Purpose
Depression is one of the most common mental disorders and substantially decreases socioemotional well-being and health-related quality of life. Analyzing temporal patterns in depressive symptoms can reveal emerging risks that require attention and have implications for mental health promotion. The present study disentangled age, period, and cohort (APC) effects on trends in depressive symptoms and their gender disparities among China’s nationally representative samples of middle-aged and older adults.
Methods
Using four-wave data (2011, 2013, 2015, and 2018) from the China Health and Retirement Longitudinal Study (N = 65455), APC effects were quantified based on the hierarchical APC model. The 10-item Center for Epidemiologic Studies Depression Scale (CES-D-10) was used to measure depressive symptoms.
Results
Depressive symptoms increased during late life and stabilized after reaching an advanced age. After further adjusting for individual characteristics, depressive symptoms exhibited a negative trend with advancing age. The mean levels of depressive symptoms remained stable during the study period. Depressive symptoms varied significantly across cohorts, with those born in 1949–1951 having the most severe depressive symptoms. Significant life-course and cohort variations existed in the gender gaps in depressive symptoms. Although women had higher mean scores on the CES-D-10 scale throughout the life course, the gender gaps in depressive symptoms gradually narrowed with age, as depressive symptoms decreased more rapidly among women. A widening trend in gender gaps in depressive symptoms was found among those born after the mid-1950s, mainly driven by a notable decline in depressive symptoms among men
Conclusions
The convergence of living conditions between genders in late life, as a result of traditional Chinese culture, may have narrowed the gender gap in depressive symptoms. However, given the widening gender disparities in depressive symptoms among younger cohorts, more attention should be paid to women’s mental health in the context of China’s rapid socioeconomic development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a common mental disorder and a major global health issue, projected to be one of the leading causes of disability by 2030 [1]. The burden of depression is widespread and diverse, including reduced overall health status [2], decreased socioemotional well-being [3], and a higher risk for suicidal behavior [4]. Research has shown a spectrum of depressive symptoms progressing to diagnosed depressive disorders, marked by increasing symptom severity and psychosocial impairment [5]. Depressive symptoms are more common than diagnosed depressive disorders but can still cause significant distress and functional impairment [6]. A recent nationally representative survey in China indicates a high prevalence of depressive symptoms (24.1%) among adults over the age of 45, surpassing rates in European countries [7]. Furthermore, significant increases in depressive symptoms were observed over the past decade, especially among the non-elderly [8]. The above findings indicate that the dynamic epidemiological status of depressive symptoms in Chinese middle-aged and older people requires attention.
Understanding trends in health outcomes over time involves estimating age, period, and cohort (APC) effects [9]. Specifically, age effects refer to developmental changes associated with aging over the life course, while time-related trends across different periods or birth cohorts represent exogenous contextual changes in broader social conditions [10]. Although some age-period-cohort (APC) analyses have been conducted in China [11, 12], these studies focused on depressive disorders and did not fully consider individual characteristics. Other research used the multilevel model framework to assess the effect of age, birth cohort, and individual characteristics in the trajectories of depressive symptoms [13, 14]. However, period effects were not controlled due to the limitations of the model. Therefore, it is necessary to decompose trends in depressive symptoms by APC effects within an integrated analytical framework, while also exploring the potential impact of individual characteristics.
Meta-analyses consistently indicate that the prevalence and levels of depressive symptoms in middle-aged and older Chinese people have risen from the 1980s to the 2010s [15, 16]. This phenomenon may be explained by the erosion of traditional culture and family values resulting from rapid industrialization and urbanization [17], which indicates a significant period effect. Cohort effects may also play a role, as younger cohorts express psychological stress more openly than older ones, influenced by changing emotional norms and reduced stigma around mental health [18, 19]. However, over the past ten years, a general downward trend in depressive symptoms was found in longitudinal studies [20, 21]. Researchers argued that improved economic conditions and social welfare may have counteracted the effects of weakened family support contributing to the increasing tendency for depressive symptoms [22]. Beyond that, little is known whether there are other potential mechanisms in terms of period and/or cohort effects responsible for this different trend.
Considering age effects, physiological changes within the body may influence mental health and increase susceptibility to depression. For instance, fluctuations in hormone levels could partially account for variations in depression rates across different age groups [23, 24]. In contrast to the positive association between age and depressive disorders, whether age plays a role in depressive symptoms in late life is unclear [25, 26]. Inconsistent results were also found in China—while some cross-sectional studies show a positive association between age and depressive symptoms after controlling for confounders [27, 28], others report no association or even a negative one [7, 29]. These inconsistent findings may be due to differences in sample representativeness, measurement methods, mortality rates, and most importantly, whether cohort effects were considered. As cohort effects may contribute more to the frequency of mental disorders in later life compared to earlier stages of life [30], the observed associations between age and depressive symptoms without controlling for cohort effects possibly reflect cohort differences in status, not necessarily age changes in status [31].
Studies in Western countries have found higher mean levels of depressive symptoms in more recent cohorts [32, 33]. Similar findings were reported in China [13], although another study showed the opposite trend [14]. Recent cohorts in China may have been better protected against the risk of depressive symptoms with the development of society over the past decade. These developments include the establishment and improvement of mental health services [34], the promulgation and implementation of mental health legislation [35], and the execution of scientific and technological projects [36] aimed at enhancing mental well-being. However, on the other hand, they might be more affected by adverse life events compared to those born earlier due to the dramatic sociocultural transformation [19]. Thus, cohort effects of depressive symptoms later in life need to be precisely, focusing on cohort variations among those born later.
The female excess in depressive symptoms has been documented in studies from different countries and populations [37,38,39]. The diathesis-stress model explains the interaction between gender and stressors in relation to mental health [40]. According to this mode, if either or both of the vulnerability [39, 41] and stressors [37, 38] are more prevalent in women, gender differences in depressive symptoms would result. However, age variations on gender disparities in depressive symptoms in later life show different patterns [42,43,44]. The gender stratification theory suggests that the cumulative effects of gendered socialization and the stabilization of gender roles after retirement may be significant factors [45]. A recent analysis of repeated cross-sectional data from European countries found that the accumulation of disadvantages for women’s mental health surpassed the effects of age-related adoption of social positions, leading to growing gender gaps in depressive symptoms among older adults [42]. In contrast, longitudinal studies have found that these gender gaps level off or even converge in old age [43, 44], which might be due to more negative changes in lifestyle and social networks among older men, counteracting previous advantages in mental health.
In studies focused on Chinese populations, evidence suggests that gender differences in depressive symptoms are less apparent in later life [18, 46]. Researchers suggested that the China’s unique social-cultural background may be one possible explanation — both women and men are treated equally as role models and knowledgeable leaders as they enter old age [47], resulting in no obvious gender differences in socioeconomic status and stressful conditions. Considering cultural factors may have a unique impact on the mental health of Chinese older populations, more evidence is needed to determine if gender differences in depressive symptoms among older Chinese adults exhibit a divergent age pattern compared to Western countries. Additionally, the unclear cohort trends in depressive symptoms among younger Chinese generation raise the question of whether gender differences vary across birth cohorts.
To effectively prevent mental disorders and promote mental health, it is crucial to gain a comprehensive understanding of how depressive symptoms are evolving and differing between genders. Analyzing these trends necessitates addressing the potential confounding effects of age, period, and cohort [10]. However, current research on these issues in China is notably insufficient. To fill this gap, using nationally representative microdata, the present study intends to simultaneously disentangle APC effects on trends in depressive symptoms among middle-aged and older adults in China and further explore whether there are gender disparities in the APC effects.
Materials and methods
Data and study sample
The data used in this study is from the China Health and Retirement Longitudinal Study (CHARLS). CHARLS is a multiple-wave panel study that follows cohorts of community-dwelling people aged 45 and over every 2–3 years beginning in 2011. Successive waves were collected in 2011, 2013, 2015, and 2018. The 2011 baseline survey conducted a stratified multi-stage probability-proportional-to-size (PPS) random sampling method to obtain a nationally representative sample of Chinese residents aged 45 years or older in 450 villages/urban communities, 150 countries/districts, and 28 provinces. Specifically, 150 county-level units were randomly selected with a PPS sampling technique from a sampling frame including all county-level units fell within 28 provinces (excluding Tibet). The sample was stratified by region, within the region by urban district and rural country, and by gross domestic product per capita. In each county-level unit, 3 primary sampling units (villages in rural areas and neighborhoods in urban areas) were randomly chosen, using PPS sampling [48]. The response rate was 80.5% in 2011 and remained at a high level in 2013, 2015, and 2018, which were 82.6%, 82.1%, and 83.8%, respectively. To maintain the sample’s age representation, refreshment samples from the baseline survey were added in the following waves. If the baseline respondents lived with someone aged between 40 and 44 in the same household, he/she was reserved for a refresh sample. In 2013 (wave 2), respondents aged 43–44 at baseline (with their spouses) were added from the refreshment sample. Using the same strategy, those who were aged 41–42 and 40 at baseline were added in 2015 (wave 3) and 2018 (wave 4) as refreshment samples, respectively [49]. The survey provides information about the respondents’ physical, mental, and cognitive functioning and sociodemographic characteristics.
As shown in Fig. 1, the raw CAHRLS data set contains 77,233 individuals (17,708, 18,612, 21,097, and 19,816 in 2011, 2013, 2015, and 2018, respectively). After excluding those under 45 years old and observations with missing information on age/assessment of depressive symptoms/survey weight, 65,455 observations were obtained as the study population (15,382, 15,733, 18,109, and 16,231 in 2011, 2013, 2015, and 2018, respectively). Among 65,455 observations, 18,567 individuals had missing values on covariates. To reduce the potential bias of missing values and maximize the utility of the available data, we imputed the missing values using the method of multiple imputations by chained equations [50].
Measurements of depressive symptoms
Depressive symptoms were measured using the 10-item Center for Epidemiologic Studies Depression Scale (CES-D-10) in CHARLS [28]. The ten items referred to the depressive feelings and behaviors of the respondents during the last week, including eight negatively oriented questions and two positive questions. Each question has four options: rarely or none of the time (< 1 day), some or a little of the time (1–2 days), occasionally or a moderate amount of the time (3–4 days), and most or all of the time (5–7 days), which were counted as 0, 1, 2 and 3 scores respectively. So the total depressive score ranges from 0 to 30, with a higher score indicating more severe depressive symptoms [51]. The validity of the CES-D-10 has been examined among Chinses adults [52], and previous studies have shown this scale has good reliability and discrimination [53].
Independent variables
Two sets of variables were included in our analyses: individual-level variables (i.e., level-1) and contextual variables (i.e., level-2). The key individual variable was age and gender. Other individual characteristics that are shown to be correlated with the risk of depressive symptoms were regarded as control variables, which were divided into three broad categories — socio-demographic characteristics, socioeconomic status (SES), and health status. The period and birth cohort were regarded as contextual variables. Specifically, the period was determined by survey years from 2011 to 2018 and generated as a four-category variable. The birth cohort was determined by three-year cohorts ranging from 1910 to 1973. To ensure an adequate sample size, the oldest cohort included twenty-one years (from 1910 to 1930), and the youngest included four years (from 1970 to 1973). As a result, respondents’ birth cohorts were divided into 15 groups. The operational definitions of all variables used in the analyses are included in Table S1 (see Appendix file).
Statistical analyses
Our analyses begin with a descriptive analysis of the study variables for each survey and then postulate the cross-classified random-effect modeling (CCREM) specification of the hierarchical APC models proposed by Yang and Land [54]. The CCREM is a two-level model — individual characteristics are level-1 variables, and period and cohort are level-2 variables. The level-1 model takes the following form:
where \({Dep}_{ijk}\) stands for the depressive score of the respondent \(i\) (for \(i=1, \dots , {n}_{jk}\)) within period \(j\) (for \(j=1, \dots , 4\)) and cohort \(k\) (for \(k=1, \dots , 15\)). \({Age}_{ijk}\) represents age, considering the non-linear relationship between depressive symptoms and age among older adults, age-cubed (\({Age}_{ijk}^{3}\)) is also added in the model [26, 55]; \({Gender}_{ijk}\) denotes gender variable; \(X\) denotes the vector of other individual variables such as control variables; \({\beta }_{1jk}, \dots , {\beta }_{pjk}\) are level-1 fixed effects and \(P\) is the maximum number of independent variables; \({\beta }_{0jk}\) is the intercept indicating the mean score on the CES-D-10 for the reference group at mean age who were nested in period \(j\) and cohort \(k\), and \({e}_{ijk}\) is the random individual effect. We can test the life course hypotheses about age variations in gender disparities in depressive symptoms by including the age-by-gender interaction term in \(X\) [56].
Level-2 random intercept and coefficient embedded in Eq. 1 may be expressed as follows:
Equation 2.1 is the model for the random intercept \({\beta }_{0jk}\) which specifies the overall mean depressive score (\({\gamma }_{0}\)) varies by time (period effect, \({\mu }_{0j}\)) or birth cohort (cohort effect, \({\nu }_{0k}\)), \({\mu }_{0j}\) and \({\nu }_{0k}\) are estimated as level-2 residual random effects. To test whether gender disparities in depressive symptoms vary by period or cohort, Eq. 2.2 specifies that the coefficient of gender (\({\beta }_{3jk}\)) has cohort effects (\({\mu }_{3j}\)) or period effects (\({\nu }_{3k}\)), with \({\gamma }_{3}\) is the fixed sex effect on the overall mean score of CES-D-10 scale.
The combined models in Eqs. 1 and 2 estimated linear CCREMs using SAS PROC GLIMMIX [57]. Bayesian Information Criterion (BIC) statistics were used to compare the goodness of fit between models. All analyses, including descriptive tables and regressions, are weighted using individual sample weights adjusting for non-response.
Results
Characteristics of the study sample
The characteristics of the study sample by survey wave are given in Table 1. The depressive scores remained stable, ranging from 7.84 to 8.50 (P = 0.149). The mean age increased monotonically, from 59.11 in 2011 to 61.67 in 2018 (P = 0.008). The distribution of sociodemographic variables did not change significantly between 2011 and 2018. In each wave, the study sample had a larger proportion of females, those who lived in the east region, lived in rural areas, and were married. SES, including education level, expenditure per capita, and health insurance coverage increased significantly by survey wave, except for occupational status. Although the percentage of nonworking declined monotonically between 2011 and 2015 (from 41.7% to 37.1%), a slight rebound was found in 2018 (39.3%). Regarding health status, the proportion of those who were dependent in activities of daily living (14.2% to 16.4%) and were dependent in instrumental activities of daily living (18.1% to 2.09%) fluctuated during the study period. A strong upward trend was found in the percentage of those who had more than one chronic disease (from 69.2% to 80.8% between 2011 and 2018).
Overall APC effects
The gross and net APC effects on depressive symptoms are shown in Table 2 (Model 1 and Model 2, respectively), and predicted depressive scores are displayed in Fig. 2 to illustrate the findings.
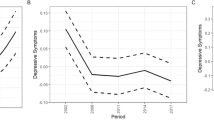
Model 1 shows a significant gross age effect (\({\beta }_{age}\) = 0.52, P < 0.001; \({\beta }_{{age}^{3}}\) = − 0.05, P = 0.003) net of period and cohort effects. Figure 2(A1) shows a curvilinear relationship between age and depressive symptoms. Predicted depressive scores increase from ages 45 to 80, but after 80, the scores stabilize and then begin to decline. As shown in Model 2, after controlling for all individual characteristics, age remains a significant predictor, but the direction of its association reverses (\({\beta }_{age}\) = – 0.40, P < 0.001), indicating a decrease in depressive symptoms with increasing age. The coefficient of age-cubed remains unchanged (\({\beta }_{{age}^{3}}\) = – 0.05, P = 0.002), reinforcing this trend. Figure 2(A2) illustrates this finding, showing a monotonic decrease in depressive symptoms as age increases, which is especially pronounced among the oldest old.
The estimates of random effects in terms of variance components in level 2 indicated no significant differences in the mean levels of depressive symptoms across periods. However, significant gross cohort effects were found (Model 1: \(\sigma\) = 0.067, P = 0.023) after adjusting for age and period effects, and the net cohort effects remained significant when control variables were considered (Model 2: \(\sigma\) = 0.093, P = 0.019). As shown in Fig. 2(B1) and (B2), depressive scores were lower in 2013 and 2015, regardless of whether controlling for individual characteristics. The estimated gross cohort effects are shown in Fig. 2(C1). Those born in 1949–1951 had the most severe depressive symptoms. After that, depressive symptoms showed a fluctuating downward trend and a rapid decline was found among those born after 1967. For those born before 1949, there was a decline in depressive scores from the pre–1930 cohort to the 1934–1936 cohort, followed by an overall upward trend, and predicted net cohort effects from Model 2 exhibit nearly the same trend (see Fig. 2(C2)). However, the cohort trends estimated from Model 2 are less fluctuating than that from Model 1.
What’s more, results from Model 2 show that all individual characteristics are significantly associated with depressive symptoms and consistent with previous studies [58]. The smaller BIC statistics indicate a better model fit for Model 2 than Model 1 (417,943 vs. 431,931).
Age variation in gender on depressive symptoms
Figure 3(A1) shows that gender differences in depressive symptoms remain stable throughout middle and old age, as the interaction effect between and age and gender is not significant (\({\beta }_{age\times female}\) = 0.04, P = 0.460) in Model 3, which only considers period and cohort effects. However, after adjusting for all individual characteristics in Model 4, a significant interaction effect emerges (\({\beta }_{age\times female}\) = − 0.15, P < 0.001). This indicates that although women have higher mean scores on the CES-D-10 scale (\({\beta }_{female}\) = 1.07, P < 0.001) throughout the life course, the gender gap in depressive symptoms gradually narrows with age. Specifically, depressive symptoms decrease more rapidly among women in late life, as illustrated in Fig. 3(A2).
Cohort variation in gender on depressive symptoms
Model 5 in Table 3 estimates the cohort variations (in terms of variance components) in gender disparities in depressive symptoms, controlling for age and period effects. Model 6 further controls for individual-level characteristics. The present study did not explore the gender disparities in depressive symptoms over time, as no significant overall period effects were found. Model 5 presents significant cohort variations in gender disparities net of age and period effects (\({\sigma }_{female}\) = 0.102, P = 0.044). In Model 6, after adjusting for individual-level variables, the cohort effects between genders remain significant (\({\sigma }_{female}\) = 0.104, P = 0.039). Based on Model 6 estimates, Fig. 4 shows that gender disparities increased from the pre–1930 birth cohort to the 1937–1939 cohort and then stabilized until the 1952–1954 cohort. After a rapid decline for birth cohorts between 1952–1954 and 1955–1957, the gender disparities in depressive symptoms widened dramatically for those born after the mid–1950s.
Sensitivity analyses
We conducted some robustness checks. First, we used list deletion for missingness on covariates to obtain a completely observed sample (N = 46,888). Then we ran all of the above models using complete cases. Comparing estimated results in Tables 2, 3 with Table S2 in Additional file and visualized results in Figs. 2, 3 with Figures S1–S3 in Additional file, we found no meaningful differences. Although the variance components of the cohort effect were smaller in models using complete cases, indicating smaller overall cohort variations and cohort effects between genders, the general pattern exhibited consistency (Fig. 4 vs. Figure S3 in Additional file). So our results were not biased by imputed values.
Second, we set age as a categorical variable for the 5-year-old group (aged 75 and over as the open-end age group) and reran the models. Table S3 and Figures S4-S6 in Additional file show that these additional analyses did not yield substantively different results, so the reported findings were confirmed again.
Third, considering the nonsignificant and nearly linear period trend, we further explored the necessity to include period effects in the analyses. Therefore, we constructed a new model based on Model 2 but excluding period effects. Table S4 in Additional file shows that, without controlling for period effects, the absolute value of \({\beta }_{age}\) decreased apparently (from 0.40 to 0.27, decreased by 32.5%), and the variance component of cohort effect increased (from 0.093 to 0.119, increased by 28.0%), which suggesting a weaker linear age effect and increased cohort variations. Moreover, the bigger BIC suggests an unimproved model fit (418,920 vs. 417,943). The above results illustrate that period effects should be controlled in the current study; otherwise, biased estimates of age and cohort effects may be generated.
Discussion
Based on four waves of panel data spanning seven years from a nationally representative community sample of people aged 45 and over, this study disentangles the confounding effects of APC and explores the trends in gender differences in depressive symptoms with age and across cohorts. While gross age effects suggest that depressive symptoms increase with age and then stabilize, adjusting for individual characteristics reveals that age trajectories turn negative. Although no significant period effects were found, the cohort effects indicate that depressive symptoms followed a U-shaped pattern, with the 1949–1951 cohort having the highest depressive scores. Significant life-course and cohort variations exist in the gender gaps in depressive symptoms. Women exhibit higher levels of depressive symptoms than men. After controlling for period effects, cohort effects, and individual characteristics, gender disparities decrease with age. However, a pronounced widening trend in gender disparities is observed among those born after the mid-1950s.
Is being older depressing? In the present study, the gross age effects indicate no more frequent depressive symptoms among the oldest age, which aligns with some longitudinal studies in China [13, 14]. The following factors may explain the findings. Firstly, sample bias may obscure the effect of aging. Specifically, both depression and its risks may reduce survival, thus selective mortality might tend to remove those with elevated depressive symptoms, which is more pronounced among the oldest old [59]. Moreover, The magnitude of depressive symptoms may be underestimated because older institutional residents, who typically exhibit significantly higher levels of depressive symptoms compared to their community-dwelling counterparts [60], were excluded from the survey. The second factor may be item bias. Older adults are more likely to attribute somatic depressive symptoms to physical illness rather than to depressed mood [61]. The CES-D-10 used in the CHARLS predominantly focuses on “depressed mood” and “negative mood” factors [53]. Therefore, we argue that the scale’s lack of assessment of somatic symptoms may lead to an underestimation of depressive symptoms in the older population. Finally, life satisfaction, a significant predictor of depressive symptoms in middle-aged and older adults [7, 62], has been shown to remain relatively stable from late adulthood to old age [63,64,65]. For Chinese older adults, cultural aspects (e.g., “Zhi Zu Chang Le” — knowing satisfaction leads to constant happiness) may lead to a greater likelihood of positive self-feelings of current life [63], but whether the above mechanisms are also present in other populations requires additional new evidence in the future.
After adjusting for individual-level characteristics, the declining curve of net age effects may suggest the “age as maturity perspective” [66]. Specifically, controlling for life course patterns of social and economic status and for progressively decline in physical function with age, the age trajectory of depressive symptoms shows a monotonically decline. The more ordered lives, more compatible relationships, and more work and life experiences that come with age may shift the quality of emotions among older adults from negative (depression) to positive (agitation) [67]. A longitudinal study based on a population of older Americans similarly found the above pattern, but linear model specifications resulted in a non-significant net age effect [33]. The present study detected more and significant age effects by using nonlinear terms for age. In summary, the balance between maturity and decline forms the basis of gains and losses over the social life cycle, with the gradual decline in physical function offsetting the gains in mental well-being from greater maturity.
Considering period and cohort effects, although no significant period effects were found in this study, the net period effect suggested a decreasing trend in depressive symptoms between 2011 and 2015 (see Fig. 2(B2)), aligning with previous studies using the same datasets [20, 21]. However, regardless of whether individual characteristics were controlled for, levels of depressive symptoms rebounded in 2018, approaching the levels observed in 2011. We speculate that the higher SES levels in the 2018 sample compared to 2011, combined with significantly poorer overall health status, may have resulted in similar gross and net period effects between the two survey waves (see Table 1). Overall, the U-shaped trend in depressive symptom levels observed between 2010 and 2018 was similarly confirmed in another Chinese population survey [8]. The increasing trend in depressive symptoms found among those born before 1949 may reflect the negative impact of war and social unrest during their early life, as early exposure to these adversities increases the risk of mental disorders in later life [68]. The highest levels of depressive symptoms in the 1949–1951 cohort may reflect the “cumulative effect” of depression risks across their lifespan [69, 70], such as the Great Famine of 1959–1961 in childhood, the Cultural Revolution of 1966–1976 in youth, and the Financial Crisis of 1998 and 2008 in adulthood. The decrease in depressive symptoms across successive cohorts born after 1960 may benefit from rapid economic expansion, improved health services, and enhanced living conditions. However, the downward trend was less evident among those born before the 1970s, which may have been affected by the Cultural Revolution [71].
In examining the age effect and its gender differentiation, results suggest that gender disparities in depressive symptoms remain stable with age when considering only period and cohort effects. This aligns with meta-analyses of data from over 90 nations, showing similar trends in gender gaps during adulthood [72]. After controlling for individual characteristics, the gender gaps decrease slowly with age. This finding suggests that the long Chinese tradition of honoring older family members may lead to a convergence of life conditions for women and men in old age, thus attenuating the effect of gender stratification in earlier life stages [46]. Moreover, the overall reduction in gender differences in depressive symptoms after controlling for individual characteristics suggests that women’s greater exposure to social and material disadvantages and poor health over the life course leads to higher levels of depressive symptoms, which supports the differential exposure hypothesis [39]. However, which exposure factors play a major role in gender gaps, and what are the mechanisms behind the unexplained parts of the differences? More focused longitudinal studies are needed in the future.
Recent studies in China and high-income countries have shown that gender gaps in depressive symptoms are narrowing among younger cohorts [14, 73, 74]. However, our study found the opposite pattern—gender disparities have widened among those born after 1955, primarily due to a decrease in depressive symptoms among men. A longitudinal study of U.S. adults similarly found that gender differences in depressive symptoms widened across cohorts born in 1925–1964, due to steeper increases in mean CES-D-10 scores among women [75]. Authors suggested that women born around the 1940s experienced unanticipated social pressures related to balancing work and family, marital dissolution, and financial constraints, reducing their perceived well-being. The applicability of the above mechanisms to Chinese women should be treated with caution due to differences between the two countries. The U.S. study only considered the linear form of the cohort effect, thus missing the pattern of rapid decline in depressive symptoms for men in the most recent cohort, as found in our study. Considering that the overall cohort effect showed a decreasing trend in the younger cohort (Fig. 2(C2)), we speculate that although social modernization and rising living standards from economic reforms benefited many, the distinctly gendered nature of family and social roles may have prevented women from equally enjoying these positive effects [76]. Future research may need to further explain cohort variations in gender gaps in depressive symptoms from a macro perspective, considering factors like macro-level gender equality and relative gendered social position [42, 73].
A weakness of this study is that we used data that only covered an observation window of seven years, which is difficult to provide a whole picture of recent period effects. Future studies drawing on longer periods would be required to detect a more accurate representation of the differences in depressive symptoms by period. Furthermore, we cannot examine exogenous factors that may explain overall and gender-specific cohort patterns due to the lack of macro-level data before the founding of the People’s Republic of China. Further research should focus on the relationships between macroeconomic variables, cohort characteristics, and cohort changes in depression [10]. Finally, given the significance of mental health issues among younger adults, understanding the prevalence and determinants of depressive symptoms in this demographic is crucial for informing targeted interventions and policies. Without data on individuals under 45 years of age, our study may not fully capture the nuances of depressive symptomatology across the adult lifespan. Future research endeavors should consider utilizing datasets that encompass a broader age range or conducting dedicated studies targeting this demographic to provide a more comprehensive understanding of trends in depressive symptoms in China. Nevertheless, in the absence of APC analyses of trends in depressive symptoms based on individual-level data in China, this study offers significant insights into the age and cohort effects of depressive symptoms among middle-aged and older adults, as well as their gender differences. These findings will aid in understanding the specific impact of individual factors on depressive symptoms in later life and the influence of major historical events and social developments on the mental well-being of different generations.
Conclusion
Our findings provide more comprehensive evidence of life course and birth cohort changes in depressive symptoms and its gender disparities among middle-aged and older Chinese. Results suggest no more frequent depressive symptoms among the oldest age net of period and cohort effects, which may be caused by methodological biases or a reflection of a long and healthy living among the oldest old in China. Depressive symptoms did not show a significant trend during the study period. The social chaos during the founding of new China resulted in significantly higher levels of depressive symptoms in those born then. As socioeconomic development progressed, symptoms decreased, especially for those born after 1960. Significant age and cohort variations in gender disparities in depressive symptoms were detected. Gender gaps narrowed with age after adjusting for period effects, cohort effects, and individual-level variables. The most noteworthy finding is that a widening trend in gender gaps in depressive symptoms was found among those born after the mid-1950s, mainly driven by a notable decline in depressive symptoms among men. In the context of an overall stable level of depressive symptoms over time, we should be aware of the widening gender disparities in mental health among younger cohorts.
This study’s findings have several important policy implications. Firstly, the significant gender disparities in depressive symptoms in terms of age and cohort effects suggest the need for gender-specific mental health interventions. Policies should be designed to address the unique stressors that women face in their life course. Moreover, for younger cohorts among women, policies should additionally consider the societal pressures and changing roles due to macro-level gender equality movements. Secondly, the results indicate that different cohorts have varying levels of depressive symptoms due to their respective life experiences. This supports the implementation of policies aimed at providing additional support to those exposed to war and social adversities early in their lives. Finally, the monotonically decreasing trend in age effects after controlling for individual characteristics suggests the necessity of alleviating the negative impact of deteriorating socioeconomic status and physical functioning on mental health well-being in old age by enhancing older people’s community, economic and social involvement.
Data availability
This study was based on the dataset from the CHARLS, and the CHARLS are freely available to all researchers. Information about the data source and access to data can be found at https://charls.charlsdata.com/pages/data/111/zh-cn.html.
References
World Health Organization (2011) Global burden of mental disorder and the need for comprehensive, coordinated response from the health and social sectors at the country level. World Health Organization, Geneva
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B (2007) Depression, chronic diseases, and decrements in health: results from the World health surveys. Lancet 370(9590):851–858. https://doi.org/10.1016/S0140-6736(07)61415-9
The enduring psychosocial consequences of mania and depression (1993). Am J Psychiatry 150(5):720–727. https://doi.org/10.1176/ajp.150.5.720
Melhem NM, Porta G, Oquendo MA, Zelazny J, Keilp JG, Iyengar S, Burke A, Birmaher B, Stanley B, Mann JJ, Brent DA (2019) Severity and variability of depression symptoms predicting suicide attempt in high-risk individuals. JAMA Psychiat 76(6):603–613. https://doi.org/10.1001/jamapsychiatry.2018.4513
Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S (2010) From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry 196(5):365–371. https://doi.org/10.1192/bjp.bp.109.071191
Cloninger CR (2012) Healthy personality development and well-being. World Psychiatry 11(2):103–104. https://doi.org/10.1016/j.wpsyc.2012.05.019
Fan X, Guo X, Ren Z, Li X, He M, Shi H, Zha S, Qiao S, Zhao H, Li Y, Pu Y, Liu H, Zhang X (2021) The prevalence of depressive symptoms and associated factors in middle-aged and elderly Chinese people. J Affect Disord 293:222–228. https://doi.org/10.1016/j.jad.2021.06.044
Luo W, Zhao M (2021) Trends and socioeconomic differentials in depression prevalence in China, 2010–2018. Chin J Sociol 7(4):535–556. https://doi.org/10.1177/2057150X211043008
Bell A (2020) Age period cohort analysis: a review of what we should and shouldn’t do. Ann Hum Biol 47(2):208–217. https://doi.org/10.1080/03014460.2019.1707872
Yang Y, Land KC (2013) Age-period-cohort analysis: new models, methods, and empirical applications. CRC Press, USA
He J, Ouyang F, Li L, Qiu D, Li Y, Xiao S (2021) Incidence trends of major depressive disorder in China: an age-period-cohort modeling study. J Affect Disord 288:10–16. https://doi.org/10.1016/j.jad.2021.03.075
Bai R, Dong W, Peng Q, Bai Z (2022) Trends in depression incidence in China, 1990–2019. J Affect Disord 296:291–297. https://doi.org/10.1016/j.jad.2021.09.084
Zhang Y, Zhao M (2021) Gender disparities and depressive symptoms over the life course and across cohorts in China. J Affect Disord 295:620–627. https://doi.org/10.1016/j.jad.2021.08.134
Chiao C, Weng LJ, Botticello A (2009) Do older adults become more depressed with age in Taiwan? The role of social position and birth cohort. J Epidemiol Commun Health 63(8):625–632. https://doi.org/10.1136/jech.2008.082230
Shao J, Li D, Zhang D, Zhang L, Zhang Q, Qi X (2013) Birth cohort changes in the depressive symptoms of Chinese older adults: a cross-temporal meta-analysis. Int J Geriatr Psychiatry 28(11):1101–1108. https://doi.org/10.1002/gps.3942
Li D, Zhang D, Shao J, Qi X, Tian L (2014) A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch Gerontol Geriatr 58(1):1–9. https://doi.org/10.1016/j.archger.2013.07.016
Lim LL, Chang W, Yu X, Chiu H, Chong M-Y, Kua E-H (2011) Depression in Chinese elderly populations. Asia Pac Psychiatry 3(2):46–53. https://doi.org/10.1111/j.1758-5872.2011.00119.x
Yu J, Li J, Cuijpers P, Wu S, Wu Z (2012) Prevalence and correlates of depressive symptoms in Chinese older adults: a population-based study. Int J Geriatr Psychiatry 27(3):305–312. https://doi.org/10.1002/gps.2721
Sun J, Ryder AG (2016) The Chinese experience of rapid modernization: sociocultural changes, psychological consequences? Front Psychol 7:477. https://doi.org/10.3389/fpsyg.2016.00477
Wang Q, Tapia Granados JA (2019) Economic growth and mental health in 21st century China. Soc Sci Med 220:387–395. https://doi.org/10.1016/j.socscimed.2018.11.031
Zeng J, Jian W (2019) Changes in income-related inequalities of depression prevalence in China: a longitudinal, population study. Soc Psychiatry Psychiatr Epidemiol 54(9):1133–1142. https://doi.org/10.1007/s00127-019-01710-0
Tang T, Jiang J, Tang X (2021) Prevalence of depressive symptoms among older adults in mainland China: a systematic review and meta-analysis. J Affect Disord 293:379–390. https://doi.org/10.1016/j.jad.2021.06.050
Winczyk K, Kostka T, Pisarek H, Pisarek H, Guligowska A, Guligowska A, Piechocka-Wochniak E, Piechocka-Wochniak E, Świętosławski J, Świętosławski J, Pawlikowski M (2022) Hormones and depression in the advanced age. Endokrynol Pol 73(6):917–921
Pataky MW, Young WF, Nair KS (2021) Hormonal and metabolic changes of aging and the influence of lifestyle modifications. Mayo Clin Proc 96(3):788–814. https://doi.org/10.1016/j.mayocp.2020.07.033
Jorm AF (2000) Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol Med 30(1):11–22. https://doi.org/10.1017/S0033291799001452
Blazer DG (2003) Depression in late life: review and commentary. J Gerontol Ser A-Biol Sci Med Sci 58(3):M249–M265. https://doi.org/10.1093/gerona/58.3.M249
Tang X, Qi S, Zhang H, Wang Z (2021) Prevalence of depressive symptoms and its related factors among China’s older adults in 2016. J Affect Disord 292:95–101. https://doi.org/10.1016/j.jad.2021.04.041
Lei X, Sun X, Strauss J, Zhang P, Zhao Y (2014) Depressive symptoms and SES among the mid-aged and elderly in China: evidence from the China health and retirement longitudinal study national baseline. Soc Sci Med 120:224–232. https://doi.org/10.1016/j.socscimed.2014.09.028
Pan A, Franco OH, Wang YF, Yu ZJ, Ye XW, Lin X (2008) Prevalence and geographic disparity of depressive symptoms among middle-aged and elderly in China. J Affect Disord 105(1–3):167–175. https://doi.org/10.1016/j.jad.2007.05.003
Blazer DG (1994) Is Depression more frequent in late life?: An honest look at the evidence. Am J Geriatr Psychiatry 2(3):193–199. https://doi.org/10.1097/00019442-199400230-00003
Clarke P, Marshall V, House J, Lantz P (2011) The social structuring of mental health over the adult life course: advancing theory in the sociology of aging. Soc Forces 89(4):1287–1313. https://doi.org/10.1093/sf/89.4.1287
Kasen S, Cohen P, Chen H, Castille D (2003) Depression in adult women: age changes and cohort effects. Am J Public Health 93(12):2061–2066. https://doi.org/10.2105/AJPH.93.12.2061
Yang Y (2007) Is old age depressing? Growth trajectories and cohort variations in late-life depression. J Health Soc Behav 48(1):16–32. https://doi.org/10.1177/002214650704800102
Xu Z, Gahr M, Xiang Y, Kingdon D, Rüsch N, Wang G (2022) The state of mental health care in China. Asian J Psychiatr 69:102975. https://doi.org/10.1016/j.ajp.2021.102975
Phillips MR, Chen H, Diesfeld K, Xie B, Cheng HG, Mellsop G, Liu X (2013) China’s new mental health law: reframing involuntary treatment. Am J Psychiatry 170(6):588–591. https://doi.org/10.1176/appi.ajp.2013.12121559
Que J, Lu L, Shi L (2019) Development and challenges of mental health in China. Gen Psychiatry 32(1):e100053. https://doi.org/10.1136/gpsych-2019-100053
Eugenia Alvarado B, Victoria Zunzunegui M, Béland F, Sicotte M, Tellechea L (2007) Social and gender inequalities in depressive symptoms among urban older adults of latin America and the Caribbean. J Gerontol Ser B 62(4):S226–S236. https://doi.org/10.1093/geronb/62.4.S226
Zunzunegui MV, Minicuci N, Blumstein T, Noale M, Deeg D, Jylhä M, Pedersen NL (2007) Gender differences in depressive symptoms among older adults: a cross-national comparison. Soc Psychiatry Psychiatr Epidemiol 42(3):198–207. https://doi.org/10.1007/s00127-007-0158-3
Marchand A, Bilodeau J, Demers A, Beauregard N, Durand P, Haines VY (2016) Gendered depression: vulnerability or exposure to work and family stressors? Soc Sci Med 166:160–168. https://doi.org/10.1016/j.socscimed.2016.08.021
Broerman R (2017) Diathesis-stress model. In: Zeigler-Hill V, Shackelford TK (eds) Encyclopedia of personality and individual differences. Springer International Publishing, Cham, pp 1–3. https://doi.org/10.1007/978-3-319-28099-8_891-1
Anderson LR, Monden CWS, Bukodi E (2021) Stressful life events, differential vulnerability, and depressive symptoms: critique and new evidence. J Health Soc Behav 63(2):283–300. https://doi.org/10.1177/00221465211055993
Bracke P, Delaruelle K, Dereuddre R, Van de Velde S (2020) Depression in women and men, cumulative disadvantage and gender inequality in 29 European countries. Soc Sci Med 267:113354. https://doi.org/10.1016/j.socscimed.2020.113354
Abrams LR, Mehta NK (2019) Changes in depressive symptoms over age among older Americans: differences by gender, race/ethnicity, education, and birth cohort. SSM Popul Health 7:100399. https://doi.org/10.1016/j.ssmph.2019.100399
Barefoot JC, Mortensen EL, Helms MJ, Avlund K, Schroll M (2001) A longitudinal study of gender differences in depressive symptoms from age 50 to 80. Psychol Aging 16(2):342–345. https://doi.org/10.1037/0882-7974.16.2.342
Mirowsky J (1996) Age and the gender gap in depression. J Health Soc Behav 37(4):362–380. https://doi.org/10.2307/2137263
Zhang B, Li J (2011) Gender and marital status differences in depressive symptoms among elderly adults: the roles of family support and friend support. Aging Ment Health 15(7):844–854. https://doi.org/10.1080/13607863.2011.569481
Davis D (1991) Long lives: Chinese elderly and the communist revolution. Stanford University Press, Redwood City
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G (2014) Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol 43(1):61–68. https://doi.org/10.1093/ije/dys203
Zhao Y, Strauss J, Chen X, Wang Y, Gong J, Meng Q, Wang G, Wang H (2020) China health and retirement longitudinal study wave 4 user’s guide. Peking University, Beijing
Royston P, White IR (2011) Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw 45(4):1–20. https://doi.org/10.18637/jss.v045.i04
Andresen EM, Malmgren JA, Carter WB, Patrick DL (1994) Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 10(2):77–84. https://doi.org/10.1016/s0749-3797(18)30622-6
Boey KW (1999) Cross-validation of a short form of the CES-D in Chinese elderly. Int J Geriatr Psychiatry 14(8):608–617. https://doi.org/10.1002/(SICI)1099-1166(199908)14:8%3c608::AID-GPS991%3e3.0.CO;2-Z
Huang Q, Wang X, Chen G (2015) Reliability and validity of 10-item CES-D among middle aged and older adults in China. Chin J Health Psychol 23(07):1036–1041. https://doi.org/10.13342/j.cnki.cjhp.2015.07.023
Yang Y, Land KC (2006) A mixed models approach to the age-period-cohort analysis of repeated cross-section surveys, with an application to data on trends in verbal test scores. Sociol Methodol 36(1):75–97. https://doi.org/10.1111/j.1467-9531.2006.00175.x
Blazer D, Burchett B, Service C, George LK (1991) The association of age and depression among the elderly: an epidemiologic exploration. J Gerontol Ser A-Biol Sci Med Sci 46(6):M210–M215. https://doi.org/10.1093/geronj/46.6.M210
Yang Y (2008) Social inequalities in happiness in the United States, 1972 to 2004: an age-period-cohort analysis. Am Sociol Rev 73(2):204–226. https://doi.org/10.1177/000312240807300202
SAS Institute Inc (2017) The GLIMMIX procedure. In: SAS Institute Inc (ed) SAS/STAT® 14.3 user’s guide. SAS Institute Inc, Cary, NC, pp 3371–3494
Vink D, Aartsen MJ, Schoevers RA (2008) Risk factors for anxiety and depression in the elderly: a review. J Affect Disord 106(1–2):29–44. https://doi.org/10.1016/j.jad.2007.06.005
Zhao K, Huang C, Xiao Q, Gao Y, Liu Q, Wang Z, Li Y, Xie Y (2012) Age and risk for depression among the elderly: a meta-analysis of the published literature. CNS Spectr 17(3):142–154. https://doi.org/10.1017/S1092852912000533
Li N, Chen G, Zeng P, Pang J, Gong H, Han Y, Zhang Y, Zhang E, Zhang T, Zheng X (2016) Prevalence of depression and its associated factors among Chinese elderly people: a comparison study between community-based population and hospitalized population. Psychiatry Res 243:87–91. https://doi.org/10.1016/j.psychres.2016.05.030
Gallo JJ, Anthony JC, Muthen BO (1994) Age differences in the symptoms of depression: a latent trait analysis. J Gerontol Ser B-Psychol Sci Soc Sci 49(6):P251–P264. https://doi.org/10.1093/geronj/49.6.P251
Ryu H-S, Chang S-O, Song J-A, Oh Y (2013) Effect of domain-specific life satisfaction on depressive symptoms in late adulthood and old age: results of a cross-sectional descriptive survey. Arch Psychiatr Nurs 27(2):101–107. https://doi.org/10.1016/j.apnu.2012.07.003
Zeng Y, Vaupel JW (2002) Functional capacity and self–evaluation of health and life of oldest old in China. J Soc Issues 58(4):733–748. https://doi.org/10.1111/1540-4560.00287
Diener E, Lucas RE, Scollon CN (2006) Beyond the hedonic treadmill: revising the adaptation theory of well-being. Am Psychol 61(4):305–314. https://doi.org/10.1037/0003-066X.61.4.305
Gerstorf D, Ram N, Röcke C, Lindenberger U, Smith J (2008) Decline in life satisfaction in old age: longitudinal evidence for links to distance-to-death. Psychol Aging 23(1):154–168. https://doi.org/10.1037/0882-7974.23.1.154
Mirowsky J, Ross CE (1992) Age and depression. J Health Soc Behav 33(3):187–205. https://doi.org/10.2307/2137349
Ross CE, Mirowsky J (2008) Age and the balance of emotions. Soc Sci Med 66(12):2391–2400. https://doi.org/10.1016/j.socscimed.2008.01.048
Xie P, Wu K, Zheng Y, Guo Y, Yang Y, He J, Ding Y, Peng H (2018) Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. J Affect Disord 228:41–48. https://doi.org/10.1016/j.jad.2017.11.011
Kraaij V, Arensman E, Spinhoven P (2002) Negative life events and depression in elderly persons: a meta-analysis. J Gerontol Ser B-Psychol Sci Soc Sci 57(1):P87–P94. https://doi.org/10.1093/geronb/57.1.P87
Lee S, Guo W, Tsang A, Mak ADP, Wu J, Ng KL, Kwok K (2010) Evidence for the 2008 economic crisis exacerbating depression in Hong Kong. J Affect Disord 126(1):125–133. https://doi.org/10.1016/j.jad.2010.03.007
Lester D (2005) Suicide and the Chinese cultural revolution. Arch Suicide Res 9(1):99–104. https://doi.org/10.1080/13811110590512994
Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143(8):783–822. https://doi.org/10.1037/bul0000102
Platt JM, Bates LM, Jager J, McLaughlin KA, Keyes KM (2020) Changes in the depression gender gap from 1992 to 2014: cohort effects and mediation by gendered social position. Soc Sci Med 258:113088. https://doi.org/10.1016/j.socscimed.2020.113088
Bramajo ON (2022) An age-period-cohort approach to analyse late-life depression prevalence in six European countries, 2004–2016. Eur J Popul Revue Eur Demogr. https://doi.org/10.1007/s10680-022-09610-x
Yang Y, Lee LC (2009) Sex and race disparities in health: cohort variations in life course patterns. Soc Forces 87(4):2093–2124. https://doi.org/10.1353/sof.0.0183
Attané I (2014) Being a woman in China today: a demography of gender. In: Attané I, Gu B (eds) Analysing China’s population: social change in a new demographic era. Springer, Dordrecht, pp 95–110. https://doi.org/10.1007/978-94-017-8987-5_6
Funding
This work was funded by Postdoctoral Fellowship Program of CPSF (Grant no. GZC20240155), Peking Union Medical College Education Foundation, Chinese Academy of Medical Sciences Basic Fund-Talent Introduction and Development Project No. 2023-RC330-01, Population and Aging Health Science Program (Grant no. WH10022023035), National Key Research and Development Program of China (Grant no. 2022YFC3600800).
Author information
Authors and Affiliations
Contributions
Shuai Guo and Xiao-Ying Zheng initially conceived the research idea; Shuai Guo designed the study, performed data collection, management, and analysis, and drafted the original manuscript; Xiao-Ying Zheng and Chang-Biao Chu revised the draft. All authors interpreted the findings and gave their final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All methods used in this study were implemented in accordance with relevant CHARLS guidelines and regulations and the Declaration of Helsinki. All participants joined CHARLS voluntarily and signed informed consent before participation. Moreover, written informed consent was obtained from their literate, legally authorized representatives/guardians for interviewees with no formal education. Ethical approval for all the CHARLS waves was granted by the Institutional Review Board at Peking University (IRB00001052-11015).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Chu, CB. & Zheng, XY. Changes in gender disparities of depressive symptoms among middle-aged and older adults in China: an age-period-cohort analysis. Soc Psychiatry Psychiatr Epidemiol (2024). https://doi.org/10.1007/s00127-024-02747-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00127-024-02747-6