least 2 weeks prior to use –10 ± 1°C; pH 7.5; 12 h: 12 h light/dark cycle – in tanks at the University of Oslo. Spirit Ørret 300 (Skretting, Norway) was used as daily food in amounts corresponding to 0.5 % of total body mass. Hepatocytes were isolated from juvenile rainbow trout liver following a two-step perfusion method described by Tollefsen et al. (2003). Viability and cell yield were determined by trypan blue exclusion. Isolates with a viability of 90 % or more were used in the experiments. Cell density was adjusted to a final concentration of 0.5 million cells/mL (0.2 mL/well) in 96-well Falcon Primaria plates (Becton–Dickinson, Franklin Lakes, NJ, USA) using serum-free L-15 medium containing penicillin (100 Units/mL), streptomycin (100 μg/L), amphotericin (0.25 μg/mL), l-glutamine (2 mM) and sodium bicarbonate (0.0375 %), and incubated at 15°C. Hepatocytes were left for 24 h to allow the establishment of a monolayer. All isolates were examined with an inverted light microscope before being used in experiments. All glassware and instruments were autoclaved and solutions were sterilized by filtration (0.22 µm) prior to liver perfusion. Fresh stock solutions of Cu were prepared daily.
For the determination of ROS, trout hepatocytes were loaded with CM-H2DCFDA and DHR 123. A CM-H2DCFDA (10 mM) stock solution was prepared immediately before the experiment by dissolving the powder in DMSO. The working solutions of fluorescent probes were prepared in TBS (DMSO final concentration <0.01 %). Cell culture media (above) were removed from the wells, exchanged with 200 μL of TBS-containing either probe and incubated for 30 min in a dark cold room (15°C). Afterwards, the cells were washed with TBS and exposed to different concentrations of Cu (1, 10 and 100 µM) in L-15 or TBS. Reading of fluorescence was done immediately after adding the exposure solutions using a Syngergy MX plate reader (BioTek Instruments Inc., Winooski, VT, USA) over 15 min (exposure time) at intervals of 45 s. Excitation and emission wavelengths were 485/530 and 485/535 for CM-H2DCFDA and DHR 123, respectively. The sensitivity was set to 80 %. Each well was measured 10 times from the top with the average calculated by the instrument. Gen5 was used for data collection (BioTek Instruments Inc., USA).
Cytotoxic effects were assessed through quantifying membrane integrity and intracellular glutathione using CFDA-AM and mBCl, respectively (Jos et al. 2009; Schreer et al. 2005). Exposure media were removed from the wells, exchanged with 100 μL of TBS containing 4 μM CFDA-AM and 275 μM mBCl and incubated on an orbital shaker (85 rpm) for 30 min in a dark cold room (15°C). The fluorescent signal for CFDA-AM (excitation/emission 493/541 nm) and mBCl (excitation/emission 394/490 mn) were measured simultaneously using a Synergy MX plate reader.
Five replicate juvenile rainbow trout were used for exposure experiments. Three of the replicate fish were used for the cytotoxicity assay and GSH content. Three technical replicates on each microtiter plate were used for blank (wells containing L-15 or TBS without cells), control (cells loaded with probe in L-15 or TBS without test compounds) and each test concentration with Cu. ROS was quantified using slopes calculated from the kinetics of probe fluorescence for each treatment. ROS concentrations in cells from different treatments following Cu exposure were analysed using a two-way analysis of variance (ANOVA) with medium and incubation time as factors after testing for homogeneous variances (heterogeneous variances were log10-transformed). For cytotoxicity and GSH content, the statistical significance of differences between the control and treatment groups was determined using a one-way ANOVA (Zar 2010). A p value of <0.05 was considered significant.
Results and Discussion
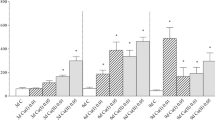
Intracellular ROS development was significantly different for CM-H2DCFDA in 1-day-old hepatocytes exposed to Cu in L-15 compared to cells exposed to Cu in TBS (Fig. 1a). A two-way ANOVA indicated a significant interaction between Cu concentration and medium for fluorescent product formation. ROS development was elevated at lower concentrations and then declined with increasing concentration of Cu in L-15. In contrast, a dose–response pattern was observed when the cells were exposed to Cu in TBS. There was no significant effect from Cu concentration and medium in the two-way ANOVA when DHR 123 was used (Fig. 1b).
The development of ROS in cells was compared following exposure to copper (Cu) in TBS or serum-free L-15 medium supplemented with l glutamine, PSA and sodium bicarbonate. At the cellular level, high Cu concentrations have been linked to the formation of ROS formation, giving rise to inhibition of ATP production, enzyme dysfunction and membrane peroxidation (Bopp et al. 2008; Manzl et al. 2004). As a result, Cu has been used as a model compound with concentrations ranging from µM to mM in cell culture studies to investigate oxidative stress (Ellesat et al. 2011; Farmen et al. 2010; Krumschnabel et al. 2005; Manzl et al. 2004; Pourahmad and O’Brien 2000; Rau et al. 2004). L-15 is a complex medium with salts, free amino acids, galactose, phosphates, folic acid, flavin mononucleotide and phenol red. Ascorbate, flavonoids, other polyphenolic compounds and thiols in commonly used cell culture media have been shown to have the potential to generate H2O2 and ROS (Halliwell 2003). Earlier studies suggest that interaction of culture medium components with xenobiotics can enhance the production of ROS (Weglarz and Bartosz 1991). Grzelak et al. (2001) identified riboflavin as the main component in cell media causing ROS generation in cells exposed to light, and suggested that metal ions, cysteine and methionine in cell media would be light-independent sources of ROS.
In contrast to CM-H2DCFDA, there was no significant interaction between Cu concentration and medium for DHR 123. This might be explained by the different types of ROS detected by the two probes: DCDHF-DA and its derivatives (CM-H2DCFDA) are thought to quantify the presence of HO·, H2O2 and RO ·2 (Gomes et al. 2005; Hempel et al. 1999), although some authors have used it specifically as a marker for intracellular H2O2 (Rico et al. 2009; Roy et al. 2009). DHR 123 has been suggested to be specific for peroxynitrite (Kooy et al. 1994) but it has been indicated to reflect ROS development following exposure to H2O2/HRP and HOCl (Gomes et al. 2005).
None of the exposures in L-15 or TBS caused increased cytotoxicity or changed glutathione content in 1-day-old rainbow trout hepatocytes (data not shown). This is in contrast to the results found by Wright et al. (2000). These authors observed a significant reduction in H2O2 cytotoxicity when an epithelioma cell line from carp was grown in L-15 medium as compared to culture in Dulbecco’s Modified Eagle Medium (DMEM). They linked this effect to elevated cellular glutathione and metallothionein levels and higher activities of GSH-dependent detoxification systems following culture in L-15. The different results can be explained by different sensitivity of species, exposure medium and model compound type.
The age of primary hepatocyte cultures significantly affected the development of ROS measured using DHR 123 and CM-H2DCFDA (Fig. 2a, b) following exposure to Cu. The development of ROS decreased with increasing age of the culture. Both probes had a dose–response relationship of ROS development following exposure to Cu at all days whereas the development of ROS appeared to be declining with culture time for each concentration of Cu.
Responses in primary cultures are generally thought to be comparable to in vivo responses in terms of metabolic and antioxidative capacity (Croci and Wolliams 1985). Bonney (1974) reported the maintenance of many specific functions characteristic of the adult rat liver in vivo when they cultured hepatocytes for 4 days. In a microarray-based study with several in vitro rat hepatocyte systems (liver slices, monolayer, and sandwich cultures), changes in gene expression were observed in the preparations by Boess et al. (2003) when compared with intact liver. Their results predominantly indicated an up-regulation of gene expression in all culture systems, including genes involved in apoptosis, regulation of growth and differentiation. Primary cultures of rainbow trout hepatocytes have been reported to maintain their in vivo-like metabolic activity for 3–8 days (Ferraris et al. 2002). In that study, however, increases in ROS generation and decreases in GSH content and catalase (CAT) activity were reported after the first 4 h of culture. ROS and GSH content returned to original levels after 24 and 48 h, respectively.
The age of cultures had no significant influence on the cell death or changes in GSH content in trout hepatocyte cultures exposed to Cu (data not shown). Ruch et al. (1989) investigated the effects of culture age (0–96 h) on primary cultured mouse hepatocytes exposed to H2O2 (1, 2 and 5 mM) in L-15 supplemented with glucose, dexamethasone, fetal bovine serum, and gentamicin for 2 h. They reported a decreased sensitivity of the hepatocyte cultures to H2O2-induced cell death and lipid peroxidation with increased culture age and linked the results to elevated GSH and/or vitamin E levels and decreased membrane polyunsaturated fatty acids as possible causes. This could have been caused by the differences in method, sensitivity of species, exposure medium and model compound type.
Conditions for using any fluorescent probe will clearly need to be optimized for each application or cell type, including buffer system or medium, temperature, concentrations and duration (Bracey et al. 1998). On the basis of the initial tests for the present study, optimal loading concentration and time for both probes at 15°C incubation were 10 μM and 30 min, respectively. This finding is in agreement with results reported by Misra and Niyogi (2009). They loaded the hepatocytes with 10 µM CM-H2DCFDA dissolved in dimethylformamide for 45 min at 15°C to measure ROS in cells exposed to selenite. The optimal conditions found in this study differ from the conditions used by Farmen et al. (2010), who loaded primary cultures of rainbow trout hepatocytes with 20 μM CM-H2DCFDA for 30 min at room temperature. Boone and Vijayan (2002) reported higher hsp70 accumulation over a 24-h period when primary cultures of trout hepatocytes were exposed to +15°C for 1 h as a heat shock. In our study, the final concentration of the solvent (DMSO) in the medium was also of concern, and never exceeded 0.01 %. DMSO is reported to be a radical scavenger and it was used to that effect by Manzl et al. (2004) in a study using primary cultures of rainbow trout hepatocytes exposed to Cu. Bopp et al. (2008) suggested it as a solvent for the fluorescent dyes, however, since they could not find any significant effect of DMSO in a dilution series from 7 up to 100 mM on ROS formation. Therefore, it may be used as solvent for the fluorescent probes.
In conclusion, there was a significantly higher ROS response in rainbow trout hepatocytes exposed to Cu in L-15 compared to TBS, using CM-H2DCFDA but not DHR 123. For some applications, a buffer may be preferable to cell medium during exposure to xenobiotics. The age of primary hepatocyte cultures significantly affected the development of ROS following Cu exposure in TBS, using DHR 123 and CM-H2DCFDA. The use of 1-day-old primary hepatocyte cultures is preferable to older cultures, at least when investigating oxidative stress.
References
Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L (2003) Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci 73:386–402. doi:10.1093/toxsci/kfg064
Bonney R (1974) Adult liver parenchymal cells in primary culture: characteristics and cell recognition standards. In Vitro 10:130–142. doi:10.1007/bf02615346
Boone AN, Vijayan MM (2002) Constitutive heat shock protein 70 (HSC70) expression in rainbow trout hepatocytes: effect of heat shock and heavy metal exposure. Comp Biochem Physiol Part C Toxicol Pharmacol 132:223–233. doi:10.1016/S1532-0456(02)00066-2
Bopp SK, Abicht HK, Knauer K (2008) Copper-induced oxidative stress in rainbow trout gill cells. Aquat Toxicol 86:197–204
Bracey D, Holyoak CD, Nebe-von Caron G, Coote PJ (1998) Determination of the intracellular pH (pHi) of growing cells of Saccharomyces cerevisiae: the effect of reduced-expression of the membrane H+-ATPase. J Microbiol Methods 31:113–125. doi:10.1016/s0167-7012(97)00095-x
Britton RS (1996) Metal-induced hepatotoxicity. Semin Liver Dis 16:3–12
Croci T, Wolliams GM (1985) Activities of several phase I and phase II xenobiotic biotransformation enzymes in cultured hepatocytes from male and female rats. Biochem Pharmacol 34:3029–3035
Ellesat KS, Yazdani M, Holth TF, Hylland K (2011) Species-dependent sensitivity to contaminants: an approach using primary hepatocyte cultures with three marine fish species. Mar Environ Res 72:216–224. doi:10.1016/j.marenvres.2011.09.003
Farmen E, Olsvik PA, Berntssen MHG, Hylland K, Tollefsen KE (2010) Oxidative stress responses in rainbow trout (Oncorhynchus mykiss) hepatocytes exposed to pro-oxidants and a complex environmental sample. Comp Biochem Physiol Part C Toxicol Pharmacol 151:431–438. doi:10.1016/j.cbpc.2010.01.008
Ferraris M, Radice S, Catalani P, Francolini M, Marabini L, Chiesara E (2002) Early oxidative damage in primary cultured trout hepatocytes: a time course study. Aquat Toxicol 59:283–296. doi:10.1016/s0166-445x(02)00007-3
Gille JJP, Joenje H (1992) Cell culture models for oxidative stress: Superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res/DNAging 275:405–414
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80. doi:10.1016/j.jbbm.2005.10.003
Grzelak A, Rychlik B, Bartosz G (2001) Light-dependent generation of reactive oxygen species in cell culture media. Free Radic Biol Med 30:1418–1425. doi:10.1016/S0891-5849(01)00545-7
Halliwell B (2003) Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett 540:3–6. doi:10.1016/S0014-5793(03)00235-7
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine. Oxford University Press, Oxford
Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM (1999) Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27:146–159. doi:10.1016/s0891-5849(99)00061-1
Heyne B, Beddie C, Scaiano JC (2007) Synthesis and characterization of a new fluorescent probe for reactive oxygen species. Org Biomol Chem 5:1454–1458
Jos A, Cameán AM, Pflugmacher S, Segner H (2009) The antioxidant glutathione in the fish cell lines EPC and BCF-2: response to model pro-oxidants as measured by three different fluorescent dyes. Toxicol In Vitro 23:546–553. doi:10.1016/j.tiv.2009.01.013
Kooy NW, Royall JA, Ischiropoulos H, Beckman JS (1994) Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16:149–156. doi:10.1016/0891-5849(94)90138-4
Krumschnabel G, Manzl C, Berger C, Hofer B (2005) Oxidative stress, mitochondrial permeability transition, and cell death in Cu-exposed trout hepatocytes. Toxicol Appl Pharmacol 209:62–73
Manzl C, Enrich J, Ebner H, Dallinger R, Krumschnabel G (2004) Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology 196:57–64
Misra S, Niyogi S (2009) Selenite causes cytotoxicity in rainbow trout (Oncorhynchus mykiss) hepatocytes by inducing oxidative stress. Toxicol In Vitro 23:1249–1258. doi:10.1016/j.tiv.2009.07.031
Nawaz M, Manzl C, Krumschnabel G (2005) Toxicity of copper, cadmium, and chromium to isolated hepatocytes from carp, Cyprinus carpio L. Bull Environ Contam Toxicol 75:652–661. doi:10.1007/s00128-005-0802-0
Nishimoto M, Roubal WT, Stein JE, Varanasi U (1991) Oxidative DNA damage in tissues of English sole (Parophrys vetulus) exposed to nitrofurantoin. Chem-Biol Interact 80:317–326. doi:10.1016/0009-2797(91)90091-K
Otto DME, Moon TW (1996) Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and non-polluted systems. Arch Environ Contam Toxicol 31:141–147. doi:10.1007/bf00203918
Pedrajas JR, Peinado J, López-Barea J (1995) Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu, Zn-superoxide dismutase as potential biomarkers. Chem-Biol Interact 98:267–282. doi:10.1016/0009-2797(95)03651-2
Pesonen M, Andersson TB (1997) Fish primary hepatocyte culture; an important model for xenobiotic metabolism and toxicity studies. Aquat Toxicol 37:253–267. doi:10.1016/S0166-445X(96)00811-9
Pourahmad J, O’Brien PJ (2000) A comparison of hepatocyte cytotoxic mechanisms for Cu2+and CD2+. Toxicology 143:263–273
Radice S, Fumagalli R, Chiesara E, Ferraris M, Frigerio S, Marabini L (2004) Estrogenic activity of procymidone in rainbow trout (Oncorhynchus mykiss) hepatocytes: a possible mechanism of action. Chem-Biol Interact 147:185–193. doi:10.1016/j.cbi.2003.12.006
Rau MA, Whitaker J, Freedman JH, Di Giulio RT (2004) Differential susceptibility of fish and rat liver cells to oxidative stress and cytotoxicity upon exposure to prooxidants. Comp Biochem Physiol Part C Toxicol Pharmacol 137:335–342
Rico D, Martín-González A, Díaz S, de Lucas P, Gutiérrez J-C (2009) Heavy metals generate reactive oxygen species in terrestrial and aquatic ciliated protozoa. Comp Biochem Physiol Part C Toxicol Pharmacol 149:90–96. doi:10.1016/j.cbpc.2008.07.016
Roy DN, Mandal S, Sen G, Biswas T (2009) Superoxide anion mediated mitochondrial dysfunction leads to hepatocyte apoptosis preferentially in the periportal region during copper toxicity in rats. Chem-Biol Interact 182:136–147. doi:10.1016/j.cbi.2009.08.014
Ruch RJ, Crist KA, Klaunig JE (1989) Effects of culture duration on hydrogen peroxide-induced hepatocyte toxicity. Toxicol Appl Pharmacol 100:451–464. doi:10.1016/0041-008X(89)90293-7
Schreer A, Tinson C, Sherry JP, Schirmer K (2005) Application of Alamar blue/5-carboxyfluorescein diacetate acetoxymethyl ester as a noninvasive cell viability assay in primary hepatocytes from rainbow trout. Anal Biochem 344:76–85. doi:10.1016/j.ab.2005.06.009
Sorg O (2004) Oxidative stress: a theoretical model or a biological reality? Comptes Rendus Biol 327:649–662
Tarpey MM, Wink DA, Grisham MB (2004) Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol 286:R431–R444. doi:10.1152/ajpregu.00361.2003
Tollefsen KE, Mathisen R, Stenersen J (2003) Induction of vitellogenin synthesis in an Atlantic salmon (Salmo salar) hepatocyte culture: a sensitive in vitro bioassay for the oestrogenic and anti-oestrogenic activity of chemicals. Biomarkers 8:394–407
Weglarz L, Bartosz G (1991) Hydralizine stimulates production of oxygen free radicals in eagle’s medium and cultured fibroblasts. Free Radic Biol Med 11:149–155. doi:10.1016/0891-5849(91)90165-Y
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161. doi:10.1016/0166-445x(91)90033-6
Wright J, George S, Martinez-Lara E, Carpene E, Kindt M (2000) Levels of cellular glutathione and metallothionein affect the toxicity of oxidative stressors in an established carp cell line. Mar Environ Res 50:503–508. doi:10.1016/s0141-1136(00)00125-2
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice-Hall/Pearson, Saddle River
Acknowledgments
This research was funded by the Faculty of Mathematics and Natural Sciences, University of Oslo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazdani, M., Paulsen, R.E., Gjøen, T. et al. Reactive Oxygen Species and Cytotoxicity in Rainbow Trout Hepatocytes: Effects of Medium and Incubation Time. Bull Environ Contam Toxicol 94, 193–198 (2015). https://doi.org/10.1007/s00128-014-1433-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1433-0