Abstract
Aims/hypothesis
South Asians have a higher risk of developing type 2 diabetes than Europeans. The underlying cause of this excess risk is still poorly understood but might be related to differences in the regulation of energy/nutrient-sensing pathways in metabolic tissues and subsequent changes in whole-body substrate metabolism. In this study, we investigated the whole-body and skeletal muscle metabolic adaptations to short-term energy restriction in South Asian and European volunteers.
Methods
Twenty-four middle-aged overweight South Asian and European men underwent a two-step hyperinsulinaemic–euglycaemic clamp, with skeletal muscle biopsies and indirect calorimetry before and after an 8 day diet very low in energy (very low calorie diet [VLCD]). Abdominal fat distribution and hepatic triacylglycerol content were assessed using MRI and MR spectroscopy.
Results
South Asian men had higher hepatic triacylglycerol content than European men, and exhibited elevated clamp insulin levels that probably reflect a lower insulin clearance rate. Despite higher insulin levels, endogenous glucose production rate was similar and glucose disposal rate (Rd) and nonoxidative glucose disposal rate (NOGD) were significantly lower in South Asian than European men, indicating impaired whole-body insulin sensitivity. Energy restriction decreased abdominal fat mass and hepatic triacylglycerol content in both groups. However, the shift induced by energy restriction from glucose towards lipid oxidation observed in European men was impaired in South Asian men, indicating whole-body metabolic inflexibility. Remarkably, although energy restriction improved hepatic insulin sensitivity in both groups, Rd improved only in South Asian men owing to higher NOGD. At the molecular level, an increase in insulin-induced activation of the skeletal muscle mTOR pathway was found in South Asian men, showing that skeletal muscle energy/nutrient-sensing pathways were differentially affected by energy restriction.
Conclusions/interpretation
We conclude that South Asian men exhibit a different metabolic adaptation to short-term energy restriction than European men.
Trial registration: Dutch trial registry (www.trialregister.nl), trial number NTR 2473.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid increase in type 2 diabetes prevalence worldwide has been associated with a Western, obesogenic lifestyle [1]. South Asians originating from the Indian subcontinent (India, Pakistan, Bangladesh, Nepal and Sri Lanka), who represent one fifth of the world’s population, seem to have an exceptionally high susceptibility to develop the metabolic syndrome and type 2 diabetes in the context of the same environmental pressure when compared with other ethnicities [2–5]. A possible explanation for this excess risk might be related to differences in the regulation of energy/nutrient-sensing pathways in metabolic tissues thereby affecting whole-body substrate homeostasis.
Among these pathways, the nutrient and energy-sensing protein kinase mammalian target of rapamycin (mTOR), which regulates cell growth according to nutrient availability and cellular energy status [6], is of major importance. The mTOR kinase interacts with several proteins to form two distinct complexes named mTOR complex 1 (mTORC1) and mTOR complex 2, which differ in their molecular composition, regulation, sensitivity to rapamycin, and downstream targets [6]. mTORC1 responds to insulin and other growth factors, stress, oxygen and nutrient levels and controls key cellular processes [6]. When active, mTORC1 promotes protein synthesis, cell growth and differentiation, and may inhibit insulin signalling by feedback regulation of the insulin receptor substrate 1 (IRS1). mTORC1 was also recently shown to play a crucial role in mitochondrial biogenesis and oxidative metabolism [7–10]. Energy restriction reduces mTORC1 activity [11], at least partly through activation of the AMP-activated protein kinase (AMPK), a key sensor of cellular energy status [12, 13].
We hypothesised that differences in the regulation of energy/nutrient-sensing pathways between people of South Asian and European descent may affect whole-body glucose and lipid metabolism, and ultimately contribute to the increased risk of type 2 diabetes in South Asian individuals. This study, therefore, assessed the effect of energy restriction (achieved through an 8 day diet very low in energy [very low calorie diet (VLCD)]) on skeletal muscle energy/nutrient-sensing pathways, with a special focus on canonical insulin signalling and mTORC1 pathways, in both middle-aged overweight South Asian men and age- and BMI-matched European men.
Methods
Participants
Twelve Dutch South Asian and 12 Dutch European overweight (BMI 25–30 kg/m2) men, aged 40–50 years, with a waist circumference of >90 cm (South Asian) or >94 cm (European) and a positive family history for type 2 diabetes, were enrolled via local advertisements. South Asian participants were all Dutch Hindostani, an ethnic group of South Asian origin composed of people who were all born in Surinam before moving to the Netherlands. Participants underwent medical screening, including their medical history, a physical examination, blood chemistry tests and an OGTT to exclude individuals with type 2 diabetes (according to ADA 2010 criteria). Other exclusion criteria were rigorous exercise, smoking and recent body weight change. The study was approved by the Medical Ethical Committee of Leiden University Medical Centre and performed in accordance with the principles of the revised Declaration of Helsinki. Written informed consent was obtained from all volunteers prior to participation.
Study design
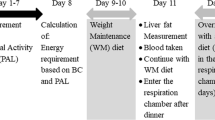
Participants were studied before and after an 8 day VLCD, consisting of three sachets of Modifast (Modifast, Nutrition & Santé Benelux, Breda, the Netherlands) per day (~1,883 kJ/day [~450 kcal/day]; ~50 g protein, 50–60 g carbohydrates, 7 g lipids and 15 g dietary fibre). They were instructed not to alter lifestyle habits, and not to perform physical activity in the last 48 h before the study days. Magnetic resonance studies were performed shortly before the start and on the 8th day of the diet, and metabolic studies were performed 1 day before and 1 day after the VLCD.
MR studies
Abdominal fat depots were quantified with turbo spin echo MRI using a 1.5 T whole-body MRI scanner (Gyroscan ACS-NT15, Philips, Best, the Netherlands) in the postprandial state [14], and hepatic triacylglycerol content was assessed by proton MR spectroscopy [15], as described previously [16].
Metabolic studies
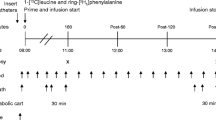
Participants underwent anthropometric measurements, a 7 h two-step hyperinsulinaemic–euglycaemic clamp with stable isotopes, skeletal muscle biopsies and indirect calorimetry after an overnight fast, as described previously [16]. Body fat mass and lean body mass (LBM) were assessed by bioelectrical impedance analysis (Bodystat 1500, Bodystat, Douglas, Isle of Man, UK). Laboratory analysis was done as described before [16]. Rates of glucose appearance and of glucose disposal (Rd), endogenous glucose production (EGP), metabolic clearance rate of insulin (MCRi), hepatic insulin resistance (HIR) index, resting energy expenditure (REE), respiratory quotient (RQ), substrate oxidation rates, and nonoxidative glucose disposal (NOGD) were calculated as described previously [16]. Metabolic flexibility was defined as the ability to increase lipid oxidation upon energy restriction (change in fasting RQ in response to VLCD) and to switch from lipid to glucose oxidation upon insulin stimulation (change in RQ from the fasted to the insulin-stimulated state) [17].
DNA/RNA isolation and real-time RT-PCR
DNA/RNA isolation and real-time RT-PCR were done as previously described [16]. In short, total RNA was isolated from skeletal muscle biopsy samples. First-strand cDNAs were synthesised from 1 μg of total RNA. Real-time PCR assays were performed using specific primer sets (sequences provided on request). mRNA expression was normalised to that of ribosomal protein S18 and expressed as arbitrary units. Genomic DNA was extracted for determination of mitochondrial (mtDNA) and nuclear (nDNA) DNA copy numbers [18].
Western blot
Skeletal muscle biopsy samples were homogenised as described previously [16]. Western blots were performed using phospho-specific (protein kinase B [PKB]-Ser473, phospho-Akt substrate, extracellular signal-regulated kinase [ERK]1/2-Thr202/Tyr204, tuberous sclerosis complex 2 [TSC2]-Thr1462, mTOR-Ser2448, ribosomal protein S6 kinase 1 [S6K1]-Thr389, eukaryotic translation initiation factor 4E-binding protein 1 [4EBP1]-Thr37/46, glycogen synthase kinase-3 [GSK3]-Ser21/9, glycogen synthase [GS]-Ser641, AMPK-Thr172 and acetyl-CoA carboxylase [ACC]-Ser79 from Cell Signaling (Cell Signaling Technology, Inc., Danvers, MA, USA); proline-rich Akt substrate of 40 kDa [PRAS40]-Thr246 from Biosource (Life Technologies Europe BV, Bleiswijk, the Netherlands)) or total primary antibodies (tubulin, PKB, Akt substrate of 160 kDa [AS160], mTOR, S6K β1 [S6K1], AMPK and ACC from Cell Signaling; insulin receptor isoform β [IRβ] and ERK1 from Santa Cruz (Santa Cruz Biotechnology, Inc., Dallas, Texas, USA); PRAS40 from Biosource; MitoProfile OXPHOS from AbCam (AbCam, Cambridge, UK)) [19]. Blots were quantified by densitometric analysis using Image J software (ImageJ, NIH, Bethesda, Maryland, USA).
Statistical analysis
Data are presented as means ± SEM or as median (interquartile range [IQR]) depending on normality distribution. A mixed effects model was applied to assess mean differences before and after the intervention within and between groups, and to determine differences in diet effects. Groups and the intervention were modelled as fixed effects and the individual specific deviances from the group means were modelled as random effects. Nonparametric tests (Wilcoxon signed-rank test within the group, Mann–Whitney U test between groups) were performed when appropriate. A p value <0.05 was considered significant. Statistical analyses were performed using SPSS for Windows version 20.0 (IBM, New York, NY, USA).
Results
Clinical characteristics
The mean age of participants was 44.6 ± 0.8 years. BMI did not differ between groups (South Asian: 28.4 ± 0.4 kg/m2 vs European: 28.1 ± 0.5 kg/m2; p = 0.65), but South Asian men tended to be lighter (p = 0.055) and were significantly shorter (Table 1). The decreases in body weight (South Asian: −4.2 ± 0.4% vs European: −4.8 ± 0.3%; p = 0.14) and fat mass (South Asian: −3.7 (3.2)% vs European: −5.3 (4.8)%; p = 0.17) induced by energy restriction were of the same extent in both groups. Fasting glucose, nonesterified fatty acid (NEFA) and triacylglycerol levels were comparable between groups, whereas HbA1c and fasting and OGTT insulin levels were significantly higher in South Asian men (Table 1). Energy restriction induced a significant similar decrease in fasting serum glucose, insulin and triacylglycerol levels. Plasma NEFAs increased in both groups, but significantly less in South Asian men.
Fat distribution
No differences between groups were observed for visceral and subcutaneous fat volumes (Table 1). However, hepatic triacylglycerol content was significantly higher in South Asian men before diet. Energy restriction led to a significant decrease in visceral and subcutaneous fat volumes and hepatic triacylglycerol content in both South Asian and European men (Table 1).
EGP and glucose disposal
During hyperinsulinaemic–euglycaemic clamp, glucose concentrations were comparable between groups, whereas insulin levels were significantly higher in South Asian men and were accompanied by a lower MCRi than that in European men (Table 2). Despite these higher insulin levels, EGP was similar between groups, indicating higher HIR in South Asian men. Furthermore, the insulin-stimulated Rd in step 2 was lower in South Asian men, indicating reduced peripheral insulin sensitivity compared with that in European men. In response to energy restriction, hepatic insulin sensitivity improved to a similar extent in both groups. However, while no effect of the diet on Rd in step 2 was observed in European men, it was significantly increased after energy restriction in South Asian men, despite a slight decrease in insulin levels. When corrected for steady state insulin levels, this effect in South Asian men was only borderline significant (p = 0.08).
REE, substrate oxidation and NOGD
Before VLCD in basal state, both REE (corrected for LBM) and substrate oxidation rates were comparable between groups (Table 3). During hyperinsulinaemic–euglycaemic clamp, insulin suppressed fat oxidation and stimulated glucose oxidation to a similar degree in both groups. NOGD in step 2 was significantly lower in South Asian men, despite higher insulin levels. Energy restriction led to a reduction in REE and a shift in substrate metabolism from glucose towards lipid oxidation. However, this shift was significantly blunted in South Asian men compared with European men. The effect of insulin on substrate oxidation rates was attenuated in South Asian men after the diet compared with before, whereas it tended to improve in European men (diet effect for South Asian vs European men p = 0.057 for both glucose and lipid oxidation). Energy restriction induced a significant increase in NOGD in step 2 only in South Asian men despite slightly reduced insulin levels in this group.
Energy/nutrient-sensing signalling pathways in skeletal muscle
Insulin canonical pathway
Before the diet, no significant differences between groups were observed in protein expression of IRβ and in both basal and insulin-induced phosphorylation of key proteins involved in the insulin canonical pathway (Fig. 1). Energy restriction induced a significant increase in protein expression of IRβ in both groups (Fig. 1b), whereas other proteins involved in insulin signalling were not affected (data not shown). The insulin-induced phosphorylation of PKB-Ser473, PRAS40-Thr246 and AS160 were slightly but significantly increased after energy restriction in European but not in South Asian men (Fig. 1c, e, f). By contrast, insulin-induced phosphorylation of ERK1/2-Thr202/Tyr204 was increased in South Asian men, whereas it tended to decrease in European men (Fig. 1d). Finally, phosphorylation of GS-Ser641 was reduced in response to energy restriction both in the basal state and during clamp in both groups, suggesting enhanced insulin sensitivity that might promote skeletal muscle glycogen synthesis (Fig. 1g).
Effect of VLCD on insulin signalling pathways in skeletal muscle from South Asian and European men in the basal state and during a hyperinsulinaemic–euglycaemic clamp. The protein expression of tubulin (a), IRβ (b) and the phosphorylation states of PKB-Ser473 (c), ERK1/2-Thr202/Tyr204 (d), PRAS40-Thr246 (e), phospho-AS160 (f), GSK3-Ser21/9 (g) and GS-Ser641 (h) were assessed by western blot in skeletal muscle from South Asian and European volunteers before (black bars) and after (white bars) an 8 day VLCD in both basal and hyperinsulinaemic states (step 2 of hyperinsulinaemic–euglycaemic clamp; labelled insulin). Representative blots for one individual per group are shown. Results are normalised to European participants in the basal state before VLCD and expressed as means ± SEM. †p < 0.05, ††p < 0.005 within group vs before diet. §p < 0.05, §§p < 0.005 within groups vs basal conditions *p < 0.05 vs Europeans. ‡p < 0.05 diet effect vs Europeans
mTOR signalling
Before the diet, in the basal state, no significant differences between groups were observed in protein expression of mTOR and S6K1 (Electronic Supplementary Material [ESM] Fig. 1), or in phosphorylation states of key upstream and downstream proteins involved in mTORC1 signalling, such as TSC2, S6K1 and 4EBP1 (Fig. 2). Energy restriction did not affect protein expression and phosphorylation states of TSC2, mTOR and S6K1 in the basal condition, whereas 4EBP1-Thr37/46 was slightly but significantly increased but only in European men. Furthermore, except for 4EBP1-Thr37/46, which was again significantly increased, the insulin-induced phosphorylation of most of the proteins involved in mTOR signalling was also not affected by energy restriction in European men. By contrast, the phosphorylation of mTOR-Ser2448, S6K1-Thr389 and 4EBP1-Thr37/46 was significantly increased during hyperinsulinaemia in South Asian men, suggesting enhanced skeletal muscle mTORC1 activity in this condition.
Effect of VLCD on mTOR signalling in skeletal muscle from South Asian and European men in the basal state and during a hyperinsulinaemic–euglycaemic clamp. The phosphorylation states of TSC2-Thr1462 (a), mTOR-Ser2448 (b), S6K1-Thr389 (c) and 4EBP1-Thr37/46 (d) were assessed by western blot in the same conditions as described in the legend to Fig. 1. Results are normalised to European participants in the basal state before VLCD and expressed as means ± SEM. †p < 0.05, ††p < 0.005 within group vs before diet. §p < 0.05, §§p < 0.005 within groups vs basal conditions *p < 0.05 vs Europeans
AMPK pathway
Before the diet, no differences were observed between groups in protein expression and phosphorylation states of AMPK and of its downstream target ACC in the basal condition (ESM Fig. 1; Fig. 3). The phosphorylation state of AMPK-Thr172 was not affected by hyperinsulinaemia in both groups, whereas a decrease in ACC-Ser79 phosphorylation was observed, but only in European men. Energy restriction did not affect protein expression of AMPKα and ACC, whereas phosphorylation of AMPK-Thr172 was significantly increased in both basal and hyperinsulinaemic conditions in South Asian men, but not in European men. Phosphorylation of ACC-Ser79 was increased in both groups in the basal state, whereas a higher degree of phosphorylation was only observed in European men during hyperinsulinaemic–euglycaemic clamp.
Effect of VLCD on AMPK signalling in skeletal muscle from South Asian and European men in the basal state and during a hyperinsulinaemic–euglycaemic clamp. The phosphorylation states of AMPK-Thr172 (a) and ACC-Ser79 (b) were assessed by western blot in the same conditions as described in the legend to Fig. 1. Results are normalised to European participants in the basal state before VLCD and expressed as means ± SEM. †p < 0.05, ††p < 0.005 within group vs before diet. §p < 0.05, §§p < 0.005 within groups vs basal conditions *p < 0.05 vs Europeans
Skeletal muscle mitochondrial respiratory chain content
Before the diet, the expression of mitochondrial respiratory chain complex 2 was significantly higher in South Asian men (data not shown). However, neither the complex II:complex I ratio (Fig. 4b) nor the mtDNA:nDNA ratio (Fig. 4c) was different between groups. Energy restriction led to a significant increase in the expression of respiratory chain complex II in European men but not in South Asian men. In both groups, the complex II:complex I ratio was significantly higher after energy restriction, suggesting a mitochondrial adaptation towards fat oxidation (Fig. 4b). The mtDNA:nDNA ratio was not affected by the diet (Fig. 4c).
Effect of VLCD on protein expression of mitochondrial respiratory chain subunits and mtDNA:nDNA ratio in skeletal muscle from South Asian and European men in the basal state and during a hyperinsulinaemic–euglycaemic clamp. The protein expression of various mitochondrial respiratory chain subunits (a; CI: NDUFB8, CII: SDHB, CIII: UQCRC2, CIV: MTCO1, CV: ATP5A) were assessed by western blot in skeletal muscle from South Asian and European volunteers in the basal state before (black bars) and after (white bars) an 8 day VLCD. Representative blots for one individual per group are shown in (a). The respiratory chain CII:CI ratio was calculated (b). The mtDNA:nDNA ratio was assessed in basal conditions by quantitative PCR (c). Results are normalised to European participants before VLCD and expressed as means ± SEM. †p < 0.05 within group vs before diet. CI–V, mitochondrial respiratory chain subunits I–V
Skeletal muscle metabolic gene expression
The skeletal muscle expression of key metabolic genes involved in the regulation of glucose and fatty acid metabolism in the basal condition was assessed (ESM Table 1). Before the diet, except for a trend for reduced INSR and SLC2A4 (also known as GLUT4) expression in South Asian men, no major differences were observed between groups. Energy restriction induced downregulation of several genes involved in glycolysis (PFKM, PKM2), glycogen synthesis (UGP2, GBE1) and glycogen breakdown (PYGM) in both groups. Of note, no obvious differences were found in mRNA levels of key genes involved in mitochondrial biogenesis and tricarboxylic acid cycle whatever the conditions. Interestingly, PPARA was differentially affected by the diet, with a significant downregulation induced by energy restriction in South Asian men but no effect in European men. In line with this finding, several PPARA target genes, such as CPT1A, ACAA2 and TXNIP, showed a similar pattern, although not always reaching a significant threshold. Furthermore, FABP3 and HMGCS2 were found to be significantly upregulated by energy restriction only in European men.
Discussion
South Asians have an exceptionally high risk of developing type 2 diabetes in the context of the same environmental pressure when compared with other ethnicities. Differences in the regulation of energy/nutrient-sensing pathways are a possible explanation for this excess risk. Intriguingly, the current study showed that middle-aged overweight South Asian men exhibited a different metabolic adaptation to an 8 day VLCD compared with age- and BMI-matched European men.
Before the diet, South Asian men were more insulin resistant than European men, as indicated by higher insulin levels (both in the fasted state and during OGTT), and lower hepatic and peripheral insulin sensitivity. In addition, hepatic triacylglycerol content was significantly higher in South Asian men. Deposition of fat in the liver is often associated with HIR [20], and previous studies also reported higher hepatic triacylglycerol content and lower HIR in South Asians than Europeans [21, 22]. The reduced peripheral insulin sensitivity in South Asians appears to be due to a reduced rate of NOGD, which suggests an impairment in glycogen storage, one of the main defects also observed in patients with type 2 diabetes [23]. To analyse this result further, we assessed the expression of key genes involved in glycolysis and glycogen synthesis in skeletal muscle but did not find relevant differences between ethnicities. As expected [24], insulin promoted skeletal muscle GSK3-Ser21/9 phosphorylation and GS-Ser641 dephosphorylation, but no differences were observed between groups. Furthermore, energy restriction similarly potentiated the insulin-induced dephosphorylation of GS-Ser641 in both South Asian men and European men, which suggests that skeletal muscle glycogen synthesis might be improved secondary to enhanced insulin sensitivity in this condition. However, the canonical insulin signalling pathway assessed by phosphorylation of PKB and its downstream targets PRAS40, which is partly involved in the control of glycogen synthesis through the GSK3–GS axis, was not found to be differently affected by energy restriction. Of note, other upstream kinases than GSK3 were shown to phosphorylate GS on this specific residue [25, 26], and may therefore explain this apparent discrepancy.
In response to the 8 day VLCD, fasting glucose, insulin and triacylglycerol levels, and abdominal fat depots were reduced in both groups, with a concomitant increase in plasma NEFAs, reflecting increased lipolysis in adipose tissue. Furthermore, in line with previous studies hepatic triacylglycerol content decreased in both groups [27], with an accompanying improvement in hepatic insulin sensitivity [28–30], although, surprisingly, the changes in hepatic triacylglycerol content were not correlated with the improvement in hepatic insulin sensitivity. In addition, European men showed a classical switch from carbohydrate to lipid oxidation in response to energy restriction together with an improved insulin effect on substrate oxidation rates, reflecting a clear improvement in metabolic flexibility [28–30]. Peripheral insulin sensitivity was not affected by the diet in European men, in line with the findings of other short-term studies of energy restriction leading to minimal weight loss [28–30]. By contrast, peripheral insulin sensitivity was increased by energy restriction in South Asian men whereas the shift in whole-body substrate oxidation rates was found to be impaired, reflecting metabolic inflexibility. Although we do not have a definitive explanation for this apparent dissociation, it is plausible that the metabolic inflexibility still present after energy restriction in South Asian men might result from impaired peripheral lipid metabolism despite better glucose Rd. Indeed, fatty acid oxidation rates, which are positively associated with plasma fatty acid levels, were reported to be an important determinant of metabolic flexibility [31, 32].
Remarkably, the skeletal muscle mTOR pathway was found to be differentially regulated in response to energy restriction, with a higher activation of the mTORC1/S6K1 axis upon insulin stimulation in South Asian than European men. Insulin, like many growth factors, induces activation of mTORC1 secondary to PKB and/or ERK1/2-mediated phosphorylation and inactivation of TSC1/2, the major upstream regulator of mTORC1 [33–35]. Conversely, energy restriction leads to mTORC1 inhibition, partly due to TSC2 phosphorylation by AMPK on different regulatory residues than the ones targeted by PKB and ERK [13]. Finally, some of these kinases can also modulate mTORC1 activity independently of TSC2 by directly phosphorylating the mTOR catalytic subunit and/or some regulatory proteins of the mTORC1 complex, like regulatory-associated protein of mTOR (Raptor) and PRAS40 [12, 36, 37]. In the present study, no diet effect was observed on PKB phosphorylation and its targeted residue on TSC2, and AMPK activity was rather increased in South Asian men after energy restriction. Therefore, mTORC1 activation upon insulin stimulation in South Asians most likely occurred via ERK, since the increase in the phosphorylation of this kinase after energy restriction was significantly higher in South Asian than European men. Interestingly, insulin resistance was reported to be associated with defective insulin regulation of ERK signalling in skeletal muscle from women with polycystic ovary syndrome [38]. Thus, it is tempting to speculate that energy restriction can restore an insulin-sensitive pathway involved in ERK regulation that was specifically impaired in the South Asian men in this study. However, the exact underlying mechanism(s) still need to be clarified and we cannot rule out that other signalling pathways are also involved.
Besides its established role in regulating cell proliferation and growth, mTORC1 also promotes lipid synthesis and storage, while inhibiting fatty acid β-oxidation [8]. In the liver, this mTORC1-induced lipid partitioning has been shown to be mediated, at least partly, by inhibition of the transcription factor peroxisome proliferator-activated receptor α (PPARα) [39], which is also a key regulator of skeletal muscle fatty acid oxidation [40]. Specifically, PPARα controls the transcription of genes involved in fatty acid uptake and mitochondrial import, as well as in β-oxidation and ketogenesis [40]. Interestingly, in the present study, we observed a significant decrease in skeletal muscle PPARA gene expression in South Asian but not European men after energy restriction. In line with this finding, several PPARA target genes were also similarly downregulated in South Asian men, including the fatty acid binding protein gene FABP3, which was previously reported to be positively associated with enhanced skeletal muscle oxidative capacity after energy restriction [41]. Taken together, we might suggest that the higher insulin-stimulated mTORC1 activity observed after energy restriction in South Asian men underlies a decrease in fatty acid oxidation secondary to inhibition of PPARα, ultimately resulting in impaired metabolic flexibility (Fig. 5). Of note, mTORC1 was shown to control lipid metabolism in various other metabolic tissues [8]. Thus, it is conceivable that mTOR signalling can also be differently regulated in the adipose tissue from South Asians, thereby promoting the storage of NEFAs by inhibiting lipolysis [8]. This promotion of storage of NEFAs by inhibiting lipolysis may be another explanation for the attenuated increase in plasma NEFAs in South Asian compared with European men, apart from suppressed fatty acid oxidation in skeletal muscle. Other possible explanations include a lower proportion of slow-twitch type 1 oxidative muscle fibres in South Asians, rendering them less efficient for fatty acid oxidation [42], or the fact that NEFAs might not be directed towards oxidation but preferentially toward storage into complex lipids, i.e. intramyocellular lipid content in South Asians. It would therefore be interesting to address these points by measuring skeletal muscle intramyocellular lipid content and muscle fibre type distribution in further experiments.
Proposed mechanism(s) underlying the different metabolic adaptations to short-term energy restriction in South Asians compared with Europeans. The differences in skeletal muscle response to energy restriction between South Asians and Europeans during step 2 of the hyperinsulinaemic–euglycaemic clamp are shown on the various signalling pathways assessed. South Asians showed an increase in mTORC1 signalling upon insulin stimulation, which most likely occurred via ERK rather than PKB activation. This higher mTORC1 activation, by inhibiting PPARα and the transcriptional regulation of its target genes, may decrease fatty acid β-oxidation and contribute to the impaired metabolic flexibility observed in South Asians. Whole-body Rd was improved in South Asians, but not in Europeans, after energy restriction. This was associated with AMPK activation, which is known to promote skeletal muscle glucose uptake by an insulin-independent, but potentially additive, mechanism that remains to be clarified. FA, fatty acid; G6P, glucose 6-phosphate; Raptor, regulatory-associated protein of mTOR; TBC1D1, TBC1 domain family member 1. Red phosphorylation sites: inhibitory; green phosphorylation sites: activating; red arrows: expression is decreased in South Asians; green arrows: expression (or level for insulin, rate for Rd) is increased in South Asians; grey boxes: similar effect of energy restriction in South Asians and Europeans
Finally, in addition to its role in lipid metabolism, mTORC1 is also known to modulate insulin sensitivity by phosphorylating IRS1 on specific serine residues, resulting in a negative-feedback loop on the canonical insulin pathway. Enhanced mTORC1 activity in livers from obese insulin-resistant mice was indeed shown to promote S6K-mediated phosphorylation of IRS1, presumably on Ser636/639 [10] and/or Ser307 [43]. Furthermore, S6K-mediated phosphorylation of IRS1 on Ser1101 was also reported to mediate hepatic and skeletal muscle insulin resistance in high-fat diet fed mice [44]. However, despite apparent enhanced mTORC1 activation upon insulin stimulation, Rd was paradoxically found to be improved after energy restriction in South Asian men in our study. Unfortunately, we were not able to detect significant phosphorylation of IRS1 on both Ser636/639 and Ser307 using commercial antibodies (data not shown), although it is worth mentioning that the existence of this regulatory feedback loop and the exact IRS1 residue(s) involved are still a matter of debate, especially in human skeletal muscle [45].
The improvement in Rd after energy restriction in South Asians is apparently primarily accounted for by increased NOGD, suggesting that glycogen storage might be improved, although no change in the insulin-induced dephosphorylation of Ser641-GS was found. AMPK activation, which is known to promote skeletal muscle glucose uptake by an insulin-independent but additive mechanism increasing translocation of GLUT4 to the plasma membrane [46], was significantly higher in South Asian than in European men, suggesting that this kinase might be involved in the improved Rd in South Asians. Although the exact mechanism by which AMPK increases glucose uptake remains incompletely understood (see [47] for recent review), it apparently involves phosphorylation of the Rab GTPase-activating proteins AS160 (also known as TBC1 domain family member 4) and/or TBC1 domain family member 1 by the kinase, on residues different from the ones targeted by PKB [48]. However, activation of AMPK was also shown to decrease the rate of GLUT4 endocytosis both in human and rat muscle in vitro [49], whereas insulin had opposite effects, showing that multiple steps in the control of glucose uptake can be differently regulated by insulin- and AMPK-dependent pathways. Interestingly, a recent study has also reported that elevated glucose transport promoted by increased AMPK activity causes an accumulation of intracellular G6P leading to allosteric activation of GS and glycogen storage in skeletal muscle, independently of changes in GS phosphorylation, notably on Ser641 [50]. Altogether, we might speculate that the higher AMPK activity observed during hyperinsulinaemic–euglycaemic clamp can underlie the improved NOGD in South Asian men after energy restriction, at least partly, secondary to an insulin-independent increase in glucose uptake. Further studies are required for clarifying this point, notably for measuring skeletal muscle glycogen content after energy restriction in both South Asians and Europeans.
Taken together, the signalling pathway analysis performed in skeletal muscle in the present study does not allow us to draw definitive conclusions on the mechanism(s) underlying the improvement in Rd observed after energy restriction in South Asians but not Europeans. Additional in depth molecular investigations are therefore clearly required, not only in skeletal muscle but also in other organs involved in whole-body Rd, such as adipose tissue. In addition, as chronic mTORC1 activation is believed to contribute to the development of insulin resistance and type 2 diabetes [8], it would be interesting to investigate the response to long-term energy restriction on mTORC1 signalling and insulin sensitivity in South Asians compared with Europeans. Of note, we have recently studied the effect of high-fat feeding on energy/nutrient-sensing pathways in young, healthy lean South Asian and European men and showed that this diet rapidly induced insulin resistance in South Asians, but had no effect on Europeans [16]. However, in contrast to the present study, we did not observe differences in mTOR, AMPK or other energy/nutrient-sensing pathways, suggesting that differences in the regulation of these pathways may develop with age and in a more disadvantageous metabolic phenotype, e.g. in overweight individuals.
In conclusion, we showed that middle-aged overweight South Asian men exhibit a different metabolic adaptation to short-term energy restriction as compared with age- and BMI-matched European men. Although metabolic flexibility was impaired after an 8 day VLCD, Rd was improved in South Asians in contrast to Europeans, and was associated with an increase in insulin-induced activation of the skeletal muscle ERK–mTOR–S6K1 axis. Additional studies are required to expand these findings, which might provide new leads in our search to elucidate the pathogenesis of type 2 diabetes in South Asians.
Abbreviations
- 4EBP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- AS160:
-
Akt substrate of 160 kDa
- EGP:
-
Endogenous glucose production
- ERK:
-
Extracellular signal-regulated kinase
- GS:
-
Glycogen synthase
- GSK3:
-
Glycogen synthase kinase-3
- HIR:
-
Hepatic insulin resistance
- IQR:
-
Interquartile range
- IRβ:
-
Insulin receptor β
- IRS1:
-
Insulin receptor substrate 1
- LBM:
-
Lean body mass
- MCRi :
-
Metabolic clearance rate of insulin
- mtDNA:
-
Mitochondrial DNA
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- nDNA:
-
Nuclear DNA
- NEFA:
-
Nonesterified fatty acid
- NOGD:
-
Nonoxidative glucose disposal
- PKB:
-
Protein kinase B
- PPARα:
-
Peroxisome proliferator-activated receptor α
- PRAS40:
-
Proline-rich Akt substrate of 40 kDa
- Rd :
-
Rate of glucose disposal
- REE:
-
Resting energy expenditure
- RQ:
-
Respiratory quotient
- S6K1:
-
Ribosomal protein S6 kinase β1
- TSC2:
-
Tuberous sclerosis complex 2
- VLCD:
-
Very low calorie diet
References
National Task Force on the Prevention and Treatment of Obesity (2000) Overweight, obesity, and health risk. Arch Intern Med 160:898–904
Mindell J, Zaninotto P (2006) Cardiovascular disease and diabetes. In: Sproston K, Mindell J (eds) Health survey for England 2004. Volume 1. The health of minority ethnic groups, 1st edn. The Information Centre, Leeds, pp 63–94
Bindraban NR, van Valkengoed IG, Mairuhu G et al (2008) Prevalence of diabetes mellitus and the performance of a risk score among Hindustani Surinamese, African Surinamese and ethnic Dutch: a cross-sectional population-based study. BMC Public Health 8:271–280
Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV (2011) Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 34:1741–1748
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:311–321
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Haruta T, Uno T, Kawahara J et al (2000) A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 14:783–794
Ricoult SJ, Manning BD (2013) The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep 14:242–251
Takano A, Usui I, Haruta T et al (2001) Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 21:5050–5062
Um SH, Frigerio F, Watanabe M et al (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310–322
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Hammer S, van der Meer RW, Lamb HJ et al (2008) Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 295:E714–E718
van der Meer RW, Hammer S, Lamb HJ et al (2008) Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab 93:2702–2708
Bakker LE, van Schinkel LD, Guigas B et al (2014) A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes 63:248–258
Corpeleijn E, Saris WH, Blaak EE (2009) Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10:178–193
Szuhai K, Ouweland J, Dirks R et al (2001) Simultaneous A8344G heteroplasmy and mitochondrial DNA copy number quantification in myoclonus epilepsy and ragged-red fibers (MERRF) syndrome by a multiplex molecular beacon based real-time fluorescence PCR. Nucleic Acids Res 29:E13
Wijngaarden MA, van der Zon GC, Willems van Dijk KW, Pijl H, Guigas B (2013) Effects of prolonged fasting on AMPK signaling, gene expression and mitochondrial respiratory-chain content in skeletal muscle from lean and obese individuals. Am J Physiol Endocrinol Metab 304:E1012–E1021
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Anand SS, Tarnopolsky MA, Rashid S et al (2011) Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6:e22112
Petersen KF, Dufour S, Feng J et al (2006) Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A 103:18273–18277
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG (1990) Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–228
Jensen J, Ruge T, Lai YC, Svensson MK, Eriksson JW (2011) Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and PKB phosphorylation in human skeletal muscle. Metabolism 60:215–226
Skurat AV, Dietrich AD (2004) Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem 279:2490–2498
Wilson WA, Skurat AV, Probst B, de Paoli-Roach A, Roach PJ, Rutter J (2005) Control of mammalian glycogen synthase by PAS kinase. Proc Natl Acad Sci U S A 102:16596–16601
van der Meer RW, Hammer S, Smit JW et al (2007) Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 56:2849–2853
Christiansen MP, Linfoot PA, Neese RA, Hellerstein MK (2000) Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes 49:1691–1699
Jazet IM, Pijl H, Frolich M, Romijn JA, Meinders AE (2005) Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism 54:705–712
Markovic TP, Jenkins AB, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ (1998) The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 21:687–694
Galgani JE, Heilbronn LK, Azuma K et al (2008) Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 57:841–845
van de Weijer T, Sparks LM, Phielix E et al (2013) Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One 8:e51648
Inoki K, Li Y, Xu T, Guan KL (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17:1829–1834
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657
Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP (2005) Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179–193
Carriere A, Romeo Y, Acosta-Jaquez HA et al (2011) ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J Biol Chem 286:567–577
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9:316–323
Rajkhowa M, Brett S, Cuthbertson DJ et al (2009) Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J 418:665–671
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM (2010) mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468:1100–1104
Rakhshandehroo M, Knoch B, Muller M, Kersten S (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Res 2010:
Blaak EE, Wagenmakers AJ, Glatz JF et al (2000) Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279:E146–E154
Kelley DE, Goodpaster B, Wing RR, Simoneau JA (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141
Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146:1473–1481
Tremblay F, Brule S, Hee US et al (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A 104:14056–14061
Copps KD, White MF (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55:2565–2582
Hardie DG (2011) Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc 70:92–99
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
Geraghty KM, Chen S, Harthill JE et al (2007) Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407:231–241
Karlsson HK, Chibalin AV, Koistinen HA et al (2009) Kinetics of GLUT4 trafficking in rat and human skeletal muscle. Diabetes 58:847–854
Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K (2011) Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60:766–774
Acknowledgements
We are greatly indebted to E. J. M. Ladan-Eygenraam (Leiden University Medical Centre, Leiden, the Netherlands) for her technical assistance during the study.
Funding
We thank Roba Metals B. V. IJsselstein (Utrecht, the Netherlands) for financial support. Funding by the Netherlands Heart Foundation (Project UL 2009-4548) is gratefully acknowledged (The Hague, the Netherlands). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors have read and approved the final version of the manuscript, and meet all three conditions as stated by the International Committee of Medical Journal Editors uniform requirements for manuscripts submitted to medical journals. LEHB contributed to the acquisition, analysis and interpretation of all data and drafted the manuscript. BG contributed to the acquisition, analysis and interpretation of skeletal muscle data and reviewed the manuscript. LDS contributed to the acquisition, analysis and interpretation of MR data and reviewed the manuscript. GCMZ contributed to the acquisition of skeletal muscle data and reviewed the manuscript. TCMS contributed to the acquisition of clamp data and reviewed the manuscript. JBK provided the statistical model, contributed to the analysis of data and reviewed the manuscript. JTJ contributed to the design of the MR protocol, contributed to the acquisition and interpretation of MR data and reviewed the manuscript. HJL contributed to the analysis and interpretation of MR data and reviewed the manuscript. JWAS contributed to the conception and design of the MR protocol, contributed to the analysis and interpretation of data and reviewed the manuscript. HP contributed to the analysis and interpretation of data and reviewed the manuscript. AEM contributed to the conception and design of the study protocol, contributed to the analysis and interpretation of all data and reviewed the manuscript. IMJ contributed to the conception and design of the protocol, contributed to the acquisition, analysis and interpretation of all data and reviewed the manuscript.
LEHB and IMJ are the guarantors of this work and, as such, have full access to all the data generated in the framework of the study and take responsibility for their integrity and the accuracy of their analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 354 kb)
ESM Table 1
(PDF 333 kb)
Rights and permissions
About this article
Cite this article
Bakker, L.E.H., Guigas, B., van Schinkel, L.D. et al. Middle-aged overweight South Asian men exhibit a different metabolic adaptation to short-term energy restriction compared with Europeans. Diabetologia 58, 165–177 (2015). https://doi.org/10.1007/s00125-014-3408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3408-4