Abstract
The effect of temperature on HgCl2 (Hg2+)-induced oxidative stress to Chinese rare minnow (Gobiocypris rarus) was evaluated in vitro. Malondialdehyde (MDA) content and superoxide dismutase, catalase and glutathione peroxidase activities were determined in whole body homogenates incubated with 0.1 mg/L Hg2+ at 15, 25 and 35°C for 60 min. The result showed that oxidative stress was at a normal level in the Hg2+ + NT (0.1 mg/L Hg2+ and normal temperature, 25°C) and Hg2+ + LT (0.1 mg/L Hg2+ and low temperature, 15°C) groups, but a significant induction in oxidative stress occurred in the Hg2+ + HT (35°C) group. This was reflected by an increased level of MDA and decreased activities of the antioxidant enzymes. The results suggest that higher temperature enhances heavy metal toxicity in aquatic systems, which should be given more attention in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Temperature as an important abiotic factor has been shown to exert a strong influence on the toxic responses of organisms exposed to chemical compounds (Li et al. 2011a). As the sensitivity of organisms to toxicants may be modified by the alteration of physiological conditions, temperature stress may enhance adverse effects, particularly close to the thermal tolerance limits (Donker et al. 1998; Heugens et al. 2003). Until now, most studies reported were directed towards the effect of a single environmental factor on organism. However in areas receiving mixed effluents from various point and non-point sources, studies on combined effects of two or more stressors may provide valuable information that more closely represents the actual exposure scenarios faced by aquatic life.
Certain heavy metals are suspected to exert their toxic action on cells through oxidative damage. Under normal physiological condition, oxidative stressors are held in check by an antioxidative defense system (Verlecar et al. 2007; Li et al. 2011b). The antioxidant enzymatic system, involving the sequential and simultaneous action of enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), may scavenge the reactive oxygen species (ROS) induced by a metal (Li et al. 2010a). Depending on metal concentration, it can either inhibit or stimulate the activities of several antioxidative enzymes (Li et al. 2010b, 2011b; Rajeshkumar et al. 2013).
In polluted environments, fish are exposed to a variety of environmental stressors, such as heavy metals, organic pollutants and thermal releases, especially in areas where clusters of industries are located. Sewage and industrial outfalls may give rise to metal contaminants, of which Hg is considered to be highly toxic. In the present study, we investigated the effects of temperature on the antioxidant responses of Hg2+ in the Chinese rare minnow (Gobiocypris rarus) in vitro, focusing on the effect of temperature on Hg2+-induced oxidative stress and the activities of antioxidative enzymes.
Materials and Methods
HgCl2 was purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Deionized water was used as the vehicle to dissolve the chemical. All of the other chemicals used in this study were of analytical grade.
Larvae (7 day post-hatch) of the rare minnow were obtained from a local hatchery (Wuhan, China), and were raised in a flow-through system with dechlorinated tap water (pH 7.2–7.6; hardness 44.0–61.0 mg CaCO3/L) at a constant temperature (25 ± 1°C) with a photoperiod of 16:8 h (light:dark). They were fed with newly hatched brine shrimp (Artemia nauplii) two times daily. Waste and residue were removed daily, while the test equipment and chambers were cleaned once a week. All procedures and animal handling were in accordance with the guidelines approved by the Chinese Association for Laboratory Animal Sciences. The study was approved by the animal ethics committee of the Institute of Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences.
After acclimation, ten larvae per sample were homogenized in 0.4 mL ELISA buffer by using an ultrasonic cell disrupter for 5 min on ice (Scientz-IID, Ningbo, China). All processes were carried out at 0–4°C.
Subsequently, the larval homogenates with a final Hg2+ concentration of 0.1 mg/L were incubated under different temperatures (15, 25 and 35°C) for 60 min. These three temperatures were considered to represent a low temperature stress, normal temperature, and a high temperature stress, respectively. One group exposed to clean buffer was used as the control. Each experimental condition was duplicated. The incubation reaction was immediately terminated by placing the homogenate on ice at the end of experiment. At the beginning and end of the test, the Hg2+ concentrations were measured as 0.10 ± 0.01 and 0.09 ± 0.01 mg/L, respectively, by using atomic fluorescence spectrometry (AFS-2000, Beijing, China).
The homogenates were centrifuged at 3,000 rpm and 4°C for 10 min (Eppendorf Centrifuge 5427R, Hamburg, Germany) to obtain the supernatant for the enzyme activity assays of SOD (EC 1.15.1.1), CAT (EC 1.11.1.6) and GPx (EC 1.11.1.9); and the measurement of MDA concentration. All the biochemical parameters were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols. Protein level was measured at 595 nm by the Bradford method with Coomassie Brilliant Blue G-250, using bovine serum albumin as a standard. All the measurements were carried out using an ultraviolet–visible spectrophotometer (Mapada-UV1800, Shanghai, China).
All statistical analyses were performed with SPSS, version 13 (IBM, Armonk, NY, USA). All values were expressed as mean ± SD and analyzed by SPSS for Win 13.0 software. One-way ANOVA followed by Tukey’s test was used to determine whether results of treatments were significantly different from the control group (p < 0.05). In addition, principal component analysis (PCA) was used to define the most important parameters, which could be used as key factors for individual variations using Statistic 6.0 (StatSoft, Inc. Tulsa, OK, USA).
Results and Discussion
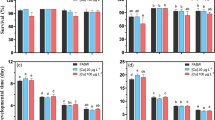
In order to verify the changes of the Hg2+-induced oxidative stress in fish larvae homogenates at the different temperature, MDA as a marker of oxidative stress and the activities of a series of antioxidant enzymes (SOD, CAT and GPx) were measured (Figs. 1, 2). Although a slight hint for an increase of oxidative stress indices was observed, there was no significant induction among control, Hg2+ + NT (0.1 mg/L Hg2+ and normal temperature, 25°C) and Hg2+ + LT (0.1 mg/L Hg2+ and low temperature, 15°C) groups during the incubation period. However, a significant induction of oxidative stress was observed in the Hg2+ + HT (0.1 mg/L Hg2+ and high temperature, 35°C) group, as reflected by an increase in MDA level and decreases in enzyme activities.
Effect of temperature on Hg2+-induced MDA level in whole body of Chinese rare minnow larvae in vitro. Note: Hg2+ + NT—0.1 mg/L Hg2+ and normal temperature, 25°C; Hg2+ + LT—0.1 mg/L Hg2+ and low temperature, 15°C; Hg2+ + HT—0.1 mg/L Hg2+ and high temperature, 35°C. n = 10. Asterisk denotes significant difference relative to the control mean (p < 0.05)
Effect of temperature on the activities of antioxidant enzymes in whole body of Chinese rare minnow larvae in vitro. Note: SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase. Other information is the same as in Fig. 1
Among abiotic factors, thermal stress is a physiological disorder in animals, which directly affects metabolism and results in the accumulation of ROS (Hwang and Lin 2002; Rajagopal et al. 2005; Li et al. 2011a). The oxidative stress results from a disturbance of the balance between ROS production and neutralization. In the Hg2+ + NT group, the antioxidant enzymes activities were induced significantly, which showed that the antioxidant defense system was evoked and could neutralize ROS under Hg2+ stress. However, the activities of all the antioxidant enzymes were inhibited in Hg2+ + HT group, especially CAT. Together with the significant higher ROS level in this group, our results indicated that temperature enhanced Hg2+-induced toxicity, likely by increasing the production of ROS and inhibiting the antioxidative defense system. Our results are in agreement with those of Verlecar et al. (2007) and Parihar et al. (1997) on oxidative stress of fish under the stress of higher temperatures. The possible reasons may be: (1) the enzymatic activity in nature at 25°C was more active than that at higher temperature; (2) higher temperature enhanced oxygen consumption, which may have increased ROS production as side-products of intensified metabolism, thereby resulting in oxidative stress (Li et al. 2011a). Moreover, the normal level of ROS was measured in Hg2+ + LT group, which may be a result of decreasing heavy metal toxicity under lower temperature by lower metal uptake at uptake sites on membranes, or a decrease in the release of facilitating molecules. However, SOD and GPx activities were induced significantly in Hg2+ + LT group after incubation.
Correlation coefficients for the parameters that were measured are presented in Table 1. Based on the data, temperature and the oxidative stress marker, MDA, were positively related, while temperature and antioxidative enzyme activities were negatively related.
In order to examine the discriminating power of the set of studied biomarkers and the variable temperature, we carried out principal component analysis (PCA). In the present study, all the parameters measured in the present study were distinguished on the ordination plots corresponding to the first (76.99 %) and second (18.17 %) principle components (Fig. 3).
In conclusion, higher temperature enhanced Hg2+- induced oxidative stress in Chinese rare minnow in vitro, through increasing the ROS level and inhibiting the antioxidant enzymes activities. However, ROS levels were in a state of near equilibrium in the normal and low temperature groups. Statistical analyses showed that temperature correlated positively with ROS production, but negatively with antioxidant activity. Based on our results, we conclude that higher temperatures may increase the toxicity of certain heavy metals in fish. As interactions between abiotic and biotic factors are common in the aquatic environment, we should give more attention to the combined effects of temperature and heavy metals in field studies.
References
Donker MH, Abdel-Lateif HM, Khalil MA, Bayoumi BM, Van Straalen NM (1998) Temperature, physiological time, and zinc toxicity in the isopod Porcellio scaber. Environ Toxicol Chem 17:1558–1563
Heugens EH, Jager T, Creyghton R, Kraak MH, Hendriks AJ, Van Straalen NM, Admiraal W (2003) Temperature-dependent effects of cadmium on Daphnia magna: accumulation versus sensitivity. Environ Sci Technol 37:2145–2151
Hwang D-F, Lin T-K (2002) Effect of temperature on dietary vitamin C requirement and lipid in common carp. Comp Biochem Physiol B Biochem Mol Biol 131:1–7
Li ZH, Li P, Randak T (2010a) Effect of a human pharmaceutical carbamazepine on antioxidant responses in brain of a model teleost in vitro: an efficient approach to biomonitoring. J Appl Toxicol 30:644–648
Li ZH, Li P, Dzyuba B, Randak T (2010b) Influence of environmental related concentrations of heavy metals on motility parameters and antioxidant responses in sturgeon sperm. Chem Biol Interact 188:473–477
Li D, Zhou D, Wang P, Li L (2011a) Temperature affects cadmium-induced phytotoxicity involved in subcellular cadmium distribution and oxidative stress in wheat roots. Ecotoxicol Environ Saf 74:2029–2035
Li ZH, Li P, Randak T (2011b) Evaluating the toxicity of environmental concentrations of waterborne chromium (VI) to a model teleost, Oncorhynchus mykiss: a comparative study of in vivo and in vitro. Comp Biochem Physiol C 153:402–407
Parihar MS, Javeri T, Hemnani T, Dubey AK, Prakash P (1997) Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature. J Therm Biol 22:151–156
Rajagopal S, Van der Gaag M, Van der Velde G, Jenner HA (2005) Upper temperature tolerances of exotic brackish-water mussel, Mytilopsis leucophaeata (Conrad): an experimental study. Mar Environ Res 60:512–530
Rajeshkumar S, Mini J, Munuswamy N (2013) Effects of heavy metals on antioxidants and expression of HSP70 in different tissues of milk fish (Chanos chanos) of Kaattuppalli Island, Chennai, India. Ecotoxicol Environ Saf 98:8–18
Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact 167:219–226
Acknowledgments
This study was supported by China Three Gorges Project Corporation (No. 07011034), by the Ministry of Education, Youth and Sports of the Czech Republic—projects “CENAKVA” (No. CZ.1.05/2.1.00/01.0024), “CENAKVA II” (No. LO1205 under the NPU I program) and the project P503/11/1130 of the Grant agency of Czech Republic.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, ZH., Li, P. & Chen, L. Temperature Affects Hg-Induced Antioxidant Responses in Chinese Rare Minnow Gobiocypris rarus Larvae In Vitro. Bull Environ Contam Toxicol 93, 666–669 (2014). https://doi.org/10.1007/s00128-014-1399-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1399-y