Abstract
Aims/hypothesis
Athletes exhibit increased muscle insulin sensitivity, despite increased intramuscular triacylglycerol content. This phenomenon has been coined the ‘athlete’s paradox’ and is poorly understood. Recent findings suggest that the subcellular distribution of sn-1,2-diacylglycerols (DAGs) in the plasma membrane leading to activation of novel protein kinase Cs (PKCs) is a crucial pathway to inducing insulin resistance. Here, we hypothesised that regular aerobic exercise would preserve muscle insulin sensitivity by preventing increases in plasma membrane sn-1,2-DAGs and activation of PKCε and PKCθ despite promoting increases in muscle triacylglycerol content.
Methods
C57BL/6J mice were allocated to three groups (regular chow feeding [RC]; high-fat diet feeding [HFD]; RC feeding and running wheel exercise [RC-EXE]). We used a novel LC-MS/MS/cellular fractionation method to assess DAG stereoisomers in five subcellular compartments (plasma membrane [PM], endoplasmic reticulum, mitochondria, lipid droplets and cytosol) in the skeletal muscle.
Results
We found that the HFD group had a greater content of sn-DAGs and ceramides in multiple subcellular compartments compared with the RC mice, which was associated with an increase in PKCε and PKCθ translocation. However, the RC-EXE mice showed, of particular note, a reduction in PM sn-1,2-DAG and ceramide content when compared with HFD mice. Consistent with the PM sn-1,2-DAG–novel PKC hypothesis, we observed an increase in phosphorylation of threonine1150 on the insulin receptor kinase (IRKT1150), and reductions in insulin-stimulated IRKY1162 phosphorylation and IRS-1-associated phosphoinositide 3-kinase activity in HFD compared with RC and RC-EXE mice, which are sites of PKCε and PKCθ action, respectively.
Conclusions/interpretation
These results demonstrate that lower PKCθ/PKCε activity and sn-1,2-DAG content, especially in the PM compartment, can explain the preserved muscle insulin sensitivity in RC-EXE mice.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Insulin resistance in skeletal muscle plays a critical role in the pathogenesis of type 2 diabetes and cardiometabolic disease and is strongly correlated with increased intramyocellular lipid (IMCL) content in sedentary individuals, which has been postulated to play a major role in causing insulin resistance [1,2,3,4,5,6]. However, contrary to these findings, athletes have been shown to maintain high skeletal muscle insulin sensitivity despite having similarly high levels of IMCL content [7, 8], a phenomenon coined the ‘athlete’s paradox’.
Novel isoforms of protein kinase C (nPKC) have been suggested as important players in lipid-induced insulin resistance [9,10,11,12]. In skeletal muscle, our group and others have shown that novel protein kinase C (PKC) isoforms PKCθ and PKCε are associated with lipid-induced insulin resistance in humans and rodents [8, 13,14,15,16,17]. In recent studies, we found that increases in plasma membrane (PM) sn-1,2-diacylglycerol (DAG) were responsible for mediating lipid-induced insulin resistance in the liver, skeletal muscle and white adipose tissue (WAT) through activation of PKCε in all of these tissues as well as PKCθ in sleletal muscle, which in contrast to the other tissues is abundant in skeletal muscle [14, 18, 19]. We went on to demonstrate that PM sn-1,2-DAG-induced PKCε activation promotes insulin resistance in these tissues through phosphorylation of threonine1150 on the insulin receptor kinase (IRK; that is, IRKT1150), which in turn leads to inhibition of IRK activity, whereas PM sn-1,2-DAG-induced PKCθ activation further contributes to insulin resistance in skeletal muscle by causing reductions in insulin-stimulated IRS-1-associated phosphoinositide 3-kinase (PI3-kinase) activity [15].
We therefore hypothesised that the paradoxical effects of physical exercise on muscle triacylglycerol (TAG) content and muscle insulin sensitivity could be explained by differences in PM sn-1,2-DAG content, and PKCε/PKCθ activation in the skeletal muscle. To address this hypothesis, we applied a novel LC-MS/MS method to assess the different stereoisomers of DAGs and ceramides in five intracellular compartments along with PKCε/PKCθ activity and insulin signalling/insulin action.
Methods
Animals
All animal studies were approved by the Yale University Institutional Animal Care and Use Committee and were performed in accordance. Male C57BL6 mice (10–12 weeks) acquired from The Jackson Laboratory (USA) were housed in the Yale Animal Resources Center in individual cages at 23°C under 12 h light (7:00 hours to 19:00 hours)/12 h dark cycles (19:00 hours to 7:00 hours). The mice were fed ad libitum with free access to water. To address the hypothesis, we studied three groups: (1) regular chow feeding (RC); (2) high-fat diet feeding (HFD); (3) RC feeding and running wheel (Columbus Instruments, USA) voluntary exercise (RC-EXE). The experimental period was 6 weeks.
OGTTs
After 6 h of fasting, a single dose of 1 g/kg dextrose was administered via gavage, and blood samples were collected from the tail vein at 0, 15, 30, 45, 60 and 120 min to measure plasma glucose and insulin levels.
Plasma and tissue biochemical measurement
Plasma glucose was measured by YSI Glucose Analyzer (Xylem, USA). Plasma insulin was measured by the Yale Diabetes Research Center Radioimmunoassay Core using radioimmunoassay. Plasma NEFA was measured using the enzymatic colorimetric method by Wako reagents (FUJIFILM; Wako Diagnostics, USA). TAG was measured by Sekisui’s Triglyceride-SL Reagent (Sekisui Diagnostics, USA). Skeletal muscle and liver TAGs were extracted with cold 2:1 chloroform:methanol (vol./vol.). Samples were then mixed with sulfuric acid (1 mol/l) and centrifuged to achieve phase separation. The organic phase was measured by Sekisui’s Triglyceride-SL Reagent.
Mouse hyperinsulinaemic–euglycaemic clamp
The surgery for the hyperinsulinaemic–euglycaemic clamp was performed under isoflurane anaesthesia (5% for induction and 2.5% for maintenance). Jugular venous catheters were placed 6–7 days before the studies. Only mice that recovered to >90% of their pre-operative weight were studied. After 6 h of fasting, awake mice under tail restraint received a 120 min infusion of [3-3H]glucose (1.800 Bq/min) to measure basal glucose turnover. The blood sample was collected by tail bleeding at the end of basal infusion to determine plasma glucose, insulin, NEFAs and 3H-glucose specific activity. Following basal infusion, a 140 min hyperinsulinaemic–euglycaemic clamp was performed with a continuous infusion of insulin at the dose of 2.5 (mU kg−1 min−1) to raise plasma insulin concentrations to simulate postprandial insulin levels. Dextrose (20%) was infused at variable rates to maintain euglycaemia (plasma glucose 5.6–6.7 mmol/l). The dextrose was enriched with [3-3H]glucose to measure insulin-stimulated glucose turnover. A bolus (370.000 Bq) of 2-[1-14C]deoxyglucose (2-DOG) was injected at 85 min to determine the rate of muscle-specific glucose uptake under insulin stimulation. During the clamp, blood samples were collected at 10–25 min intervals for immediate determination of plasma glucose levels. After 2-DOG injection, extra plasma was collected for measuring plasma 14C-glucose specific activity. At the end of the clamp, plasma was collected to measure plasma glucose, insulin, NEFAs and 3H/14C-glucose specific activity [20, 21].
DAG subcellular fractionation
The subcellular fractionation protocol has been previously described [18, 19]. Briefly, gastrocnemius tissues (~100 mg) were homogenised in cold (4°C) TES buffer (250 mmol/l sucrose, 10 mmol/l Tris-pH 7.4, 0.5 mmol/l EDTA) with a Dounce homogeniser. The homogenate was centrifuged (at 17,000 g, SS-34 rotor, 15 min, 4°C) to separate pellet A and supernatant A.
Pellet A was resuspended in TES buffer and centrifuged (at 17,000 g with SS-34 rotor, 20 min, 4°C). After that, pellet A was resuspended in TES buffer and layered on top of a 1.12 mol/l sucrose buffer. The samples were centrifuged (at 105,000 g with TLS-55 rotor, 20 min, 4°C) to separate pellet B, interface B and supernatant B. Pellet B was collected as the mitochondrial fraction. Interface B was collected and resuspended in TES buffer and centrifuged (at 60,000 g with TLA-100.2 rotor, 9 min, 4°C) to obtain pellet C. Pellet C was resuspended in TES buffer and centrifuged (at 60,000 g with TLA-100.2 rotor, 9 min, 4°C) and was used as the PM fraction.
Supernatant A was centrifuged (at 390,000 g with Ti-70.1 rotor, 75 min, 4°C) to separate pellet D and supernatant D. The top lipid layer was collected as the lipid droplet (LD) fraction. Supernatant D was collected as the cytosol fraction, while pellet D was collected as the endoplasmic reticulum (ER) fraction. Figure 1 shows the separation of the five subcellular compartments in gastrocnemius.
DAGs were extracted from the five fractions and measured by LC-MS/MS as described previously [18, 19].
Western blotting
Gastrocnemius lysates were prepared in RIPA buffer with protease inhibitors (cOmplete MINI; Roche, Switzerland) and phosphatase inhibitors (PhosSTOP; Roche, Switzerland). Protein concentration was determined by the Bradford method (Bradford Reagent; Thermo Scientific, USA). Protein was loaded and resolved by SDS-PAGE using 4–12% gradient gels (Life Technologies, USA) and proteins were transferred to PVDF membranes (MilliporeSigma, USA) by semidry transfer. Membranes were blocked in 5% BSA for 1 h at room temperature and probed overnight at 4°C with primary antibodies (1:1000). The antibody list can be found in detail in the electronic supplementary material (ESM). Films were developed within the linear dynamic range of signal intensity and then were scanned for digital analysis.
IRS-1-associated PI3-kinase activity
IRS-1-associated PI3-kinase activities were measured in gastrocnemius samples after immunoprecipitation with IRS-1 antibody (BD Biosciences, USA). The incorporation of 32P into phosphatidylinositol to yield phosphatidylinositol-3-monophosphate was measured to determine the IRS-1-associated PI3-kinase activity, as previously described [22].
PKC, CD36 and lipoprotein lipase translocation
Gastrocnemius samples were homogenised in buffer A (20 mmol/l Tris-HCl, pH 7.4, 1 mmol/l EDTA, 0.25 mmol/l EGTA, 250 mmol/l sucrose, and protease and phosphatase inhibitors). The lysate was centrifuged (60 min, 100,000g, 4°C), and the supernatant was collected as the cytosolic fraction. The pellet was resuspended in buffer B (250 mmol/l Tris-HCl, pH 7.4, 1 mmol/ EDTA, 0.25 mmol/l EGTA, 2% Triton X-100, and added protease and phosphatase inhibitors) by sonication, and incubated at 4°C for 45 min to solubilise membrane proteins. Next, samples were centrifuged (60 min, 100,000g, 4°C), and the supernatant was saved as the membrane fraction. The protein samples were subjected to western blot analysis as described above. The ratio of membrane fraction to cytosolic fraction was calculated as an index of protein translocation.
Glycogen measurements
To assess the glycogen content, gastrocnemius samples were homogenised in 0.6 mol/l perchloric acid. Part of the homogenate was saved to measure as free glucose. Another part of the homogenate was neutralised by 1 mol/l KHCO3 and digested with amyloglucosidase (2 mg/ml) dissolved in acetate buffer (0.4 mol/l, pH 4.8) for 2 h at 37–40°C. Glucose levels were analysed in free glucose samples and after the amyloglucosidase digestion (total glucose) to calculate total glycogen content [19].
Statistics
Data are presented as the mean ± SEM. Data normality was analysed by the Shapiro–Wilk W test. For the data with normal distribution, one-way ANOVA followed by Tukey’s post hoc test was used. Kruskal–Wallis nonparametric test was used for non-normal data. Two-way ANOVA and the Tukey post hoc test were adopted to compare all groups in pairs for the OGTT analyses. Differences were considered significant if p<0.05. *p<0.05, **p<0.01, ***p<0.001 vs RC mice and †p<0.05, ††p<0.01, †††p<0.001 vs HFD mice. GraphPad Prism 9.4.1 (https://www.graphpad.com/scientific-software/prism/, GraphPad Software, USA) was used for the construction of the graphs and for statistical analysis.
Results
Baseline characteristics of mice
We first evaluated the body composition of mice after 6 weeks of HFD, RC or RC-EXE [20]. As designed, the final body weight was higher in HFD mice compared with RC and RC-EXE mice (Table 1). HFD mice had significantly increased fat mass and a reduction in lean body mass compared with the other two groups (Table 1). No difference was observed in the muscle glycogen content among the three groups at baseline (Table 1).
Exercised mice have normal glucose tolerance despite similar muscle TAG content
The weekly and cumulative running distances were calculated from the running wheels during the 6 weeks of running wheel exercise for the RC-EXE mice and demonstrated that the RC-EXE mice ran voluntarily a mean of ~43 km per week and a total distance of ~260 km over 6 weeks prior to the study (Fig. 2a). Consistent with this voluntary exercise, RC-EXE mice had a slightly increased \( \dot{V}{\mathrm{O}}_{2\max } \) rate (\( \dot{V}{\mathrm{O}}_{2\mathrm{peak}} \)) (p=0.08) as well as having a higher speed and time of exhaustion on a graded treadmill test when compared with HFD and RC mice (ESM Fig. 1). We next assessed indirect calorimetry in our experimental model [20]. Under basal conditions, RC and RC-EXE mice exhibited slightly increased rates of \( \dot{V}{\mathrm{O}}_2 \) and energy expenditure than the HFD mice in the dark cycle (ESM Fig. 2). Compared with HFD mice, carbon dioxide production and the respiratory exchange ratio were higher in the RC and RC-EXE mice without prominent differences in the activity counts (ESM Fig. 2). In addition, HFD mice showed significantly increased concentrations of TAGs in the gastrocnemius and quadriceps muscles compared with RC mice but not when compared with RC-EXE (Fig. 2b, ESM Fig. 1). Conversely, RC-EXE mice demonstrated lower NEFA and TAG levels in the liver when compared with both RC and HFD mice (ESM Fig. 1). Lastly, to better understand the effects of IMCL on glucose metabolism in our model, we performed OGTTs in the three groups of mice. Despite no significant differences in muscle TAG between HFD and RC-EXE mice, the RC-EXE mice manifested normal glucose tolerance and normal plasma insulin concentrations during an OGTT, while the HFD mice showed impaired glucose tolerance and markedly higher plasma insulin concentrations (Fig. 2c,d).
Exercised mice have normal glucose tolerance despite similar muscle TAG content to HFD mice. Data are mean ± SEM. (a) Distance evolution on running wheels over 6 weeks. (b) Gastrocnemius TAG content. (c) Plasma glucose levels and OGTT glucose AUC. (d) Plasma insulin levels and OGTT insulin AUC. After data normality analysis ANOVA was used to identify the statistical differences. *p<0.05 and **p<0.01 vs RC mice; †p<0.05 and ††p<0.01 vs HFD mice. In (a), n=7 mice. In (b), n=10–12 per group. In (c) and (d), n=6 per group
Exercised mice manifest normal insulin sensitivity in muscle despite no significant differences in muscle TAG content compared with HFD mice
We next evaluated whole-body and skeletal muscle insulin sensitivity in these mice. Consistent with the OGTT results the HFD mice exhibited whole-body insulin resistance during the hyperinsulinaemic–euglycaemic clamp, as reflected by a lower glucose infusion rate (GIR) required to maintain euglycaemia during the clamp, while the RC-EXE mice showed normal insulin sensitivity compared with the RC mice (Fig. 3a,b,c). Glucose disposal rate (Rd) in the RC-EXE mice was comparable to the RC mice while being lower in the HFD mice than in RC-EXE (Fig. 3d). Skeletal muscle insulin-stimulated glucose uptake was higher in the RC-EXE mice compared with the HFD mice (Fig. 3e). In addition, no difference was observed in the endogenous glucose production (EGP); however, RC-EXE mice showed a greater suppression of plasma NEFA levels (p=0.08) by insulin during the hyperinsulinaemic–euglycaemic clamp compared with the HFD mice (Fig. 3f,g).
Exercised mice manifest normal insulin sensitivity in muscle despite no significant differences in muscle TAG content compared with HFD mice. Data are mean ± SEM, n=6 mice per group. (a) Plasma glucose. (b) GIR. (c) Mean GIR during the hyperinsulinaemic–euglycaemic clamp. (d) Mean glucose Rd. (e) Gastrocnemius 2-DOG uptake. (f) EGP. (g) Plasma NEFA concentrations during basal and clamp periods. After data normality analysis ANOVA was used to identify the statistical differences. *p<0.05 vs RC mice; †p<0.05 and ††p<0.01 vs HFD mice
Exercised mice have lower PKCε/PKCθ activation and IRKT1150 phosphorylation in skeletal muscle compared with HFD mice
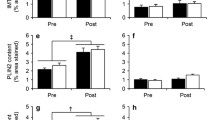
Previous studies have demonstrated that lipid-induced skeletal muscle insulin resistance is mediated by PKCε/PKCθ activation in humans and rodents [1, 9]. Consistent with these studies, HFD mice had higher activation compared with the RC mice, while the RC-EXE mice exhibited reduced PKCθ and PKCε activation compared with the HFD mice (Fig. 4a). No changes were found in PKCα, PKCβ and PKCδ translocation (ESM Fig. 3). PKCε has been shown to phosphorylate IRK at IRKT1150 in rodents (IRKT1160 in humans) which in turn leads to reduced IRK activity [14, 18, 19]. Consistent with this model, IRKT1150 phosphorylation was also higher in the HFD mice compared with the RC group, while the RC-EXE mice maintained a low level of IRKT1150 phosphorylation in the skeletal muscle (Fig. 4b). Furthermore RC-EXE mice manifested increased insulin-stimulated IRKY1162 phosphorylation and IRS-1-associated PI3-kinase activity when compared with the HFD mice (Fig. 4c,e). Next, we measured insulin-stimulated Akt phosphorylation, which was reduced in HFD mice compared with RC mice (Fig. 4d). We also assessed Akt translocation and p-PKCζT410 in our experimental model. However, no differences among the groups were observed in Akt translocation or PKCζT410 phosphorylation (ESM Fig. 3).
Exercised mice have lower PKCε/PKCθ activation and IRKT1150 phosphorylation in skeletal muscle compared with HFD mice. Data are mean ± SEM. (a) PKCθ and PKCε translocation (membrane/cytosol). (b) IRKT1150/IRK total. (c) IRKY1162/IRK total. (d) AktS473/Akt total. (e) IRS-1-associated PI3-kinase activity. After data normality analysis ANOVA was used to identify the statistical differences. *p<0.05, **p<0.01 and ***p<0.001 vs RC mice; †p<0.05 and ††p<0.01 vs HFD mice. In (a) and (b), n=8. In (c–e), RC n=4, HFD n=5 and RC-EXE n=5 mice. In (f), n=4 basal and n=6 clamp. AU, arbitrary units
sn-DAG and ceramide compartmentation is different in exercised mice compared with HFD mice
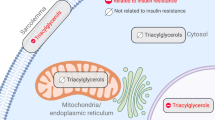
Increased concentrations of PM sn-1,2-DAGs have been demonstrated to be the key mediators of lipid-induced insulin resistance in several tissues through activation of nPKCs [9, 14, 19]. Consistent with these prior observations, we found that HFD mice had ~twofold higher levels of sn-1,2-DAGs in the PM of skeletal muscle compared with the RC mice (Fig. 5a). We also found higher levels of sn-1,2-DAGs in the LD, mitochondria (Mito) and cytosol of skeletal muscle in HFD mice compared with the RC mice (Fig. 5a). On the other hand, the RC-EXE mice maintained lower levels of sn-1,2 DAGs in the PM but no differences were observed in the other cellular compartments compared with the HFD mice (Fig. 5a). Strikingly, compared with RC mice, HFD mice showed similar increases in the other two DAG stereoisomers (Fig. 5b,c). Conversely, RC-EXE mice manifested lower levels of sn-1,3-DAGs in the PM and Mito compartments when compared with HFD mice (Fig. 5b,c). We also assessed ceramides in the same five compartments. Compared with RC, the HFD mice manifested increased ceramide content in the PM, LD and cytosol compartments (Fig. 5d). However, the RC-EXE mice had lower ceramide levels in the PM and ER when compared with HFD mice (Fig. 5d). Finally, we analysed the content of different species of ceramides and observed higher C16:0, C18:0 and C22:0 ceramides in the PM of HFD mice (ESM Fig. 4). HFD mice also had greater C18:0 levels in the LD and cytosol compartments compared with RC mice (ESM Fig. 4). In contrast, the RC-EXE mice demonstrated reductions in C16:0, C18:0, C20:0 and C22:0 ceramides in the PM as well as a lower C18:0 ceramide in the ER and Mito when compared with HFD mice (ESM Fig. 4). Since sn-DAG content and PKCε activation can be altered by changes in the fed (insulin-stimulated state) and fasted states [23], we used different sets of mice for each study. Thus, it was not possible to determine the correlation between nPKC activation and insulin signalling.
sn-DAG and ceramide compartmentation is different in exercised mice compared with HFD mice. Data are mean ± SEM, n=11–12 mice per group. (a) Gastrocnemius sn-1,2-DAG. (b) Gastrocnemius sn-1,3-DAG. (c) Gastrocnemius sn-2,3-DAG. (d) Gastrocnemius ceramide concentrations. After data normality analysis ANOVA was used to identify the statistical differences. *p<0.05, **p<0.01 and ***p<0.001 vs RC mice; †p<0.05, ††p<0.01 and †††p<0.001 vs HFD mice
Exercised mice increased DGAT1 and CD36 in skeletal muscle
To examine the potential mechanism responsible for increased TAG accumulation in the skeletal muscle of RC-EXE mice and HFD mice, we evaluated several key enzymes responsible for fatty acid uptake and TAG synthesis. Expression of diacylglycerol acyltransferase-1 (DGAT1) was higher in the RC-EXE mice compared with the other groups, whereas expression of diacylglycerol acyltransferase-2 (DGAT2) was higher in the HFD mice compared with the other two groups (Fig. 6a). RC-EXE mice showed a higher CD36 protein content compared with both RC and HFD mice (Fig. 6a). We did not observe any differences in lipoprotein lipase (LPL) expression and translocation in these mice (Fig. 6b, ESM Fig. 5). On the other hand, HFD mice showed lower CD36 translocation compared with the RC mice, while the CD36 translocation was higher in exercised mice than in HFD mice (Fig. 6c). In contrast, we did not observe any differences in adipose triglyceride lipase (ATGL) or hormone-sensitive lipase (HSL) phosphorylation (ESM Fig. 5) among the groups.
Exercised mice had increased DGAT1 and CD36 in skeletal muscle. Data are mean ± SEM (RC n=4, HFD n=5 and RC-EXE n=5 mice). (a) DGAT1/GAPDH, DGAT2/GAPDH and CD36/GAPDH. (b) LPL translocation (membrane/cytosol). (c) CD36 translocation (membrane/cytosol). After data normality analysis ANOVA was used to identify the statistical differences. *p<0.05 and **p<0.01 vs RC mice; †p<0.05, ††p<0.01 and †††p<0.001 vs HFD mice
Discussion
Increased IMCL content is strongly associated with muscle insulin resistance in sedentary individuals and is likely a biomarker for related lipid metabolites (e.g. DAGs, ceramides, etc.) that mediate insulin resistance [5]. However, an important exception to this relationship occurs in athletes who typically manifest increased IMCL content despite maintaining normal or increased muscle insulin sensitivity, a phenomenon coined the athlete’s paradox [24,25,26]. In this regard, recent studies have demonstrated that specific increases in sn-1,2-DAGs in the PM lead to increased nPKC activation and insulin resistance in the liver, WAT and skeletal muscle [14, 18, 19] independent of changes in TAG content.
To test this hypothesis in our model, we measured the translocation of PKCs/nPKCs, and the activation of key steps of the insulin signalling pathway as well as PM sn-1,2, DAG content, key activators of nPKC translocation [27, 28]. Here we show that despite similar TAG levels in skeletal muscle of HFD and RC-EXE mice, RC-EXE mice manifested lower levels of especially PM sn-1,2-DAG content, which was associated with reduced PKCε and PKCθ translocation compared with the HFD mice. Strikingly, HFD mice showed increases in sn-DAG isoforms and ceramides in multiple subcellular compartments which were not observed in the RC-EXE group. Further, we observed that this protection from lipid-induced muscle insulin resistance in the RC-EXE mice was associated with a reduction in IRKT1150 phosphorylation and increases in insulin-stimulated IRKY1162 phosphorylation consistent with the sn-1,2-DAG–PKCε–IRKT1150 phosphorylation pathway of lipid-induced insulin resistance [14, 18, 19].
In contrast to our findings, Perreault et al did not observe an association between sarcolemma sn-1,2-DAG content and insulin resistance [8]. These different results may be due to technical differences in the methods used to isolate skeletal muscle in the two studies. The muscle biopsy procedure used by Perreault et al (2018), which included extramuscular fat dissection, might result in some degree of warm ischaemia and breakdown of sn-DAGs. In our study, we performed in situ freeze-clamping of skeletal muscle with aluminium tongs precooled in liquid nitrogen to minimise DAG hydrolysis due to warm ischaemia. Furthermore, in contrast to the study by Perreault et al, we also assessed the effects of exercise and high-fat feeding on insulin signalling IRKY1162 phosphorylation and IRS-1-associated PI3-kinase. In addition we also assessed IRKT1150 phosphorylation, a direct target of PKCε and PKCθ translocation, which has been shown to mediate sn-1,2-DAG-induced muscle insulin resistance at the level of IRS-1-associated PI3-kinase [14, 22].
Alterations in muscle lipid storage have also been observed in individuals with type 2 diabetes, who were found to store lipids in the subsarcolemmal, in larger LDs, especially in type II fibres [7]. On the other hand, athletes predominately store lipids in type I fibres, in the intramyofibrillar region, but a higher number of LDs [7]. Most recently, Sarabhai et al (2022) showed that a single meal containing safflower oil and palm oil induced whole-body and hepatic insulin resistance in healthy volunteers [29]. In the skeletal muscle, the lipid meal led to an increase in DAG species in the membrane as well as in the LD compartment [29]. Interestingly, PKCθ translocation was increased in both the safflower and palm oil groups, whereas PKCε translocation increased in the palm oil-fed group and tended to increase in the safflower group [29]. The palm oil group also showed an increase in ceramides associated with greater PKCζ translocation and phosphatase 2A (PP2A) expression [29].
We also observed that the ceramide species C16:0 and C18:0 were higher in the HFD mice compared with the RC-EXE mice. Ceramide inhibitory action on insulin signalling is thought to occur further downstream of insulin signalling at the level of Akt phosphorylation, which may occur particularly through impairments in Akt translocation, dephosphorylation of Akt by protein PP2A or by PKCζ phosphorylation [19, 30, 31]. In our study, we did not observe any difference in Akt translocation and PKCζ phosphorylation among the groups, whereas the HFD mice showed lower p-AktS473 compared with RC mice. The alterations in ceramide content could also have contributed to the observed changes in insulin resistance in these mice, but cannot explain the observed changes in insulin-stimulated IRKY1162 phosphorylation, IRS-1-associated PI3-kinase activity or IRKT1150 phosphorylation.
In addition to translocation/activation of the nPKC isoforms, we also assessed translocation of the classical PKC isoforms in this study. We did not observe any differences in the classical PKC isoforms among the groups. In contrast with our findings, Jani and collaborators [30] evaluated PKCδ translocation in the soleus, extensor digitorum longus (EDL) and epitrochlearis muscles after 8 weeks of HFD. They observed that PKCδ translocation was increased in the soleus and EDL muscles, which was associated with increased accumulation of both DAG and ceramide content in those tissues [30]. Since the sn-1,3-DAGs are mostly derived from TAG lipolysis and are not able to activate nPKCs [31], our results suggested that the PM sn-1,2-DAGs are responsible for mediating the differences in insulin sensitivity in our models through the activation of nPKCs, as previously described [27, 28].
In order to understand potential mechanisms by which HFD and exercise might lead to differences in DAG and ceramide cellular compartmentation, we also analysed expression of DGAT1 and DGAT2, which are critical enzymes in the re-esterification pathway. We found that RC-EXE mice showed increases in DGAT1 protein content when compared with RC and HFD mice. On the other hand, compared with the other groups, HFD mice showed increased DGAT2 protein levels. A previous study reported that exercised mice had increased muscle diacylglycerol acyltransferase (DGAT) activity and that overexpression of DGAT1 in skeletal muscle increased the muscle TAG and protected mice from HFD-induced insulin resistance, reproducing the athlete’s paradox phenomenon [32]. Six weeks of running wheel access was also able to increase TAG levels in the skeletal muscle of female mice [33]. In contrast, overexpression of DGAT2 in skeletal muscle was associated with increased lipid content and consequently glucose intolerance [34]. Further, in agreement with our studies, a single bout of or chronic physical exercise can induce increases in CD36 expression in skeletal muscle [35,36,37]. Interestingly, CD36 knockout mice have attenuated aerobic exercise capacity, which indicates that CD36 is a crucial molecule for maintaining endurance running capacity [38,39,40]. Taken together, these data suggest that increased expression of CD36 in RC-EXE mice may contribute to increased muscle fatty acid uptake as reflected by reductions in the plasma NEFA levels. Further, increased DGAT1 expression in RC-EXE mice may promote increased muscle TAG content and reduced PM sn-1,2-DAG content in the mice by a pull mechanism.
In summary, our data demonstrate that despite TAG content in skeletal muscle, voluntary exercise in mice resulted in: (1) normal muscle insulin sensitivity in the RC-EXE mice compared with the HFD mice; (2) reductions in PM sn-1,2-DAG content and PKCε and PKCθ translocation/activation in skeletal muscle of the RC-EXE mice compared with the HFD mice; (3) reductions in PKCε translocation associated with reduced IRKT1150 phosphorylation in the RC-EXE mice compared with the HFD mice; (4) reductions in PKCε and PKCθ translocation/activation in skeletal muscle of RC-EXE mice compared with HFD mice, leading to increased insulin-stimulated IRKY1162 phosphorylation and IRS-1-associated PI3-kinase activity; and (5) lower ceramide content in RC-EXE mice compared with HFD mice, although no differences were observed among the groups for Akt translocation or PKCζT410 phosphorylation, which are thought to mediate ceramide-induced insulin resistance. Taken together, these data suggest that lower PM sn-1,2-DAG content and lower PKCε/PKCθ activity in skeletal muscle could provide an explanation for the preserved insulin sensitivity in exercised mice despite their intramuscular TAG content.
Data availability
The original data generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DAG:
-
Diacylglycerol
- DGAT1:
-
Diacylglycerol acyltransferase-1
- DGAT2:
-
Diacylglycerol acyltransferase-2
- 2-DOG:
-
2-[1-14C]deoxyglucose
- EDL:
-
Extensor digitorum longus
- EGP:
-
Endogenous glucose production
- ER:
-
Endoplasmic reticulum
- GIR:
-
Glucose infusion rate
- HFD:
-
High-fat diet feeding
- IMCL:
-
Intramyocellular lipid
- IRK:
-
Insulin receptor kinase
- LD:
-
Lipid droplet
- LPL:
-
Lipoprotein lipase
- Mito:
-
Mitochondria
- nPKC:
-
Novel protein kinase C
- PI3-kinase:
-
Phosphoinositide 3-kinase
- PKC:
-
Protein kinase C
- PM:
-
Plasma membrane
- PP2A:
-
Phosphatase 2A
- RC:
-
Regular chow feeding
- RC-EXE:
-
RC feeding and running wheel voluntary exercise
- R d :
-
Disposal rate
- TAG:
-
Triacylglycerol
- WAT:
-
White adipose tissue
References
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98(4):2133–2223. https://doi.org/10.1152/physrev.00063.2017
Laurens C, Moro C (2016) Intramyocellular fat storage in metabolic diseases. Horm Mol Biol Clin Investig 26(1):43–52. https://doi.org/10.1515/hmbci-2015-0045
Shulman GI (2014) Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371(12):1131–1141. https://doi.org/10.1056/nejmra1011035
Weiss R, Dufour S, Taksali SE et al (2003) Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362(9388):951–957. https://doi.org/10.1016/S0140-6736(03)14364-4
Krssak M, Falk Petersen K, Dresner A et al (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1 H NMR spectroscopy study. Diabetologia 42(1):113–116. https://doi.org/10.1007/s001250051123
Dresner A, Laurent D, Marcucci M et al (1999) Effects of free fatty acids on glucose transport and IRS-1–associated phosphatidylinositol 3-kinase activity. J Clin Invest 103(2):253–259. https://doi.org/10.1172/JCI5001
Daemen S, Gemmink A, Brouwers B et al (2018) Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol Metab 17:71–81. https://doi.org/10.1016/j.molmet.2018.08.004
Perreault L, Newsom SA, Strauss A et al (2018) Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 3(3):e96805. https://doi.org/10.1172/jci.insight.96805
Petersen MC, Shulman GI (2017) Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci 38(7):649–665. https://doi.org/10.1016/j.tips.2017.04.004
Batista TM, Haider N, Kahn CR (2021) Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 64(5):994–1006. https://doi.org/10.1007/s00125-021-05415-5
White MF, Kahn CR (2021) Insulin action at a molecular level – 100 years of progress. Mol Metab 52(July):101304. https://doi.org/10.1016/j.molmet.2021.101304
Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576(7785):51–60. https://doi.org/10.1038/s41586-019-1797-8
Szendroedi J, Yoshimura T, Phielix E et al (2014) Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci 111(26):9597–9602. https://doi.org/10.1073/pnas.1409229111
Song JD, Alves TC, Befroy DE et al (2020) Dissociation of muscle insulin resistance from alterations in mitochondrial substrate preference. Cell Metab 32(5):726–735.e5. https://doi.org/10.1016/j.cmet.2020.09.008
Li Y, Soos TJ, Li X et al (2004) Protein kinase C θ inhibits insulin signaling by phosphorylating IRS1 at Ser1101. J Biol Chem 279(44):45304–45307. https://doi.org/10.1074/jbc.C400186200
Itani SI, Pories WJ, MacDonald KG, Dohm GL (2001) Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism 50(5):553–557. https://doi.org/10.1053/meta.2001.22512
Griffin ME, Marcucci MJ, Cline GW et al (1999) Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes 48(6):1270–1274. https://doi.org/10.2337/diabetes.48.6.1270
Lyu K, Zhang D, Song J et al (2021) Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation. JCI insight 6(4):e139946. https://doi.org/10.1172/jci.insight.139946
Lyu K, Zhang Y, Zhang D et al (2020) A membrane-bound diacylglycerol species induces PKCϵ-mediated hepatic insulin resistance. Cell Metab 32(4):654–664.e5. https://doi.org/10.1016/j.cmet.2020.08.001
Camporez JP, Lyu K, Goldberg EL et al (2019) Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol 597(15):3885–3903. https://doi.org/10.1113/JP277270
Kim JK (2009) Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo. In: Stocker C (ed) Type 2 diabetes methods and protocols. Humana Press, New Jersey, pp 221–238
Kim JK, Fillmore JJ, Sunshine MJ et al (2004) PKC-θ knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114(6):823–827. https://doi.org/10.1172/JCI200422230
Perry RJ, Wang Y, Cline GW et al (2018) Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell 172(1–2):234–248.e17. https://doi.org/10.1016/j.cell.2017.12.001
Goodpaster BH, He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86(12):5755–5761. https://doi.org/10.1210/jcem.86.12.8075
Amati F, Dubé JJ, Alvarez-Carnero E et al (2011) Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance. Diabetes 60(10):2588–2597. https://doi.org/10.2337/db10-1221
Li X, Li Z, Zhao M et al (2019) Skeletal muscle lipid droplets and the athlete’s paradox. Cells 8(3):249. https://doi.org/10.3390/cells8030249
Boni LT, Rando RR (1985) The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem 260(19):10819–10825. https://doi.org/10.1016/S0021-9258(19)85156-6
Rando RR, Young N (1984) The stereospecific activation of protein kinase C. Biochem Biophys Res Commun 122(2):818–823. https://doi.org/10.1016/S0006-291X(84)80107-2
Sarabhai T, Koliaki C, Mastrototaro L et al (2022) Dietary palmitate and oleate differently modulate insulin sensitivity in human skeletal muscle. Diabetologia 65(2):301–314. https://doi.org/10.1007/s00125-021-05596-z
Jani S, Da Eira D, Hadday I et al (2021) Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci Rep 11(1):19160. https://doi.org/10.1038/s41598-021-98819-7
Eichmann TO, Kumari M, Haas JT et al (2012) Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J Biol Chem 287(49):41446–41457. https://doi.org/10.1074/jbc.M112.400416
Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH (2007) Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117(6):1679–1689. https://doi.org/10.1172/JCI30565
Morton TL, Galior K, McGrath C et al (2016) Exercise increases and browns muscle lipid in high-fat diet-fed mice. Front Endocrinol (Lausanne) 7:1–8. https://doi.org/10.3389/fendo.2016.00080
Levin MC, Monetti M, Watt MJ et al (2007) Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Metab 293(6):E1772–E1781. https://doi.org/10.1152/ajpendo.00158.2007
Łukaszuk B, Bialuk I, Górski J, Zajączkiewicz M, Winnicka MM, Chabowski A (2012) A single bout of exercise increases the expression of glucose but not fatty acid transporters in skeletal muscle of IL-6 KO mice. Lipids 47(8):763–772. https://doi.org/10.1007/s11745-012-3678-x
Fujitani M, Matsumura S, Masuda D, Yamashita S, Fushiki T, Inoue K (2014) CD36, but not GPR120, is required for efficient fatty acid utilization during endurance exercise. Biosci Biotechnol Biochem 78(11):1871–1878. https://doi.org/10.1080/09168451.2014.940835
Kim J, Lim K (2016) Relationship between FAT/CD36 protein in skeletal muscle and whole-body fat oxidation in endurance-trained mice. J Exerc Nutr Biochem 20(4):48–52. https://doi.org/10.20463/jenb.2016.0057
Manio MCC, Matsumura S, Masuda D, Inoue K (2017) CD36 is essential for endurance improvement, changes in whole-body metabolism, and efficient PPAR-related transcriptional responses in the muscle with exercise training. Physiol Rep 5(10):e13282. https://doi.org/10.14814/phy2.13282
Yanai H, Watanabe I, Ishii K et al (2007) Attenuated aerobic exercise capacity in CD36 deficiency. J Med Genet 44(7):445–447. https://doi.org/10.1136/jmg.2007.050070
McFarlan JT, Yoshida Y, Jain SS et al (2012) In vivo, fatty acid translocase (CD36) critically regulates skeletal muscle fuel selection, exercise performance, and training-induced adaptation of fatty acid oxidation. J Biol Chem 28:23502–23516. https://doi.org/10.1074/jbc.M111.315358
Acknowledgements
The graphical abstract was drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All the authors contributed substantially to the conception and design; data acquisition or analysis; interpretation of data; and drafting of the article or revising it critically for important intellectual content. All authors approved the final version to be published. RCG and GIS are guarantors of this study and accept full responsibility for the conduct of the study.
Funding
This study was supported by grants from the São Paulo Research Foundation (FAPESP; Process Nos. 2017/20542-3; 2019/11338-9; 2020/13443-1) and the United States Public Health Service (R01 DK116774, R01 DK119968, R01 DK113984, P30 DK045735, T32 GM007205 and R01 DK092661) and a VA Merit award (I01 BX000901).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM
(PDF 582 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gaspar, R.C., Lyu, K., Hubbard, B.T. et al. Distinct subcellular localisation of intramyocellular lipids and reduced PKCε/PKCθ activity preserve muscle insulin sensitivity in exercise-trained mice. Diabetologia 66, 567–578 (2023). https://doi.org/10.1007/s00125-022-05838-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05838-8