Abstract
Purpose
To determine the prevalence of substance use disorders (SUDs) in patients with schizophrenia in a sample from South Africa and compare the clinical and demographic correlates in those with and without co-occurring SUDs.
Methods
Patients with schizophrenia were interviewed using the Xhosa version SCID-I for DSM-IV. We used logistic regression to determine the predictors of SUDs.
Results
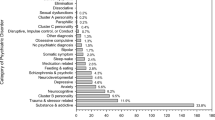
In the total sample of 1420 participants, SUDs occurred in 47.8%, with the most prevalent SUD being cannabis use disorders (39.6%), followed by alcohol (20.5%), methaqualone (6.2%), methamphetamine (4.8%) and other SUDs (cocaine, ecstasy, opioids, 0.6%). Polydrug use occurred in 40%, abuse occurred in 13.5%, and 39.6% had at least one substance dependence diagnosis. Significant predictors of any SUD were younger age (41–55 vs. 21–30: OR = 0.7, 95% CI = 0.5–0.9), male sex (OR = 8.6, 95% CI = 5.1–14.6), inpatient status (OR = 1.7, 95% CI = 1.3–2.1), post-traumatic stress symptoms (OR = 4.6, 95% CI = 1.6–13.3), legal (OR = 3.4, 95% CI = 2.0–5.5) and economic problems (OR = 1.4, 95% CI = 1.0–2.0). Methamphetamine use disorders occurred significantly less often in the Eastern compared to the Western Cape provinces. Inpatient status and higher levels of prior admissions were significantly associated with cannabis and methamphetamine use disorders. Post-traumatic stress symptoms were significantly associated with alcohol use disorders. Anxiety disorders were associated with other SUDs.
Conclusion
SUDs occurred in almost half of the sample. It is important for clinicians to identify the presence of SUDs as their presence is associated with characteristics, such as male sex, younger age, inpatient status, more prior hospitalisations, legal and economic problems, PTSD symptoms and anxiety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Substance use disorders (SUDs) often co-occur with schizophrenia (SZ) and are associated with poor treatment outcomes and prognosis [1, 2]. Depending on the study, lifetime prevalence rates of SUDs have been reported to be as low as 10% and as high as high as 74% [1, 3]. Systematic reviews and meta-analyses of SUD prevalence rates in SZ and affective psychoses have suggested that cannabis use disorders occur most frequently with a pooled median lifetime prevalence of 27.1%, followed by alcohol use disorders (median lifetime prevalent of 20.6%) and finally stimulants (amphetamine, cocaine, ecstasy and other stimulants) which occur at a pooled lifetime and recent rate of 10.4% [4,5,6].Meta-regression analyses from these and findings from other systematic reviews have suggested variables that significantly affect variation in prevalence are the diagnostic method used, male sex, stage of illness (e.g. first episode versus more established illness, inpatient vs. outpatient status) and age with higher rates of cannabis use in younger patients. In contrast, alcohol use disorders occur more frequently in older age groups [7]. Importantly, in these and other systematic reviews, geographic region has been shown to significantly influence the prevalence rate of alcohol, cannabis and amphetamine use disorders [8].
Literature has also examined additional comorbid conditions in relation to SUD in SZ. While some studies show no association between depressive symptoms and SUD in SZ [9, 10], others have shown elevated depressive symptoms [11,12,13,14], and corresponding risky behaviours, such as suicidality [9, 15]. Further anxiety symptoms, such as panic attacks and post-traumatic stress symptoms, have been found to be elevated in patients with SZ and SUDs is some studies [16, 17], while other studies have found no such associations [18, 19]. Similarly, studies have yielded varying results of risky and impulse behaviours, such as criminal involvement, and consequent legal problems. Some studies have found these to be elevated in people with SZ and co-occurring disorders [3, 15, 20]. In studies examining DSM-IV axis IV psychosocial problems, people with psychotic disorder were found to have elevated rates of problems with the primary support group, housing, educational and legal problems, although studies examining SZ spectrum disorder in particular only found significantly elevated rates of housing and access to healthcare problems [21, 22].
Within the South African context, there are few studies examining the prevalence and correlates of substance use disorder in adults with psychotic disorders and none examining the relationship between SUD and SZ in particular [23,24,25,26,27]. In turn, these studies have not used structured diagnostic instruments. Diagnoses of SUDs and other psychiatric comorbidities are improved using semi-structured interviews that make use of multiple sources of information and face-to-face clinical interviewing to validate clinical diagnoses. The SCID-I is such an instrument and has demonstrated good reliability in the diagnosis of substance use disorders [28]. To improve cross-cultural validity, this instrument has been translated into several languages worldwide [29]. In this secondary data analysis of an existing larger study investigating the genomics of SZ in Xhosa people from South Africa, we aimed to determine the prevalence and distribution of SUDs as well as associated clinical and demographic features. The study utilised the Xhosa language version of the SCID-I in a homogeneous sample of Xhosa speaking patients with a well-established history of SZ spectrum disorders (SZ or schizoaffective disorder) and measured correlates of SUDs that included mood and anxiety symptoms and disorders, risky behaviours, such as suicidality and criminal involvement, as well as other psychosocial problems.
Methods
Design, sample and setting
This study is a secondary data analysis based on data collected for a case–control study. Participants included cases from an existing larger case–control study investigating the Genomics of SZ in the South African Xhosa Population (SAX study) [30, 31]. Xhosa speaking people are one of the largest black African groups in South Africa and mainly live in the country’s Western and Eastern Cape regions [32]. During the era in South African history characterized by the rule of the Apartheid government, the Xhosa community fell within the group of racially segregated and oppressed South Africans institutionally discriminated against. Consequently, this group has had restricted access to healthcare, education and employment opportunities; a legacy that 20 years after the introduction of democracy in South Africa, continues to perpetuate gross inequalities between population groups [33]. Members of the Xhosa community diagnosed with schizophrenia are a small minority within this larger community. The SAX study involved a collaboration between a number of Universities in the United States and South Africa, funded by the National Institutes of Mental Health and was a member of the Human Heritability and Health in Africa consortium (www.h3africa.org). This study aimed to recruit over 2800 participants (1400 cases and 1400 matched controls) over 5 years and started recruitment in 2013 across various psychiatric treatment settings including community mental health clinics and psychiatric hospitals in two South African provinces (Western and Eastern Cape). Eligible participants self-identified with a Xhosa ethnic background and were IsiXhosa speaking, aged 21–60 years, had a suspected diagnosis of SZ or schizoaffective disorder with duration of illness of at least 2 years and the ability to give informed consent to the genomics study. Controls were participants without a psychotic disorder but were excluded in this analysis which focuses on SZ spectrum cases with and without co-occurring SUDs. Participants were volunteers who were approached by study staff attending community psychiatry clinics and admitted to inpatient psychiatric treatment units. In addition, mental health practitioners (psychiatric nurses and psychiatrists) referred suitable cases. The final sample consisted of 1420 cases with schizophrenia or schizoaffective disorder.
Measures and procedures
Demographics, diagnoses and related clinical characteristics were collected using the overview section of the SCID-I [28]. Demographic variables included age, sex, marital status, education level, employment, recruitment area, i.e. Western Cape or Eastern Cape provinces, inpatient or outpatient status. The diagnoses of substance use disorders (abuse or dependence) were determined using Module E of the Structured Clinical Interview for DSM-IV (SCID-I). In addition, the diagnoses of SZ or schizoaffective disorder were confirmed using the SCID-I modules A, B, C, and D. From module A of the SCID-I, we coded the presence of any current (past month) major depressive episode (MDE) as well as the presence of any current (past month) threshold or sub-threshold depressive symptoms defined as the presence of depressed mood or anhedonia, the minimum entry requirements for any mood episode in the SCID-I. We coded suicidality (current) as a separate variable. Using Module F of the SCID-I, we assessed anxiety symptoms (panic, agoraphobia without a history of panic, specific phobia, social phobia, generalised anxiety), post-traumatic stress and obsessive–compulsive disorder symptoms. For anxiety, post-traumatic and obsessive–compulsive symptoms, in addition to coding syndrome level disorders, we coded the presence of any sub-threshold or threshold symptoms that fell short of meeting criteria for a clinical disorder. The number of lifetime hospitalizations was categorized into three categories (none, ≤ 2, ≥ 3 hospitalisations) (Table 2).
Psychosocial and environmental problems which form part of Axis IV of the DSM-IV-TR were also systematically recorded in this study. Axis IV psychosocial problems were recorded under a number of headings with paired descriptors, and included:
-
“Problems with primary support group” (including death of family member, health problems in family, disruption of family by separation, divorce, or estrangement, removal from the home, remarriage of parent, sexual or physical abuse, parental overprotection, neglect of child, inadequate discipline, discord with siblings, birth of a sibling);
-
“Problems related to the social environment” (including death or loss of a friend; inadequate social support; living alone; difficulty with acculturation; discrimination; adjustment to life-cycle transition, such as retirement);
-
“Educational problems” (illiteracy, academic problems, discord with teachers or classmates, inadequate school environment),
-
“Occupational problems” (unemployment, threat of job loss, stressful work schedule, difficult work conditions, job dissatisfaction, job change, discord with boss or co-workers);
-
“Housing problems” (homelessness, inadequate housing, unsafe neighbourhood, discord with neighbours or landlord);
-
“Economic problems” (extreme poverty, inadequate finances, insufficient welfare support); “problems with access to health care” (inadequate health care services, transportation to health care facilities unavailable, inadequate health insurance);
-
“Problems related to interaction with the legal system/crime” (arrest, incarceration, litigation, victim of crime) and,
-
“Other psychosocial and environmental problems” (exposure to disasters, war, other hostilities, discord with non-family caregivers, such as counsellor, social worker, or physician; unavailability of social service agencies).
Global functioning was assessed by means of the Global Assessment of Functioning Scale (GAF scale), which assesses functioning on a 100-point scale [34].
Participants were interviewed by bilingual (isiXhosa and English speaking) psychiatric research assistants (a psychiatrist, a research fellow in psychiatry and psychiatric nurses) with extensive experience in working with patients with serious mental illness. In addition, research staff underwent extensive training in structured clinical interviewing (that included scoring of GAF scale) and participated in weekly supervision and inter-rater reliability meetings to discuss diagnostic issues. Training and supervision were conducted by a senior psychiatrist (HT) a doctorate level medical practitioner (GS) and a psychologist (MC).
The SCID-I was translated into isiXhosa in accordance with guidelines recommended by the World Health Organization [35]. The translation design included a forward and back-translation approach where materials were first translated into isiXhosa by a group of 5 first language isiXhosa speaking, experienced mental health practitioners, and then back-translated by an independent isiXhosa speaking psychiatrist. Initial translations were piloted and compared to the SCID by a native English-speaking researcher and then improved through an iterative process. Where there were discrepancies in the translation, these were discussed in group meetings, and consensus was reached on the final translation. Inter-rater reliability obtained on a smaller sample of participants (N = 22) was substantial for the principle psychotic disorder diagnosis (kappa = 0.74, p < 0.001). Similarly, for the SCID-I DSM-IV substance use disorder (abuse or dependence) diagnoses, inter-rater reliability ranged from substantial to near perfect (any SUD, kappa = 0.82; alcohol use disorder, kappa = 0.84, p < 0.001; cannabis use disorder, kappa = 0.85, p < 0.001, methamphetamine use disorder, kappa = 0.71, p < 0.001). Typical SCID-I interviews lasted 1.5–4.5 h. In addition to the patient interview, additional information was considered in the diagnostic process from referral notes, including urine drug tests conducted on hospital admissions where available, past and current clinical records, interviews with other members of the multidisciplinary teams and information from family members or other associates of the patients.
Ethics
All participants gave written informed consent to participate in the study. The consent procedure included the administration of the University of California, San Diego Brief Assessment of Capacity to Consent Questionnaire (UBACC) [36]. This instrument was used to screen for decisional capacity and improve the understating of study elements during the consent process [30]. The study was approved by the University of Cape Town Human Research Ethics Committee, The Walter Sisulu University Research Ethics and Biosafety Committee, The Rhodes University Ethical Standards Committee, The Columbia University Internal Review Board and the University of Washington Institutional Review Board.
Statistical analysis
We calculated the 95% confidence intervals of the various SUD prevalence’s using the normal approximation of the binomial distribution. We constructed six separate dichotomous dependent variables denoting the presence or absence of any lifetime, alcohol, cannabis, methaqualone, methamphetamine and other drug (cocaine and hallucinogens) SUDs (abuse or dependence). For the association between SUDs and demographic and clinical variables, we first conducted bivariate logistic regression analyses for each dependent variable separately onto each of the different independent demographic and clinical predictor variables. We then constructed multivariable logistic regression models with the dependent variables and the various SUDs (any SUDS, alcohol, cannabis, methamphetamine, methaqualone, and other drug use disorders) and entered independent variables (including other co-occurring SUDs) that were significant in the bivariate analyses at a p ≤ 0.10 level into the final models. We inspected models for multi-collinearity using variance inflation and tolerance measures and removed redundant and collinear variables to obtain parsimonious models. There were some variables with missing data in the final dataset (percentage of missing data: marital status 10%, education level 1.1%, employment status 22.8%, treatment setting 0.14%; number of hospitalizations 11.1%, GAF score 16.2%). (See Tables 1 and 2) To handle missing at random (MAR) data, multiple imputation models with chained equations (MICE) were constructed utilising variables selected for final models including auxiliary variables, to derive 20 imputed datasets with estimation models from which parameter estimates were then pooled using Rubin’s rules [37]. We report the associations between independent variables and dependent (SUD) variables as adjusted odds ratios (ORs) with their 95% confidence intervals. All analyses were two-tailed and considered significant at the 5% level. We used Stata version 16 for Windows for all analyses [38].
Results
Sample characteristics
The total sample consisted of 1420 participants. The mean age was 36.2 years (SD = 9.2), and the majority of the sample were male (87.7%). Tables 1 and 2 present the sample demographics and clinical characteristics. The majority of the sample were never married, had less than 12 years of education and were unemployed. Over half resided in the Eastern Cape Province, and two-thirds were inpatients. The majority had SZ, and only a small group were diagnosed with schizoaffective disorder. The occurrence of past month depressive symptoms and MDE was very low as were the presence of suicidal ideation, plans or attempts. Lifetime anxiety, OCD and PTSD occurred infrequently, with the most common disorder being PTSD and PTSD symptoms. Problems with education and economic problems occurred in almost half the sample, followed by problems with housing. Other psychosocial problems occurred at lower levels. More than half the sample had a history of at least one axis IV psychosocial problem and almost all participants have had at least one hospital admission. The average GAF score (Global Assessment of Functioning) was in the moderate symptom level and functional impairment range.
Prevalence, patterns and distribution of substance use disorders
Of the 1420 participants, the prevalence of any SUD was 47.8% (95% CI = 45.1–50.4%). The most prevalent SUD was cannabis use disorders (39.6%, 95% CI = 37.1–42.2%), followed by alcohol use disorders (20.5%, 95% CI = 18.4–22.7%), and methaqualone use disorders (sedative–hypnotic) (6.2%, 95% CI = 5.0–7.6%). Methamphetamine use disorders occurred much less frequently (4.8%, 95% CI = 3.8–6.1%) as did other SUDs (hallucinogens, cocaine and opioids) (0.6%, 95% CI = 0.2–1.1%). Of all participants with SUDs, 40.1% used two or more substances, 13.5% had a diagnosis of substance abuse and 39.1% a diagnosis of substance dependence. Participants who used cannabis had significantly increased odds of also using alcohol, methaqualone and methamphetamine. In turn cannabis, methamphetamine and methaqualone often occurred together and using one of these substances was associated with significantly increased odds for using any of the other (Table 3).
Demographic and clinical correlates of substance use disorders
After adjustment for demographic and clinical covariates in multivariable logistic regression models, some variables remained significantly associated with SUDs (Tables 4 and 5). For any SUD, there was a significant association with younger age, male sex, inpatient status, lifetime PTSD symptoms, economic problems and legal or criminal involvement. For alcohol use disorders, male sex and PTSD symptoms were significant in the final models. In turn, younger age, male sex, inpatient status, legal/criminal involvement and having had three or more prior hospitalisations had significant positive associations with having a cannabis use disorder in the adjusted analyses. Younger age had a significant positive association with methaqualone use disorder, whereas having a methaqualone use disorder was significantly less likely if living in the Eastern Cape compared to the Western Cape. Similarly, having a methamphetamine use disorder was also significantly less likely for those residing in the Eastern Cape than the Western Cape, but significantly more likely with younger age and having inpatient status. Having a diagnosis of schizoaffective disorder, a lifetime anxiety disorder diagnosis and at least 1–2 prior hospitalisations had significantly increased odds of having “other SUDs” diagnosis (i.e. cocaine, hallucinogens, and opioids). In the multivariable models in Tables 4 and 5, after adjustment for demographic and clinical variables and other SUDs, the associations between the various SUDs (alcohol, cannabis, methaqualone, methamphetamine and others) remained significant.
Discussion
Main findings and comparison with other studies
Our study has yielded several important findings and is the first study in South Africa to examine SUDs in SZ using a validated and rigorous diagnostic instrument (SCID-I), translated into the Xhosa language. First, in a sample of Xhosa speaking patients with an established diagnosis of SZ, as expected, we found a high lifetime prevalence (47.8%) of any SUD. Second, we found the most prevalent SUDs were cannabis use disorders, followed by alcohol use disorders, methaqualone use disorders, but with methamphetamine use disorder having a relatively low prevalence (4.8%). Third, substance dependence was more common than substance abuse. Fourth, correlates of SUDs included, age, sex, inpatient status, geographical regions, PTSD and anxiety symptoms and frequency of inpatient admissions. Each of these findings is elaborated below drawing comparisons with previous literature.
Our prevalence estimate of 47.8% of any SUD corresponds with findings from previous local and international cross-sectional studies that typically find about half of patients with SZ have a SUD [3, 27]. Weich et al., who studied sample of both first episode and patients with more established severe mental illness from the Western Cape, and included a heterogenous group in terms of race and diagnosis and found a rate of any SUD of 51%, similar rates of cannabis and alcohol use to our sample, but with higher rates of methamphetamine use [27]. In turn, two studies in patients with a first-episode psychosis from the Eastern Cape and Kwa-Zulu Natal and found substantially higher rates of cannabis, alcohol and methamphetamine usage [24, 39]. These studies differed from ours in that they studied first-episode psychosis of various aetiologies, included heterogenous racial groupings, excluded established schizophrenia and did not use validated diagnostic instruments to measure substance use and only recorded substance use as opposed to abuse or dependence. These and our finding of high SUD prevalence in SZ population are in contrast to those from a nationally representative South African general population sample that found any substance use disorder in only 13.3%, with alcohol use disorders to be the most prevalent substance used (abuse 4.5% and dependence 1.2%), followed drug abuse (3.9%) and drug dependence (0.6%) [40]. Reasons for higher SUD prevalence in schizophrenia are severalfold, and may include increased risk for developing psychosis in substance users (substance induced theories), alleviation of dysphoria associated with SZ (self-medication theory) and common risk factors theories, such as common underlying genetic and environmental (i.e. trauma and stress) risk factors, for SUDs and SZ [41].
In turn, our findings are corroborated by meta-analyses of prevalence studies of SUDs in SZ, that have found that the most prevalent SUD is cannabis use disorders, although our prevalence of 39.6% is somewhat higher than the pooled prevalence of 27% from one meta-analysis [5]. Several theories have been proposed to explain the high co-occurrence between cannabis and schizophrenia. Whilst prospective studies have demonstrated an association between cannabis use in adolescence and the development of schizophrenia [42], some studies have shown that people with schizophrenia use cannabis to self-medicate, to “arrange thoughts” and “to decrease hallucinations and suspiciousness” [43]. We could speculate that in our sample of patients with well-established schizophrenia, for some patients, this may also be the case. Our finding of a significant association between inpatients status and cannabis use, and the fact that two-thirds of our sample consisted of inpatients, may also explain the high prevalence of cannabis use in our sample. We found a prevalence of 20% for alcohol use disorders in our sample, a rate similar to pooled prevalence rates from one meta-analysis (20%) [4].
In our sample, a diagnosis of dependence was more common compared to a diagnosis of abuse, which is the opposite found in a general community South African sample and other studies in the international literature [40]. However, our results are similar to the findings of some meta-analyses of SUDs in SZ, which have also found higher levels of dependence compared to abuse for cannabis and alcohol use disorders [4, 5]. Other meta-analyses have found similar rates for abuse and dependence in SZ [8]. As dependence criteria in DSM-IV includes “continued use despite having a psychological problems that is likely to have been caused or exacerbated by the substance used”, it may be that patients score more frequently on this item as they may be more vulnerable and sensitive to the effects of substances on their mental health (including symptoms of psychosis).
Polydrug use (2 or more substances) occurred in 40% of those who had a SUD, and “other substance use disorders” occurred in less than 1% of patients. There was a significant association between having a methamphetamine use disorder and cannabis use disorder, similar to findings from extant literature [44]. Interestingly, there was an even stronger significant association between having cannabis or methamphetamine use disorder and a methaqualone (sedative hypnotic) use disorder.
Consistent with meta-analyses of prevalence studies, we found a strong association between male sex and any SUD [3,4,5,6, 27, 45]. This association held across most multivariable models for different substances. Consistent with other studies, we found significant associations with younger age and SUDs, with the exception of alcohol use disorders which, similar to findings from meta-analyses, typically occur more equally spread across age groups, with high prevalence in older groups [4,5,6, 15].
We found that patients from the Eastern Cape Province were significantly less likely to use methamphetamines or methaqualone, perhaps due to the fact that methamphetamine use is currently particularly prevalent to the Western Cape [46]. This is echoed in the international literature with meta-analyses showing significant differences between geographic regions (i.e. USA and Australia vs. Europe and UK) and stimulant use prevalence among patients with psychotic disorders [6]. As the Western Cape sample was from the densely urbanized Cape Town compared to the less urbanized Eastern Cape, urbanization and associated social circumstances, such as the drug and gang culture in certain urbanized neighbourhoods, with rife and easy access to methamphetamines, may have also contributed to the higher rates of methamphetamine and methaqualone use seen in the Western Cape [47, 48]. The fact that in the total sample methamphetamine use disorders occurred in a somewhat lower rate of 4.7%, less than half of that found in meta-analyses of prevalence studies of amphetamines [6], may also be as a result of the fact that over half of our samples are from the lower urbanized Eastern Cape Province.
Moreover, there was a significant association with being an inpatient as opposed to an outpatient and a having any SUD, cannabis use disorder and methamphetamine use disorders, which perhaps reflects the illness severity of those people with a dual diagnosis. Of note, participants with cannabis use disorders had significant higher odds of having three of more compared to no prior hospitalisations. In one meta-analysis, cannabis use disorders were more prevalent among inpatients (31.3% vs. 25.2%), but this difference did not reach statistical significance [5]. Similarly, in studies with hospitalised patients, there was a significantly higher prevalence of stimulant use disorders (cocaine and amphetamines) compared to studies with community samples [6].
For diagnosis, we found a positive association with having a schizoaffective disorder and a diagnosis of other SUDs (cocaine, ecstasy and heroin). Interestingly, in one meta-analysis of prevalence studies (that included a high number of studies with cocaine, ecstasy and mixed stimulant use), there was a significant association between stimulant disorders and an affective psychosis diagnosis in univariate meta-regression which was no longer significant after adjusting for geographic and treatment setting in meta-regression models.
Axis IV economic problems were significantly more prevalent in those with any SUD and only legal problems remained significantly associated with cannabis use disorders in multivariable models. These findings are somewhat different from one other study that found significant economic and legal problems in unadjusted models, with only economic problems remaining significant in adjusted models [21]. The significant association in our sample with legal problems and cannabis use may relate to arrests as result of illicit drug use or trade but could also be as a result of being the victim of crime.
Despite the low prevalence of anxiety disorders, PTSD and OCD, we found a significant association between having PTSD symptoms and a SUD, alcohol use disorders in particular. Anxiety disorders were significantly associated with having an “other SUD”. This finding resonates with that of the South African Stress and health study that found a strong association with anxiety disorders and PTSD in particular and substance use disorders notably alcohol use disorders [49]. Studies in patients with severe mental illness (including schizophrenia spectrum disorders) have shown that subjective distress and negative coping styles mediate the relationship between PTSD symptoms and alcohol consumption, providing evidence for the self-medication hypothesis [50, 51]. We could speculate that similar mechanism may be at play in our sample; however, the possibility of alternative explanations remains, such as impairments in executive control and impulsivity associated with schizophrenia and co-occurring alcohol and drug use, which may lead to risky behaviours and place patients at risk of trauma and potential PTSD.
Implications for clinicians
Our findings have a number of potential implications for clinicians treating patients with SZ. Within this population of Xhosa speaking patients from a variety of treatment settings varying from inpatient settings and community health clinics across two provinces in South Africa, almost half the participants had a SUD. Screening for these disorders would be of particular importance, and significant clinical predictors were younger age and male sex. The fact that both cannabis and methamphetamine use disorders were associated with inpatient status may underline concerns that these substances may be related to increasing admissions due to their potential for triggering underlying psychotic states [52, 53], and association with higher hospitalisation rates [54]. Another important finding was the significant association of PTSD symptoms with any substance used disorder, alcohol use disorders in particular. Screening for trauma and related PTSD symptoms would be particularly important in this population, specifically patients with alcohol use disorders. Economic problems (extreme poverty, inadequate finances, insufficient welfare support) and legal problems (i.e. involvement with the law police arrests) are also significantly elevated, underlying the importance of an assessment of problems with finances (and its relationship with drug use) and liaison with the criminal justice system when patients have problems in this area.
Study strengths and limitations
This study is the first large clinical survey investigating the prevalence of substances in the Xhosa speaking schizophrenia population in the South African setting using a semi-structured clinical interview. To improve validity, this study utilised an isiXhosa language version of the SCID-I to determine clinical and substance related diagnoses. Nevertheless, there are some limitations to our findings. First, the sample was selected from volunteers with a clinical diagnosis of SZ who attended treatment centres. We cannot rule out that the exclusion of some patients who were not treatment seeking or in treatment may have had an impact on the estimation of the prevalence of SUDs. Our sample is also heavily skewed towards male participants, possibly as a result of the study recruitment procedures that focused on participants admitted to psychiatric hospitals, where male sex predominated. This makes the findings less generalizable to females with schizophrenia. In addition, the lack of biological validation of substance use is an important shortcoming of this study. Nevertheless, validation of drug use in diverse samples such this one that contains inpatients at hospitals as well as community patients, are less than ideal, with several issues, such as short detection windows of drug tests and limited practical methods for assessment of alcohol use disorders. Furthermore, multiple sources of information (clinical interview, case notes) were used to assess substance use, and studies have demonstrated that clinical methods can be accurate in diagnosing substance use disorders. The use of the SCID-I to determine comorbid anxiety, post-traumatic and obsessive disorders could have led to lower frequencies of anxiety disorders due to the use of diagnostic hierarchy rules. This, however, could have been offset to a degree by including in our analyses, sub-threshold anxiety symptoms. Furthermore, we did not have data on lifetime depressive symptoms and our analysis was limited to current (past month) MDE and depressive symptoms.
Conclusion
This study demonstrated a high occurrence of comorbid SUD in people with SZ and underlines the importance of conducting an assessment for SUDs in patients with SZ. Having a dual diagnosis was also associated with certain characteristics, such as younger age, male sex, higher inpatient status, more prior admissions, economic and legal problems, as well as high levels of post-traumatic stress disorder symptomology. This underscores the importance of also assessing the presence of these factors in patients who have both SZ and a SUD.
References
Lambert M, Conus P, Lubman DI, Wade D, Yuen H, Moritz S, Naber D, McGorry PD, Schimmelmann BG (2005) The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand 112(2):141–148. https://doi.org/10.1111/j.1600-0447.2005.00554.x
Wade D, Harrigan S, McGorry PD, Burgess PM, Whelan G (2007) Impact of severity of substance use disorder on symptomatic and functional outcome in young individuals with first-episode psychosis. J Clin Psychiatry 68(5):767–774
Cantor-Graae E, Nordstrom LG, McNeil TF (2001) Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Res 48(1):69–82
Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J (2009) Prevalence of alcohol use disorders in schizophrenia—a systematic review and meta-analysis. Acta Psychiatr Scand 120(2):85–96. https://doi.org/10.1111/j.1600-0447.2009.01385.x
Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J (2010) Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull 36(6):1115–1130. https://doi.org/10.1093/schbul/sbp031
Sara GE, Large MM, Matheson SL, Burgess PM, Malhi GS, Whiteford HA, Hall WD (2015) Stimulant use disorders in people with psychosis: a meta-analysis of rate and factors affecting variation. Aust N Zeal J Psychiatry 49(2):106–117. https://doi.org/10.1177/0004867414561526
Ayano G (2019) Co-occurring medical and substance use disorders in patients with schizophrenia: a systematic review. Int J Ment Health 48(1):62–76. https://doi.org/10.1080/00207411.2019.1581047
Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB (2018) Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: systematic review and meta-analysis. Drug Alcohol Depend 191:234–258. https://doi.org/10.1016/j.drugalcdep.2018.07.011
Gut-Fayand A, Dervaux A, Olie JP, Loo H, Poirier MF, Krebs MO (2001) Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry Res 102(1):65–72
Kamali M, Kelly L, Gervin M, Browne S, Larkin C, O'Callaghan E (2000) The prevalence of comorbid substance misuse and its influence on suicidal ideation among in-patients with schizophrenia. Acta Psychiatr Scand 101(6):452–456
Cuffel BJ, Heithoff KA, Lawson W (1993) Correlates of patterns of substance abuse among patients with schizophrenia. Hosp Community Psychiatry 44(3):247–251
Jimenez-Castro L, Hare E, Medina R, Raventos H, Nicolini H, Mendoza R, Ontiveros A, Jerez A, Munoz R, Dassori A, Escamilla M (2010) Substance use disorder comorbidity with schizophrenia in families of Mexican and Central American ancestry. Schizophr Res 120(1–3):87–94. https://doi.org/10.1016/j.schres.2010.02.1053
Kerfoot KE, Rosenheck RA, Petrakis IL, Swartz MS, Keefe RS, McEvoy JP, Stroup TS (2011) Substance use and schizophrenia: adverse correlates in the CATIE study sample. Schizophr Res 132(2–3):177–182. https://doi.org/10.1016/j.schres.2011.07.032
Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K (2004) Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res 67(2–3):157–166. https://doi.org/10.1016/s0920-9964(02)00523-6
Rush B, Koegl CJ (2008) Prevalence and profile of people with co-occurring mental and substance use disorders within a comprehensive mental health system. Can J Psychiatry 53(12):810–821. https://doi.org/10.1177/070674370805301207
Goodwin RD, Amador XF, Malaspina D, Yale SA, Goetz RR, Gorman JM (2003) Anxiety and substance use comorbidity among inpatients with schizophrenia. Schizophr Res 61(1):89–95
Scheller-Gilkey G, Moynes K, Cooper I, Kant C, Miller AH (2004) Early life stress and PTSD symptoms in patients with comorbid schizophrenia and substance abuse. Schizophr Res 69(2–3):167–174
Seedat F, Roos JL, Pretorius HW, Karayiorgou M, Nel B (2007) Prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive compulsive symptoms in Afrikaner schizophrenia and schizoaffective disorder patients. Afr J Psychiatry (Johannesbg) 10(4):219–224
Shoval G, Zalsman G, Apter A, Diller R, Sher L, Weizman A (2007) A 10-year retrospective study of inpatient adolescents with schizophrenia/schizoaffective disorder and substance use. Compr Psychiatry 48(1):1–7. https://doi.org/10.1016/j.comppsych.2006.05.002
Carra G, Crocamo C, Borrelli P, Popa I, Ornaghi A, Montomoli C, Clerici M (2015) Correlates of dependence and treatment for substance use among people with comorbid severe mental and substance use disorders: findings from the “Psychiatric and Addictive Dual Disorder in Italy (PADDI)” Study. Compr Psychiatry 58:152–159. https://doi.org/10.1016/j.comppsych.2014.11.021
Compton MT, Simmons CM, Weiss PS, West JC (2011) Axis IV psychosocial problems among patients with psychotic or mood disorders with a cannabis use disorder comorbidity. Am J Addict 20(6):563–567. https://doi.org/10.1111/j.1521-0391.2011.00184.x
Compton MT, Weiss PS, West JC, Kaslow NJ (2005) The associations between substance use disorders, schizophrenia-spectrum disorders, and Axis IV psychosocial problems. Soc Psychiatry Psychiatr Epidemiol 40(12):939–946. https://doi.org/10.1007/s00127-005-0964-4
Helseth V, Lykke-Enger T, Johnsen J, Waal H (2009) Substance use disorders among psychotic patients admitted to inpatient psychiatric care. Nord J Psychiatry 63(1):72–77. https://doi.org/10.1080/08039480802450439
Paruk S, Burns JK, Caplan R (2013) Cannabis use and family history in adolescent first episode psychosis in Durban, South Africa. J Child Adolesc Ment Health 25(1):61–68. https://doi.org/10.2989/17280583.2013.767264
Taukoor B, Paruk S, Karim E, Burns JK (2017) Substance use in adolescents with mental illness in Durban, South Africa. J Child Adolesc Ment Health 29(1):51–61. https://doi.org/10.2989/17280583.2017.1318395
Vos PJ, Cloete KJ, le Roux A, Kidd M, Jordaan GP (2010) A retrospective review of trends and clinical characteristics of methamphetamine-related acute psychiatric admissions in a South African context. Afr J Psychiatry 13(5):390–394
Weich L, Pienaar W (2009) Occurrence of comorbid substance use disorders among acute psychiatric inpatients at Stikland Hospital in the Western Cape, South Africa. Afr J Psychiatry 12(3):213–217
First M, RL S, M G, JBW W (1994) Structured clinical interview for DSM-IV Axis I disorders-Patient Edition (SCID-I-P, version 2)
https://www.scid4.org Structured Clinical Interview for DSM-IV. Biometrics Research Department. Accessed 15 Jan 2018
Campbell MM, Susser E, Mall S, Mqulwana SG, Mndini MM, Ntola OA, Nagdee M, Zingela Z, Van Wyk S, Stein DJ (2017) Using iterative learning to improve understanding during the informed consent process in a South African psychiatric genomics study. PLoS ONE 12(11):e0188466. https://doi.org/10.1371/journal.pone.0188466
Mall S, Platt JM, Temmingh H, Musenge E, Campbell M, Susser E, Stein DJ (2019) The relationship between childhood trauma and schizophrenia in the Genomics of Schizophrenia in the Xhosa people (SAX) study in South Africa. Psychol Med. https://doi.org/10.1017/s0033291719001703
Koen L, Niehaus DJ, Wright G, Warnich L, De Jong G, Emsley RA, Mall S (2012) Chromosome 22q11 in a Xhosa schizophrenia population. S Afr Med J 102(3 Pt 1):165–166. https://doi.org/10.7196/samj.5326
South African Institute of Race Relations. South African Survey Online (2003) www.irr.org.za. Accessed 15 Jan 2014
Endicott J, Spitzer RL, Fleiss JL, Cohen J (1976) The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33(6):766–771
Sartorius N, Janca A (1996) Psychiatric assessment instruments developed by the World Health Organization. Soc Psychiatry Psychiatr Epidemiol 31(2):55–69
Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kim K, Meeks T, Kraemer HC (2007) A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry 64(8):966–974. https://doi.org/10.1001/archpsyc.64.8.966
Rubin D (1987) Multiple imputation for nonresponse in surveys. Wiley, New York
StataCorp (2013) Stata statistical software: release 13. StataCorp LP, College Station
Thungana Y, Zingela Z, van Wyk S (2019) First-episode psychosis and substance use in Nelson Mandela Bay: findings from an acute mental health unit. S Afr J Psychiatry 25:1372. https://doi.org/10.4102/sajpsychiatry.v25i0.1372
Herman AA, Stein DJ, Seedat S, Heeringa SG, Moomal H, Williams DR (2009) The South African Stress and Health (SASH) study: 12-month and lifetime prevalence of common mental disorders. S Afr Med J 99(5 Pt 2):339–344
Gregg L, Barrowclough C, Haddock G (2007) Reasons for increased substance use in psychosis. Clin Psychol Rev 27(4):494–510. https://doi.org/10.1016/j.cpr.2006.09.004
Gage SH, Hickman M, Zammit S (2016) Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiat 79(7):549–556. https://doi.org/10.1016/j.biopsych.2015.08.001
Mané A, Fernández-Expósito M, Bergé D, Gómez-Pérez L, Sabaté A, Toll A, Diaz L, Diez-Aja C, Perez V (2015) Relationship between cannabis and psychosis: reasons for use and associated clinical variables. Psychiatry Res 229(1–2):70–74. https://doi.org/10.1016/j.psychres.2015.07.070
Sara GE, Burgess PM, Malhi GS, Whiteford HA, Hall WC (2014) Stimulant and other substance use disorders in schizophrenia: prevalence, correlates and impacts in a population sample. Aust N Zeal J Psychiatry 48(11):1036–1047. https://doi.org/10.1177/0004867414533838
Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, Canive JM, Miller DD, Reimherr F, McGee M, Khan A, Van Dorn R, Rosenheck RA, Lieberman JA (2006) Substance use in persons with schizophrenia: baseline prevalence and correlates from the NIMH CATIE study. J Nerv Ment Dis 194(3):164–172. https://doi.org/10.1097/01.nmd.0000202575.79453.6e
Pluddemann A, Dada S, Parry CD, Kader R, Parker JS, Temmingh H, van Heerden S, de Clercq C, Lewis I (2013) Monitoring the prevalence of methamphetamine-related presentations at psychiatric hospitals in Cape Town, South Africa. Afr J Psychiatry (Johannesbg) 16(1):45–49
Hobkirk AL, Watt MH, Myers B, Skinner D, Meade CS (2016) A qualitative study of methamphetamine initiation in Cape Town, South Africa. Int J Drug Pol 30:99–106. https://doi.org/10.1016/j.drugpo.2015.10.006
Morgan N, Mall S (2019) Pathways between urbanization and harmful substance use. Curr Opin Psychiatry 32(3):218–223. https://doi.org/10.1097/yco.0000000000000488
Saban A, Flisher AJ, Grimsrud A, Morojele N, London L, Williams DR, Stein DJ (2014) The association between substance use and common mental disorders in young adults: results from the South African Stress and Health (SASH) Survey. Pan Afr Med J 17(Suppl 1):11. https://doi.org/10.11694/pamj.supp.2014.17.1.3328
O'Hare T, Sherrer MV, Shen C (2006) Subjective distress from stressful events and high-risk behaviors as predictors of PTSD symptom severity in clients with severe mental illness. J Trauma Stress 19(3):375–386. https://doi.org/10.1002/jts.20131
O'Hare T, Sherrer M (2011) Drinking motives as mediators between PTSD symptom severity and alcohol consumption in persons with severe mental illnesses. Addict Behav 36(5):465–469. https://doi.org/10.1016/j.addbeh.2011.01.006
Andrade C (2016) Cannabis and neuropsychiatry, 2: the longitudinal risk of psychosis as an adverse outcome. J Clin Psychiatry 77(6):e739–742. https://doi.org/10.4088/JCP.16f10918
Hermens DF, Lubman DI, Ward PB, Naismith SL, Hickie IB (2009) Amphetamine psychosis: a model for studying the onset and course of psychosis. Med J Aust 190(4 Suppl):S22–25
Ouellet-Plamondon C, Abdel-Baki A, Salvat E, Potvin S (2017) Specific impact of stimulant, alcohol and cannabis use disorders on first-episode psychosis: 2-year functional and symptomatic outcomes. Psychol Med 47(14):2461–2471. https://doi.org/10.1017/s0033291717000976
Acknowledgements
We wish to acknowledge the support of Dr Adele Pretorius, project manager of the SAX study as well as the Cape Town and New York based SAX data teams, notably Megan Malan, Bronwyn Malagas, Howard Andrews and Kim Fader for their assistance with data management, quality control and access. We would like to thank Dr. Adam Baldinger, Prof. Mo Nagdee and Prof. Zuki Zingela for their support and groundwork in the Western and Eastern Cape SAX study sites.
Funding
The SAX study received funding from the National Institute of Mental Health (NIMH: Grant No. 5UO1MH096754) and is a member of the Human Heredity and Health in Africa Consortium (H3 Africa) (https://www.h3africa.org/). SM has received support from the Harry Crossley and National Research Foundation of South Africa’s post-doctoral research fellowships, a Claude Leon Foundation early career research award and a seed award from the School of Public Health, University of the Witwatersrand, Johannesburg, South Africa. SM has received funding from the Columbia University Southern African AIDS International Training/Research Program.
Author information
Authors and Affiliations
Contributions
HT conceptualised the study, analysed the data and wrote the first and subsequent drafts. All other authors contributed to the first and subsequent drafts.
Corresponding author
Ethics declarations
Conflict of interest
HT, ES, SM, MC, and GS and have not received any financial or commercial related compensation that can be considered to represent a conflict of interest. DJS is supported by the Medical Research Council of South Africa and has received research grants and/or consultancy honoraria from: Abbott, Astrazeneca, Eli-Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Johnson & Johnson, Lundbeck, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, Sun, Takeda, Tikvah, and Wyeth. DJS is also on the scientific advisory board of the TLC Foundation for Body-Focused Repetitive Behaviours, the Anxiety and Depression Association of America (ADAA), and the South African Depression and Anxiety Support Group. SM has received incentive funding for rated researchers from the South African National Research Foundation (NRF) (Grant No. 119375).
Ethics approval
The Human Ethics Committee of the Faculty of Health Sciences, University of Cape Town, South Africa granted approval to the study (HREC No. 049/2013). In addition, ethical approval was also obtained from the Walter Sisulu University Research Ethics and Biosafety Committee, The Rhodes University Ethical Standards Committee, The Columbia University Internal Review Board and the University of Washington Institutional Review Board. To ensure that participants understood and had sufficient decisional capacity to understand the nature of the study, the University of California, San Diego Brief Assessment of Capacity to Consent (UBACC) questionnaire [30] was administered to all participants.
Rights and permissions
About this article
Cite this article
Temmingh, H., Susser, E., Mall, S. et al. Prevalence and clinical correlates of substance use disorders in South African Xhosa patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol 56, 695–706 (2021). https://doi.org/10.1007/s00127-020-01942-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00127-020-01942-5