Abstract
Aims/hypothesis
The aim of this study was to compare the effectiveness of stand-alone intermittently scanned continuous glucose monitoring (isCGM) with or without a structured education programme and blood glucose monitoring (BGM) in adults with type 2 diabetes on multiple daily insulin injections (MDI).
Methods
In this 24 week randomised open-label multicentre trial, adults with type 2 diabetes on intensive insulin therapy with HbA1c levels of 58–108 mmol/mol (7.5–12.0%) were randomly assigned in a 1:1:1 ratio to isCGM with a structured education programme on adjusting insulin dose and timing according to graphical patterns in CGM (intervention group), isCGM with conventional education (control group 1) or BGM with conventional education (control group 2). Block randomisation was conducted by an independent statistician. Due to the nature of the intervention, blinding of participants and investigators was not possible. The primary outcome was change in HbA1c from baseline at 24 weeks, assessed using ANCOVA with the baseline value as a covariate.
Results
A total of 159 individuals were randomised (n=53 for each group); 148 were included in the full analysis set, with 52 in the intervention group, 49 in control group 1 and 47 in control group 2. The mean (± SD) HbA1c level at baseline was 68.19±10.94 mmol/mol (8.39±1.00%). The least squares mean change (± SEM) from baseline HbA1c at 24 weeks was −10.96±1.35 mmol/mol (−1.00±0.12%) in the intervention group, −6.87±1.39 mmol/mol (−0.63±0.13%) in control group 1 (p=0.0367 vs intervention group) and −6.32±1.42 mmol/mol (−0.58±0.13%) in control group 2 (p=0.0193 vs intervention group). Adverse events occurred in 28.85% (15/52) of individuals in the intervention group, 26.42% (14/53) in control group 1 and 48.08% (25/52) in control group 2.
Conclusions/interpretation
Stand-alone isCGM offers a greater reduction in HbA1c in adults with type 2 diabetes on MDI when education on the interpretation of graphical patterns in CGM is provided.
Trial registration
ClinicalTrials.gov NCT04926623.
Funding
This study was supported by Daewoong Pharmaceutical Co., Ltd.
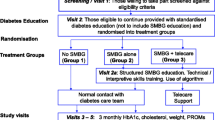
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Continuous glucose monitoring (CGM) provides real-time information on high and low glucose patterns, glucose change directions and glycaemic variability that cannot be obtained by HbA1c or a limited number of daily fingerstick blood glucose monitoring (BGM) [1,2,3,4,5,6]. RCTs in individuals with type 1 diabetes have consistently demonstrated the advantages of both real-time CGM [7,8,9,10,11,12,13] and intermittently scanned CGM (isCGM) [14] in either lowering HbA1c levels [7,8,9,10,11,12,13] or minimising hypoglycaemia [14,15,16,17,18,19,20]. One study that lacked adequate educational support, however, failed to show these benefits [21], thus highlighting the importance of sufficient education in leveraging CGM for effective glycaemic control in type 1 diabetes.
In individuals with type 2 diabetes managed by multiple daily insulin injections (MDI), Beck et al reported that the use of CGM as an adjunct to BGM could improve glycaemic control [22]. However, Haak et al found no reduction in HbA1c in an RCT assessing the efficacy of stand-alone CGM to replace BGM [5]. These inconsistent results may be associated with the provision of a structured education in CGM. Hermanns et al demonstrated the efficacy of a structured education and treatment programme designed for individuals with diabetes using CGM [23]. In fact, we recently showed that a structured education programme on the adjustment of insulin dose and timing according to the graphical patterns of CGM was a requisite for the sustained benefit of real-time CGM in individuals with type 1 diabetes on MDI [24]. In contrast, an RCT involving individuals with type 2 diabetes not using prandial insulin demonstrated the effectiveness of CGM in glycaemic control without the need for additional educational input beyond standard clinical practice [25]. This suggests that the necessity for educational support in optimising CGM use may vary according to factors such as endogenous insulin secretion and insulin treatment regimens. It remains to be determined whether individuals with type 2 diabetes on MDI would benefit from a structured education programme that goes beyond standard clinical practice, especially considering their potential residual endogenous insulin secretion that might compensate for insulin dosing errors to some extent.
The Freestyle Libre-based Education on MDI in type 2 diabetes (FreEdoM-2) trial assessed whether isCGM could replace BGM effectively in individuals with type 2 diabetes on intensive insulin therapy. The FreEdoM-2 trial also evaluated the need for a structured education programme on the adjustment of insulin dose and timing according to the graphical patterns of CGM. We compared groups using either isCGM with a structured education programme, isCGM with conventional education, or BGM with conventional education.
Methods
Study design and participants
This was a prospective open-label multicentre RCT. The trial was conducted at eight tertiary medical centres in South Korea in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent. This study was approved by the institutional review board (IRB) of Samsung Medical Center (IRB approval no. 2020-11-083-002) and registered at ClinicalTrials.gov (registration no. NCT04926623). The trial protocol is provided in electronic supplementary material (ESM) Methods.
We enrolled participants aged 19–74 years old with type 2 diabetes who were treated with MDI or insulin pump for 12 weeks or more and had HbA1c levels of 58–108 mmol/mol (7.5–12.0%). MDI was defined as an injection of basal insulin plus two or more prandial insulin injections per day. Basal insulin included ultra-long-acting insulin and long-acting insulin but not intermediate-acting insulin. If ultra-long-acting insulin was used in a premixed form, the use of two or more injections of prandial insulin was required for eligibility. People with diabetes who met the current reimbursement criteria of the Korean National Health Insurance service for type 1 diabetes were not eligible. According to these criteria, type 1 diabetes could be diagnosed in insulin users who met at least one of the following criteria: fasting C-peptide ≤0.2 nmol/l; glucagon or meal stimulated C-peptide ≤0.6 nmol/l; positive for glutamic-acid-decarboxylase and/or other autoantibodies; 24 h urine C-peptide <30 µg/day; or a history of diabetic ketoacidosis at the time of diabetes diagnosis [26]. Key exclusion criteria included severe comorbidities preventing participation in education, acute diabetic complications requiring emergent treatment in the preceding 12 weeks, and pregnancy (ESM Methods provides a complete list of the exclusion criteria). The enrolled participants were considered representative of the target trial population with respect to sex and age.
Randomisation and masking
Participants were centrally randomised in a 1:1:1 ratio to isCGM with a structured education programme (intervention group), isCGM with conventional education (control group 1), and BGM with conventional education (control group 2). Prior to the commencement of the study, an independent statistician, not associated with the study, generated a randomisation table with a block size of either 6 or 9. Block randomisation was conducted using SAS (Version 9.4; SAS Institute, Cary, NC, USA). After the screening, eligible participants were randomised by an interactive web response system. Due to the nature of the intervention, blinding of participants and investigators was not possible.
Study procedures
A flow diagram of the study design is shown in ESM Fig. 1. After informed consent was provided by participants, age, sex and history of comorbidity and medication were investigated. Sex was determined based on self-report. Participants underwent anthropometric measurements, including height and weight measurements, vital sign measurements, and laboratory tests including HbA1c levels. All participants underwent blinded CGM, which concealed glucose measurements from participants but allowed investigators to review them retrospectively, for 2 weeks immediately before the baseline visit. Then, the participants in the intervention group and control group 1 underwent isCGM from the baseline visit for 24 weeks. Participants in control group 2 were asked to do self-monitoring of blood glucose for 22 weeks and then underwent blinded CGM for 2 weeks (from week 22 to week 24). FreeStyle Libre 1 (Abbott Diabetes Care, Witney, Oxon, UK) was given for both blinded CGM and isCGM during the study period. BGM devices were not provided but the participants were asked to use a glucometer of their own.
A structured education programme was given at baseline (week 0), 4, 8, 12 and 18 weeks. Education at 4, 8 and 18 weeks could be given via telephone calls. The education could be omitted if the percentage of time in range (TIR; the time that blood glucose readings are within the target glucose range [3.9–10.0 mmol/l]) during that time was ≥80%, upon the judgement of the investigators. The structured education included individual education on adjustment of insulin dose and timing according to blood glucose level and the graphical patterns of the CGM [27]. The summary of education contents was as follows: the ideal dose of basal insulin will result in a flat glucose trend overnight within the target range; appropriate dose and timing of pre-meal rapid-acting insulin bolus will result in postprandial glucose excursion ≤10.0 mmol/l with return to target over 4 h; if postprandial glucose excursion is above the target range but returns to target after 4 h, the timing of insulin injection and/or meal composition needs to be adjusted; and, on the contrary, if the postprandial glucose excursion does not return to target after 4 h, the dose of insulin and/or meal composition needs to be adjusted. The time consumed by structured education was recorded on iKooB (iKooB, Seoul, South Korea), a digital patient education platform. For control groups 1 and 2, conventional education was given at baseline and 12 weeks. Conventional education included instructions regarding adjustment of insulin dose according to blood glucose level and how to use the isCGM device. Both structured and conventional education were provided by the diabetes educators at each centre.
HbA1c was measured with HLC-723G11 (Tosoh Corporation, Tokyo, Japan) in the central laboratory at baseline, 12 and 24 weeks. Treatment satisfaction was measured by the Diabetes Treatment Satisfaction Questionnaire (DTSQ) at baseline, 12 and 24 weeks.
Study outcomes
The primary outcome was the change in HbA1c from baseline to week 24. The key secondary outcomes were as follows: change in HbA1c from baseline to week 12; change in percentage of TIR from baseline to weeks 22–24; change in percentage of time above range (TAR) >10.0 mmol/l from baseline to weeks 22–24; change in percentage of TAR >13.9 mmol/l from baseline to weeks 22–24; change in percentage of time below range (TBR) <3.9 mmol/l from baseline to weeks 22–24; change in percentage of TBR <3.0 mmol/l from baseline to weeks 22–24; change in mean glucose level from baseline to weeks 22–24; change in the glucose CV from baseline to weeks 22–24; and change in treatment satisfaction estimated by DTSQ from baseline to 12 and 24 weeks.
Exploratory secondary outcomes included the number of sensor scans (intervention group and control group 1) and frequency of glucose finger-sticks (control group 2). Safety outcomes included all adverse events.
Statistical analysis
We calculated that a sample size of 45 per group was necessary to provide at least 80% power to detect a difference in mean HbA1c level between treatment groups (intervention vs control group 2), assuming a population difference of 5.9 mmol/mol (0.54%), SD of 9.8 mmol/mol (0.90%), and a significance level of 5%, based on previous trials [28,29,30]. Considering the 15% potential loss to follow-up, the sample size was set at 53 per group. We also aimed to compare the intervention group and control group 1; thus, a total of 159 participants were planned to be enrolled.
Analyses followed the intention-to-treat principle and were conducted in a full analysis (FA) set. The FA set included participants who received primary outcome measurements. Per-protocol set analyses were also performed for those who did not violate the study protocol. Safety analysis was done for participants receiving at least one education session (safety set). Missing values in the FA set were imputed using the last-observation-carried-forward approach. Safety set and per-protocol analyses only used the available data.
Data distributions were tested for normality using the Shapiro–Wilk test. The continuous variables at baseline were compared between groups by one-way ANOVA or the Kruskal–Wallis test. For outcome analyses, changes were compared using ANCOVA, with the baseline value as a covariate. Additionally, ANCOVA with both the baseline value and study centre as covariates (not included in the initial statistical analysis plan) was conducted. We first calculated the p values for within-group differences. Next, we compared the changes between groups. Multiple comparisons were also done to compare the intervention group with control group 1, and the intervention group with control group 2. Categorical variables are presented as n (%), and Pearson’s χ2 test or Fisher’s exact test was used for comparisons between groups. For exploratory secondary analyses, Pearson’s correlation analyses were done between the number of sensor scans and CGM metrics.
All statistical analyses were performed using SAS Version 9.4 (SAS Institute). Statistical significance was set at a two-sided p value <0.05. Since the multiple comparisons were exploratory analyses, correction for significance level was not performed.
Results
Study recruitment and baseline characteristics of participants
Between 1 July 2021 and 27 October 2022, 159 participants were randomised to the intervention group, control group 1 or control group 2 in a 1:1:1 ratio. A total of 148 individuals were included in the FA set, with 52 in the intervention group, 49 in control group 1 and 47 in control group 2. Among them, 52 in the intervention group, 47 in control group 1 and 46 in control group 2 completed the study. The flowchart of study participation is shown in ESM Fig. 2. The mean (± SD) total amount of time spent on the structured education programme was 3.12±0.95 h for each participant.
Table 1 shows the participants’ baseline characteristics, which were well-balanced between groups. The mean (± SD) age was 57.73±10.58 years and 85/159 (53.46%) were men. The mean (± SD) duration of diabetes was 17.09±10.10 years and 44/159 (27.67%) had previous experience of using CGM. The mean (± SD) fasting C-peptide level was 0.52±0.52 nmol/l. There was one insulin pump user in control group 2. The most common glucose-lowering drug used by participants, other than insulin, was metformin (86.16%), followed by dipeptidyl peptidase-4 inhibitor (42.77%), sodium–glucose cotransporter 2 inhibitor (41.51%), thiazolidinedione (11.95%), sulfonylurea (8.18%) and glucagon-like peptide-1 receptor agonist (3.77%).
Change in HbA1c
Figure 1 and Table 2 present the change in HbA1c levels. The mean (± SD) HbA1c level at baseline was 68.19±10.94 mmol/mol (8.39±1.00%). At week 12, the least squares (LS) mean change (± SEM) from baseline in HbA1c was −10.74±1.28 mmol/mol (−0.98±0.12%) in the intervention group, −6.18±1.34 mmol/mol (−0.57±0.12%) in control group 1 (p=0.0152 vs intervention group) and −4.45±1.34 mmol/mol (−0.41±0.12%) in control group 2 (p=0.0009 vs intervention group). For the primary outcome, the LS mean change in HbA1c from baseline to 24 weeks was −10.96±1.35 mmol/mol (−1.00±0.12%) in the intervention group, −6.87±1.39 mmol/mol (−0.63±0.13%) in control group 1 (p=0.0367 vs intervention group) and −6.32±1.42 mmol/mol (−0.58±0.13%) in control group 2 (p=0.0193 vs intervention group). Additional analyses, including ANCOVA with baseline values and study centre as covariates (ESM Table 1) and per-protocol set analyses (data not shown), yielded similar results.
Change in HbA1c levels over time. Data are presented as mean ± SEM. At week 12, LS mean change ± SEM from baseline in HbA1c was −10.74±1.28 mmol/mol (–0.98±0.12%) in the intervention group, −6.18±1.34 mmol/mol (–0.57±0.12%) in control group 1 and −4.45±1.34 mmol/mol (–0.41±0.12%) in control group 2. At week 24, LS mean change in HbA1c from baseline was −10.96±1.35 mmol/mol (–1.00±0.12%) in the intervention group, −6.87±1.39 mmol/mol (–0.63±0.13%) in control group 1 and −6.32±1.42 mmol/mol (–0.58±0.13%) in control group 2. Intervention group, isCGM with structured education; control group 1, isCGM with conventional education; control group 2, BGM with conventional education. **p<0.01 and ***p<0.001 for within-group differences; †p<0.05 and †††p<0.001 for between-group differences
ESM Fig. 3 presents the proportion of participants achieving the target HbA1c level of less than 53 mmol/mol (7%) at weeks 12 and 24. At week 24, 38.46% of participants in the intervention group achieved the target whereas 16.33% in control group 1 (p=0.0153 vs intervention group) and 19.15% in control group 2 (p=0.0467 vs intervention group) achieved the target HbA1c levels.
Change in CGM metrics
Figure 2 and Table 2 show the change in CGM metrics during the study period. TIR gradually increased as the education on the interpretation of the graphical patterns in CGM was repeated in the intervention group, with the improvement more prominent in the latter half of the study period (Fig. 2a). At week 24, LS mean change (± SEM) in TIR was +11.65±2.61% in the intervention group (p<0.0001 for within-group difference). In contrast, participants in control group 1 showed an increase in TIR at weeks 2, 12 and 18, but not at week 24. The difference in TIR at week 24 between the groups was not statistically significant.
Change in CGM metrics over time. (a) TIR. (b) TAR >10.0 mmol/l. (c) TAR >13.9 mmol/l. (d) TBR <3.9 mmol/l. (e) TBR <3.0 mmol/l. Data are presented as mean ± SEM. At baseline and 12 weeks, conventional education, Education (C), and structured education, Education (S), were provided face-to-face (represented by triangles with solid lines). Structured education at 4, 8 and 18 weeks could be delivered either face-to-face or via telephone calls, or it could be omitted if the percentage of time spent in range (3.9–10.0 mmol/l) was ≥80%, as determined by the investigators (indicated by triangles with dotted lines). Intervention group, isCGM with structured education; control group 1, isCGM with conventional education; control group 2, BGM with conventional education. *p<0.05, **p<0.01 and ***p<0.001 for within-group differences
In the intervention group, TAR showed a gradual decrease from baseline, with a more notable reduction in the latter half of the study period (Fig. 2b,c), although the differences in TAR at week 24 between groups were not significant. In control group 1, TAR significantly decreased from baseline at weeks 2 and 12 but this improvement was not maintained later in the study. At week 24, the LS mean (± SEM) change in TAR >10.0 mmol/l and TAR>13.9 mmol/l in the intervention group was −10.42±2.75% (p=0.0002 for within-group difference) and −7.90±2.08% (p=0.0002 for within-group difference), respectively.
TBR <3.9 mmol/l during the whole follow-up period (weeks 2–24) was in an acceptable range (<4%) [1] in all groups (Fig. 2d). However, at week 24, only participants in the intervention group had a reduced TBR <3.9 mmol/l compared with baseline (LS mean ± SEM: −1.15±0.44%; p=0.0093 for within-group difference). TBR <3.0 mmol/l decreased from baseline and remained in an acceptable range (<1%) [1] during the whole follow-up period (weeks 2–24) in both the intervention group and control group 1 (Fig. 2e). CV for glucose values also significantly decreased from baseline in both intervention and control group 1 at the end of the trial (Table 2).
Other secondary outcomes
Treatment satisfaction estimated by DTSQ significantly increased from baseline in both the intervention group and control group 1, at weeks 12 and 24 (ESM Fig. 4). The intervention group showed a greater improvement in DTSQ compared with control group 2.
The mean number of sensor scans per day was higher in the intervention group than in control group 1, with a similar clock-time distribution between groups (ESM Fig. 5a, 11.34±5.36 vs 9.51±6.25, p=0.0207). The number of daily mean frequency of sensor scans was correlated with TIR in both the intervention group (r=0.50, p=0.0002) and control group 1 (r=0.28, p=0.0481) (ESM Fig. 5b). The mean frequency of glucose finger-sticks in control group 2 was 2.43±1.20 per day.
Adverse events
Adverse events occurred in 28.85% (15/52) of individuals in the intervention group, 26.42% (14/53) in control group 1, and 48.08% (25/52) in control group 2 (ESM Table 2). A device adhesion problem occurred in one participant in control group 1. Diabetic ketoacidosis did not occur in any of the three groups.
Discussion
This study demonstrates that when BGM is replaced with stand-alone CGM, improved glycaemic control is achievable with structured education in individuals with type 2 diabetes on MDI. For the primary outcome of a reduction in HbA1c, isCGM with a structured education programme was superior not only to BGM but also to isCGM with conventional education. The superiority in HbA1c reduction was explained by a gradual decrease in TAR; this effect was more prominent in the latter half of the study period, as education on the interpretation of the graphical patterns of CGM was repeated in the intervention group. Importantly, this was achieved in the intervention group with a significantly reduced TBR <3.9 mmol/l and CV from baseline.
To the best of our knowledge, this is the first multicentre RCT achieving the primary endpoint of reducing HbA1c levels with stand-alone isCGM in individuals with type 2 diabetes on MDI. Although Beck et al reported that CGM use improved glycaemic control in individuals with type 2 diabetes receiving MDI [22], this study used CGM as an adjunct to BGM performed at least four times a day, not as stand-alone CGM. A previous RCT by Haak et al, assessing the efficacy of stand-alone isCGM, found no reduction in HbA1c, which was designated as the primary outcome [5]. Although one RCT showed a greater reduction of HbA1c as a secondary outcome with stand-alone isCGM, the primary endpoint of the study was patient satisfaction, which did not meet statistical significance during the trial [30]. In contrast to the study by Haak et al, our study found that stand-alone isCGM could effectively control blood glucose levels if it is provided with structured education on the interpretation of the graphical patterns of CGM. The benefit of stand-alone isCGM in individuals with type 2 diabetes who are on MDI is encouraging because many people do not check their blood glucose level frequently enough to optimally adjust insulin regimens with BGM [31].
Although previous studies have reported that structured education on the interpretation of CGM can improve glycaemic control in type 1 diabetes [32, 33], we did not know whether this is also important in type 2 diabetes. While Hermanns et al included individuals with type 2 diabetes in their study, most of the participants had type 1 diabetes [23]. The current study demonstrates that a structured education programme on the adjustment of insulin dose and timing according to the graphical patterns of CGM [27] is also essential for achieving profound and sustained benefits from stand-alone isCGM in individuals with type 2 diabetes on MDI. At least in part, these findings could be explained by the effect of educating appropriate timing for mealtime bolus insulin injection, by interpreting the graphical patterns of the CGM. Even with the same insulin doses, a mealtime bolus 15–20 min before a meal reduces postprandial glucose excursion by approximately 30% and also reduces postprandial hypoglycaemia compared with insulin administration immediately before the meal or after the meal [34]. Moreover, participants who had adjusted their prandial insulin based on peak postprandial glucose obtained by BGM would have benefited from CGM-based prandial insulin dose adjustment, which uses 4–5 h of postprandial glucose to differentiate the problem in timing and prandial insulin dose. This could explain the intervention group’s gradual improvement of TIR and TAR, which was achieved along with a reduction in TBR during the study period.
A notable distinction of our study is that we compared three groups: isCGM with a structured education programme; isCGM with conventional education; and BGM with conventional education. By comparing these three groups, we demonstrated that a structured education programme is essential for significant and sustained glycaemic benefits from stand-alone isCGM in individuals with type 2 diabetes on MDI. A reduction in HbA1c of −10.96±1.35 mmol/mol (−1.00±0.12%) in the intervention group in this study is impressive because CGM was associated with a modest reduction in HbA1c of 1.9 mmol/mol (0.17%) in a meta-analysis of RCTs comparing CGM with BGM in type 1 diabetes and type 2 diabetes [35]. This was consistent with the results of our previous study with individualised education for individuals with type 1 diabetes using CGM [24]. In that study, the difference in TIR between groups was remarkable (15.3%) [24] compared with those of a previous study providing group education (3.8%) [23]. The intervention group in the current study benefited from diabetes education tailored to individual needs, with a mean education duration of 3.1 h per participant. However, it might prove challenging to implement such a long education duration in busy clinical practice. Therefore, we advocate for policies that support time-intensive education, such as government funding for a systematic education programme.
Notably, a significant difference was observed in TBR <3.9 mmol/l and CV when comparing the intervention group with control group 2 but not when comparing the intervention group with control group 1. Given that Haak et al also reported a reduction in hypoglycaemia with isCGM [5], we speculate that isCGM alone might be effective for reducing hypoglycaemia regardless of the intensity of education.
In contrast to the current study, research on individuals with type 2 diabetes using basal insulin or oral glucose-lowering agents without prandial insulin [25, 36] has shown that CGM can successfully lower HbA1c even in a primary care setting with conventional education [25]. The discrepancy would be explained by the relative importance of CGM-based adjustment of prandial insulin in individuals with type 2 diabetes on intensive insulin therapy, this being more complicated than CGM-based lifestyle modification in those not on prandial insulin.
A limitation of our study is that the trial was conducted in tertiary medical centres where systematic education was delivered by specialists in diabetes, thus the effect of isCGM with education in primary care settings could be different. Second, the study was conducted only in South Korea, which could limit the generalisability of the results. Additionally, we did not conduct sex-based analyses. However, based on a previous study [23], structured education seems to be generally effective across different settings and among ethnically diverse populations. Third, although we planned to allow enrolment of insulin pump users, only one insulin pump user participated in this study, limiting the extrapolation of the results to insulin pump users. Fourth, study centres were not used as stratifying variables during randomisation; this could influence the effectiveness of isCGM with education across different centres. However, efficacy endpoint analyses using ANCOVA with both baseline values and study centre as covariates (ESM Table 1) yielded results consistent with the primary analyses using ANCOVA with only the baseline value as a covariate (Table 2). Finally, this study was not powered for comparison of CGM metrics between groups, and could only clarify the significant superiority of isCGM combined with structured education in terms of HbA1c improvement.
In conclusion, this RCT demonstrates that stand-alone isCGM combined with structured education offers a greater reduction in HbA1c in adults with type 2 diabetes on MDI compared with either isCGM with conventional education or BGM with conventional education. Unlike in type 2 diabetes without the requirement of prandial insulin, educational support beyond conventional diabetes education covering the adjustment of insulin dose and timing according to the graphical patterns of CGM would be a requisite for such benefit.
Abbreviations
- BGM:
-
Blood glucose monitoring
- CGM:
-
Continuous glucose monitoring
- DTSQ:
-
Diabetes Treatment Satisfaction Questionnaire
- FA:
-
Full analysis
- FreEdoM-2:
-
Freestyle Libre-based Education on MDI in type 2 diabetes
- isCGM:
-
Intermittently scanned CGM
- LS:
-
Least squares
- MDI:
-
Multiple daily insulin injections
- TAR:
-
Time above range
- TBR:
-
Time below range
- TIR:
-
Time in range
References
ElSayed NA, Aleppo G, Aroda VR et al (2023) 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1):S97–S110. https://doi.org/10.2337/dc23-S006
Yoo JH, Kim JH (2020) Time in range from continuous glucose monitoring: a novel metric for glycemic control. Diabetes Metab J 44(6):828–839. https://doi.org/10.4093/dmj.2020.0257
Yoo JH, Kim JH (2023) Advances in continuous glucose monitoring and integrated devices for management of diabetes with insulin-based therapy: improvement in glycemic control. Diabetes Metab J 47(1):27–41. https://doi.org/10.4093/dmj.2022.0271
Aleppo G, Ruedy KJ, Riddlesworth TD et al (2017) REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 40(4):538–545. https://doi.org/10.2337/dc16-2482
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6
Cappon G, Vettoretti M, Sparacino G, Facchinetti A (2019) Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J 43(4):383–397. https://doi.org/10.4093/dmj.2019.0121
Beck RW, Riddlesworth T, Ruedy K et al (2017) Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 317(4):371–378. https://doi.org/10.1001/jama.2016.19975
Lind M, Polonsky W, Hirsch IB et al (2017) Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 317(4):379–387. https://doi.org/10.1001/jama.2016.19976
Tumminia A, Crimi S, Sciacca L et al (2015) Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev 31(1):61–68. https://doi.org/10.1002/dmrr.2557
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW et al (2008) Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 359(14):1464–1476. https://doi.org/10.1056/NEJMoa0805017
Battelino T, Conget I, Olsen B et al (2012) The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 55(12):3155–3162. https://doi.org/10.1007/s00125-012-2708-9
Deiss D, Bolinder J, Riveline JP et al (2006) Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 29(12):2730–2732. https://doi.org/10.2337/dc06-1134
O’Connell MA, Donath S, O’Neal DN et al (2009) Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 52(7):1250–1257. https://doi.org/10.1007/s00125-009-1365-0
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R (2016) Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 388(10057):2254–2263. https://doi.org/10.1016/S0140-6736(16)31535-5
Hermanns N, Schumann B, Kulzer B, Haak T (2014) The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial. J Diabetes Sci Technol 8(3):516–522. https://doi.org/10.1177/1932296814524105
van Beers CA, DeVries JH, Kleijer SJ et al (2016) Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 4(11):893–902. https://doi.org/10.1016/S2213-8587(16)30193-0
Heinemann L, Freckmann G, Ehrmann D et al (2018) Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 391(10128):1367–1377. https://doi.org/10.1016/s0140-6736(18)30297-6
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch IB et al (2009) The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 32(8):1378–1383. https://doi.org/10.2337/dc09-0108
Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J (2011) Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 34(4):795–800. https://doi.org/10.2337/dc10-1989
Seyed Ahmadi S, Westman K, Pivodic A et al (2020) The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care 43(9):2017–2024. https://doi.org/10.2337/dc19-2606
Sequeira PA, Montoya L, Ruelas V et al (2013) Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther 15(10):855–858. https://doi.org/10.1089/dia.2013.0072
Beck RW, Riddlesworth TD, Ruedy K et al (2017) Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 167(6):365–374. https://doi.org/10.7326/m16-2855
Hermanns N, Ehrmann D, Schipfer M, Kröger J, Haak T, Kulzer B (2019) The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system: results of a randomized controlled trial. Diabetes Res Clin Pract 150:111–121. https://doi.org/10.1016/j.diabres.2019.03.003
Yoo JH, Kim G, Lee HJ, Sim KH, Jin SM, Kim JH (2022) Effect of structured individualized education on continuous glucose monitoring use in poorly controlled patients with type 1 diabetes: a randomized controlled trial. Diabetes Res Clin Pract 184:109209. https://doi.org/10.1016/j.diabres.2022.109209
Martens T, Beck RW, Bailey R et al (2021) Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 325(22):2262–2272. https://doi.org/10.1001/jama.2021.7444
Baek JH, Lee WJ, Lee BW et al (2021) Age at diagnosis and the risk of diabetic nephropathy in young patients with type 1 diabetes mellitus. Diabetes Metab J 45(1):46–54. https://doi.org/10.4093/dmj.2019.0134
Ajjan RA, Cummings MH, Jennings P, Leelarathna L, Rayman G, Wilmot EG (2019) Optimising use of rate-of-change trend arrows for insulin dosing decisions using the FreeStyle Libre flash glucose monitoring system. Diab Vasc Dis Res 16(1):3–12. https://doi.org/10.1177/1479164118795252
Yoo HJ, An HG, Park SY et al (2008) Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 82(1):73–79. https://doi.org/10.1016/j.diabres.2008.06.015
Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA (2011) The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 5(3):668–675. https://doi.org/10.1177/193229681100500320
Yaron M, Roitman E, Aharon-Hananel G et al (2019) Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 42(7):1178–1184. https://doi.org/10.2337/dc18-0166
Ong WM, Chua SS, Ng CJ (2014) Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence 8:237–246. https://doi.org/10.2147/ppa.S57567
Pemberton JS, Kershaw M, Dias R et al (2021) DYNAMIC: dynamic glucose management strategies delivered through a structured education program improves time in range in a socioeconomically deprived cohort of children and young people with type 1 diabetes with a history of hypoglycemia. Pediatr Diabetes 22(2):249–260. https://doi.org/10.1111/pedi.13155
Schlüter S, Freckmann G, Heinemann L, Wintergerst P, Lange K (2021) Evaluation of the SPECTRUM training programme for real-time continuous glucose monitoring: a real-world multicentre prospective study in 120 adults with type 1 diabetes. Diabet Med 38(2):e14467. https://doi.org/10.1111/dme.14467
Slattery D, Amiel SA, Choudhary P (2018) Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med 35(3):306–316. https://doi.org/10.1111/dme.13525
Maiorino MI, Signoriello S, Maio A et al (2020) Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 43(5):1146–1156. https://doi.org/10.2337/dc19-1459
Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM (2012) Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 35(1):32–38. https://doi.org/10.2337/dc11-1438
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The authors thank all the healthcare professionals at each centre who provided education, including J. H. Kim of The Catholic Smart Healthcare Center, Seoul St Mary’s Hospital, The Catholic University of Korea (Seoul, Republic of Korea), S. Park of Seoul National University Bundang Hospital, Seoul National University College of Medicine (Seongnam, Republic of Korea), H. Ju and Y. Hyun of Diabetes Center, Severance Hospital, Yonsei University College of Medicine (Seoul, Republic of Korea), J. M. Kim of Diabetes Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine (Seoul, Republic of Korea) and M. Yoon, H. Kim and K. Kim of Diabetes Center, Korea University Anam Hospital, Korea University College of Medicine (Seoul, Republic of Korea).
Data availability
Data are available from the corresponding author on reasonable request.
Funding
This study was supported by Daewoong Pharmaceutical Co., Ltd. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Author’s relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
S-MJ and JHK contributed to the conception and design of the study. JYK, S-MJ, KHS, B-YK, JHC, JSM, SL, ESK, C-YP, SGK and JHK conducted data collection. JYK, S-MJ and JHK interpreted the results. JYK and S-MJ wrote the initial draft of the manuscript. JYK, S-MJ, KHS, B-YK, JHC, JSM, SL, ESK, C-YP, SGK and JHK contributed to reviewing the work critically for important intellectual content. All authors approved the final version to be published. JHK is the guarantor of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.Y., Jin, SM., Sim, K.H. et al. Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial. Diabetologia 67, 1223–1234 (2024). https://doi.org/10.1007/s00125-024-06152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06152-1