Abstract
Key message
Crosses of parents that differ in their DNA methylation states leads to progressive demethylation in the F1 hybrids.
Abstract
In plant breeding research, hybrid vigor in F1 hybrids is known to be a very important phenomenon. Hybrid vigor, or heterosis, refers to the fact that F1 hybrids from crosses with a certain combination of parents have traits that are superior to those of the parents. In addition, DNA methylation is an important factor that affects gene expression in plant genomes and contributes to hybrid vigor. We introduced the 35S promoter sequence into the cucumber mosaic virus (CMV)-based vector and inoculated the GFP-expressing transgenic Nicotiana benthamiana line 16c with the recombinant virus specifically to induce DNA methylation on the 35S promoter. For plants that had transcriptional gene silencing (TGS) of GFP established by methylation of the 35S promoter (35S-TGS), TGS was fully maintained in their later self-pollinated generations. When the 35S-TGS plants were crossed with 16c, which does not contain DNA methylation in the 35S promoter, the F1 hybrids unexpectedly became progressively DNA demethylated as the plants grew. We hypothesis that in F1 hybrids that are produced by a cross between parents with extremely different gene methylation states, the methylation state of the genes in question may shift more and more to hypomethylation as the plants grow. This progressive demethylation phenomenon observed in this study may be important in plant breeding to reactivate the genes which were silenced by DNA methylation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DNA methylation is a highly conserved epigenetic modification that regulates gene expression. In plants, DNA methylation occurs in three sequence contexts: CG, CHG, and CHH (H for A, C, and T). Establishment of DNA methylation in all cytosine contexts mediated through the RNA-directed DNA methylation (RdDM). In RdDM pathway, two plant-specific RNA polymerases, Pol IV and Pol V, play an important role in de novo DNA methylation in plants. 24-nucleotide (nt) siRNAs are generated by Pol IV, RNA-DEPENDENT RNA POLYMERASE 2 (RDR2), and DICER- LIKE 3 (DCL3). After the 24-nt siRNA is load to Argonaute 4 (AGO4), the AGO4-siRNA complex eventually interacts with the Pol V subunit, NUCLEAR RNA POLYMERASE E1 (NRPE1), and forms a complex with DRM2 to initiate DNA methylation (Matzke et al. 2015; Dalakouras and Vlachostergios 2021). CG methylation is maintained by METHYLTRANSFERASE1 (MET1), CHG and CHH methylation are maintained by CHROMOMETHYLASE3 (CMT3) and CHROMOMETHYLASE2 (CMT2), respectively (Baulcombe. 2004; Zhang et al. 2018). In addition, DOMAINS REARRANGED METHYLTRANSFERASE2 (DRM2), a de novo DNA methyltransferase, is involved in both maintenance and initiation of DNA methylation (Cao et al. 2003).
Over the years, many crops have been bred to generate hybrid vigor in offspring with traits superior to their parents, but the molecular mechanisms responsible for hybrid vigor have been unclear (Fujimoto et al. 2018). However, DNA methylation in F1 hybrids has been associated with hybrid vigor. For example, analyses of DNA methylation changes in protein-coding genes or transposable elements (TEs) in Arabidopsis F1 hybrids showed that methylation levels tended to be higher than in the parents, but in some cases, the methylation level decreased (Greaves et al. 2012, 2014; Groszmann et al. 2013; Shen et al. 2012). Thus, progressive demethylation of various genes in F1 hybrids as a result of hybridization was considered to be an important factor underlying the molecular mechanism of hybrid vigor (Greaves et al. 2015). F1 hybrids between Arabidopsis Col-0 and C24 were larger than their parents, and various genes were demethylated. In particular, increased expression of chlorophyll biosynthetic genes, due to demethylation, at an early stage of plant growth was reported to be the cause of hybrid vigor (Fujimoto et al. 2012; Kawanabe et al. 2016). The changes to the hybrid methylome occur through two related processes, trans-chromosomal methylation (TCM) and trans-chromosomal demethylation (TCdM), where the methylation state of one parental allele is altered to resemble that of the other parental allele (Greaves et al. 2012). TCdM tends to be observed in 1-kb upstream regions of genes (Groszmann et al. 2011a, b; Zhang et al. 2016). In addition to TCdM, TCM was also reported in Arabidopsis F1 hybrids (Greaves et al. 2016). However, Arabidopsis hybrids that contained nrpd1 or nrpe1 mutations maintained the heterostic phenotype despite the loss of TCM and TCdM, suggesting that DNA methylation was not a determining factor for the heterosis (Zhang et al. 2016). Thus, it was unclear whether DNA methylation level was a key factor for hybrid vigor.
Although induction of DNA methylation and demethylation have both been reported to be associated with hybrid vigor, the majority of such findings indicate that DNA demethylation has progressed in the F1 hybrids (Fujimoto et al. 2018). For example, in Arabidopsis, elevated DNA methylation has been reported in the F1 hybrid between the ecotype Col-0 and C24 (Shen et al. 2012). On the other hand, substantially reduced DNA methylation has been found in the F1 hybrid between rice lines 9311 and PA64 (Zhou et al. 2021). However, the factors that determine whether the methylation state of F1 hybrids is elevated or decreased compared to parental methylation levels are not well understood. Zhou et al. (2021) previously discussed that the methylation was reduced in Arabidopsis F1 hybrids at sites where methylation levels differed between the parents. On the other hand, rice F1 hybrids showed increased methylation at sites where methylation levels were homologous between the parents. An important question that arises may provide a clue for elucidating the trigger for DNA methylation or demethylation: If the DNA methylation state that is involved in heterosis differs greatly between two parents, what is the effect on DNA methylation in the F1 hybrid? It is almost impossible to determine using naturally occurring plants.
In our experiments using the transgenic line 16c of Nicotiana benthamiana, which expresses GFP under the control of the 35S promoter, we noticed that the cucumber mosaic virus (CMV) vector induced transcriptional gene silencing (TGS) at the 35S promoter (Otagaki et al. 2006). We previously reported that TGS-induced plants could have two phenotypes in the next generation: those with complete TGS autofluoresced red (RED), and those with incomplete TGS fluoresced orange (ORN) (Matsunaga et al. 2019). In present study, in the third generation of selfing, all individuals had the RED phenotype (RED16c). We were curious what would happen when RED16c was crossed with an individual that lacks TGS in the 35S promoter. Our cross of RED16c with 16c, which does not have methylation on the 35S promoter, yielded only a few RED types among the F1 hybrids, and the TGS of the GFP gene (GFP-TGS) was gradually eliminated as demethylation progressed as the plants grew. Thus, the strong TGS state inherited by selfing was destabilized by crossing with unmethylated 16c. We hypothesized that alleles with extremely different methylation levels between the two parents might have caused the progressive demethylation of DNA in the F1 plants.
Materials and methods
Plant materials
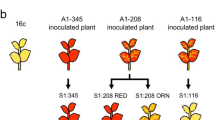
Nicotiana benthamiana line 16c was a kind gift from David Baulcombe at University of Cambridge, UK. RED16c was developed from three rounds of self-pollination of a 16c plant that had been inoculated with a CMV-A1 vector that contained a 208-nt fragment of the 35S promoter (Matsunaga et al. 2019) (Figs. S1, S2). All plants were grown in a plant growth room with 16 h light/8 h dark at 24 °C and 50% relative humidity.
GFP observation
The GFP fluorescence phenotype was assessed using fully developed leaves at the top of the three independent plants at 20, 30, 40, 50, and 65 days after germination (DAG). GFP fluorescence was observed with a Microscope VB-7000 (Keyence, JP) fitted with the GFP filter (OP-42313) or using a hand-held fluorescence excitation system (ExF-B) with a yellow filter (SCG-Y) (Bio Tools, JP).
DNA methylation assay
DNA was extracted from a bulk sample of fully developed leaves from the top of the three independent plants each at 20, 40, and 65 DAG using the Illusta DNA extraction Kit PhytoPure (GE Healthcare, USA). DNA was treated with bisulfite using EZ DNA Methylation-Lightning Kit (Zymo Research, USA). For PCR amplification, primer pair 35S-346F-bisuT (5′-ATTGAGAYTTTTYAAYAAAGGGT-3′) and 35S + 1A-bisuA (5′-CTCTCCAAATGAAATGAACTTC-3′) were designed for the plus-strand. Primer pair 35S(−)-5-BS (5′-TTATATAGAGGAAGGGTYTTGYGAAG-3′) and 35S(−)-3-BS (5′-CAATTRARACTTTTCAACAAAR-3′) were designed for the minus-strand. The primers were synthesized using the program MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi). To amplify the entire (~ 345 bp) core sequence, we synthesized degenerate primers, which can hybridize to the bisulfite-treated DNA. The bisulfite-treated DNA was amplified using Takara Epi-taq (Takara, Japan) with the following conditions by GeneAtlas thermocycler (astec, JP): 40 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. The PCR fragments were cloned into the pTAC1 vector (Dyna Express TA PCR Cloning Kit, Bio Dynamics Laboratory, JP). For bisulfite sequencing, we used 10–12 clones for each DNA preparation. The sequences were aligned using MEGA7 (Kumar et al. 2016), and the graphs and heatmap were created using Microsoft (USA) Excel.
Pollen DNA extraction
RED16c pollen grains were suspended in TE buffer, and DNA was extracted using the conventional phenol–chloroform method. The extracted DNA was then treated with RNase A (Sigma-Aldrich, USA) and ethanol-precipitated.
Results
Phenotype of 35S-TGS plants
As we showed previously (Matsunaga et al. 2019), after the 16c plants were inoculated with the CMV-A1 vector, which harbors the 208-nt fragment of the 35S promoter (CMV-208), virus-induced gene silencing (VIGS) was evident because the plants emitted weak GFP fluorescence from the petiole or veins of leaves. The S1 progeny plants from the CMV-208-inoculated plants showed two different phenotypes for the GFP fluorescence level. The RED-type plants had completely lost GFP fluorescence, while the ORN-type plants seemed to retain a low level of GFP fluorescence, which masked the red autofluorescence. After three rounds of self-pollination of the RED-type plants, all individuals stably showed the RED phenotype. Because the RED plants appeared to be epigenetically fixed, we called the S3 line RED16c (Figs. 1a, S1, S2). When we analyzed the DNA methylation pattern in the 35S promoter region of RED16c using bisulfite sequencing for the entire core sequence of the 35S promoter (~ 345 bp) (Fig. 1b), we found that the entire region of the 35S promoter in the original 16c was barely methylated on both the plus- and minus- strands (Fig. 1c), whereas the region was highly methylated in RED16c (Fig. 1d). We used these lines that were epigenetically “isogenic” for the 35S-GFP gene as parental lines to develop F1 hybrids. We found a small level of methylation at the CHH sites in the 35S promoter of 16c. For this reason, we considered the possibility that some CHH sites might not be modified by our bisulfite treatment due to incomplete bisulfite reaction; the CHH methylation may have been falsely detected as a background in 16c.
GFP fluorescence and DNA methylation level of 16c and RED16c. a GFP fluorescence of 16c and RED16c. b Schematic diagram of the 35S promoter sequence for bisulfite sequencing. The red arrow shows the region corresponding to the 208-nt fragment contained in the CMV vector for induction of DNA methylation. The black line shows the position of subdomain A1, which is important for transcriptional activity of the 35S promoter (Benfey and Chua 1990). c–d DNA methylation levels on the 35S promoter. c 16c, plus- and minus-strands (N = 10 and 11, respectively). d RED16c, plus- and minus-strands (N = 12 and 10, respectively). The sequenced regions of the plus- and minus-strands were slightly different because the PCR-amplified regions were different. The x-axis shows the position of the 35S promoter where the transcription start site is set to + 1 (positions at −321 to −23 in plus-strand, and at −324 to −50 in minus-strand); the y-axis shows the DNA methylation rate. The black line shows the position for the subdomain A1
Next, to confirm that any serious PCR bias did not occur with the primers used for bisulfite sequencing, we performed another methylation analysis of the 35S promoter of RED using primer pair different from those used in Fig. 1. In addition, because we wanted to also confirm that any serious cloning bias, which is sometimes found in bisulfite sequencing depending on the target sequence, did not occur, we here used one of the next generation sequencing tools, Amplicon-seq. As a result, the methylation patterns of the plus- and minus-strands obtained by Amplicon-seq were in good agreement with those obtained by the method used in the clone-based bisulfite sequencing (Fig. S3). Based on these results, we concluded that neither PCR bias nor cloning bias occurred.
Progressive cancellation of TGS in the 35S promoter-driven GFP fluorescence
The phenotypes of GFP fluorescence were here classified into three types as shown in Fig. 2a and Matsunaga et al. (2019). The same phenotype as the original 16c was classified as the yellow (YEL) type, while the RED type had red autofluorescence due to complete TGS of 35S-GFP such as in RED16c. The intermediate type with GFP fluorescence weaker than 16c was classified as the ORN type. In the F1 hybrids derived from the cross between RED16c as the female parent and 16c as the male parent (RED16c × 16c), the majority of the F1 hybrids had the ORN phenotype, an incomplete TGS state, but ca 10% had the RED phenotype at 40 DAG (Fig. 2b, Table S1). However, the GFP-TGS was gradually lost as the plants grew, and at 65 DAG, all the RED phenotypes had changed into the ORN phenotypes. Most of the F1 hybrids (around 90%) had returned to the original 16c phenotype (YEL) (Fig. 2b, Table S1). In the F1 hybrids derived from the cross of 16c × RED16c, RED was no longer observed even at the early growth stage examined (20 DAG) (Fig. S4a). In addition, ORN had been predominant until 40 DAG, but at 50 DAG, YEL was more frequent than ORN. All the F1 hybrids were YEL by 65 DAG (Table S1, Fig. S4a). Thus, the robust 35S-TGS state in RED16c that is inherited and maintained by self-fertilization was quite readily cancelled by the cross with unmethylated 16c.
Phenotype populations for GFP fluorescence level in F1 plants from RED16c × 16c cross. a Representative phenotype for GFP fluorescence level: YEL, same phenotype as the original 16c; ORN, intermediate type with weaker GFP fluorescence than 16c; RED, same phenotype as RED16c. b Schematic diagram of ratio of GFP phenotypes for the F1 hybrids. The x-axis shows the number of days after germination (DAG); the y-axis shows the ratio of the GFP phenotypes
Analysis of the methylation of the 35S promoter in F1 hybrid between RED16c and 16c
The phenotypic ratio of YEL, ORN and RED in the F1 hybrids (RED16c × 16c) was almost the same at 20, 30 and 40 DAG (Fig. 2b). However, the YEL phenotype had increased to 70% of the total plants by 50 DAG and to 90% by 65 DAG. We then conducted bisulfite sequencing to analyze DNA methylation level of the 35S promoter of the F1 hybrids (RED16c × 16c). In the plus-strand, methylation was rarely detected in YEL at 20 and 40 DAG (Fig. 3a, d). For ORN phenotypes, the methylation level was slightly higher at 40 DAG than at 20 DAG (Fig. 3b, e). For RED, the 35S promoter was highly methylated at 20 and 40 DAG; the CHH methylation level had greatly increased in the entire promoter region in particular (Fig. 3c, f). In the plants that were ORN at 40 DAG and had shifted to YEL by 65 DAG, the methylation level had slightly increased in the entire promoter region even though the GFP phenotype was YEL (Fig. 3e, g). In the plants that were RED at 40 DAG and had shifted to ORN by 65 DAG, the methylation level had decreased in the entire promoter region (Fig. 3f, h). We found little methylation in the minus-strand in YEL plants at 20 and 40 DAG (Fig. 3a, d). On the other hand, in ORN plants, the methylation level gradually increased (Fig. 3b, e). In RED plants, high DNA methylation levels were maintained at 20 and 40 DAG, and the CHH methylation level was slightly higher at 40 DAG (Fig. 3c, f). In the plants that were ORN at 40 DAG and had shifted to YEL by 65 DAG, the methylation level was much lower (Fig. 3e, g). In the plants that were RED at 40 DAG and had shifted to ORN by 65 DAG, the methylation level had also decreased in the entire promoter region (Fig. 3f, h). These results together indicate some correlation between the GFP phenotype and the methylation level in the minus-strand, but not in the plus-strand. To further clarify the correlation between phenotype and methylation level, the difference in methylation percentage between the plants that were RED at 40 DAG (Fig. 3f) but ORN at 65 DAG (Fig. 3h) and between the plants that were ORN at 40 DAG (Fig. 3e) but YEL at 65 DAG (Fig. 3g) were visualized by subtracting values at 40 DAG from those at 65 DAG (Fig. S5). According to our expectation, the methylation levels seem to decrease when the phenotype shifted from RED to ORG or from ORG to YEL between 40 and 65 DAG. As shown in Fig. S5, the values after the calculation of YEL65 − ORN40 were never negative for the four methylation types in the plus-strand. On the other hand, in the minus strand, both values from the calculations were almost always negative, indicating that there is good correlation between phenotype and methylation state, especially in the minus strand.
DNA methylation patterns in the plus- and minus-strands of the 35S promoter of each F1 plant from RED16c × 16c cross showing the different phenotypes at 20, 40 and 65 days after germination (DAG). a YEL at 20 DAG (Plus-strand, N = 12) (Minus-strand, N = 12). b ORN at 20 DAG (Plus-strand, N = 12) (Minus-strand, N = 12). c RED at 20 DAG (Plus-strand, N = 12) (Minus-strand, N = 11). d YEL at 40 DAG (Plus-strand, N = 10) (Minus-strand, N = 10). e ORN at 40 DAG (Plus-strand, N = 12) (Minus-strand, N = 10). f RED at 40 DAG (Plus-strand, N = 12) (Minus-strand, N = 11). g ORN plants at 40 DAG that shifted to YEL at 65 DAG (Plus-strand, N = 12) (Minus-strand, N = 10). h RED plants at 40 DAG that shifted to ORN at 65 DAG (Plus-strand, N = 12) (Minus-strand, N = 12). The x-axis shows the position of the 35S promoter where the transcription start site is set to + 1; the y-axis shows the DNA methylation rate. The black line shows the position for the subdomain A1
Most of the F1 hybrids (16c × RED16c) had the ORN phenotype at 40 DAG (Fig. S4a). We examined DNA methylation levels of the 35S promoter in ORN at 40 DAG and found that it was hypomethylated compared to that in the 16c control (Fig. 1c, S4b). On the other hand, the methylation states in both the plus- and minus-strands were low. However, subdomain A1 was still methylated to some extent (Fig. S4b). The promoter in the F1 hybrids (16c × RED16c) was significantly hypomethylated at 40 DAG, and by 65 DAG, GFP fluorescence had fully returned to the state in the original 16c (Table S1, Fig. S4a). These results therefore suggest that the combination of the parents affected the appearance of the phenotype and the level of DNA demethylation.
To confirm that the results of our bisulfite sequencing really reflect the methylation status in the 35S promoter, we conducted another experiment to compare methylation levels at the CCGG (positions at −316 and −315) site recognized by methylation-sensitive and methylation-insensitive restriction enzymes (HpaII and MspI) among 16c, YEL and RED plants. In addition, with those enzymes, we could analyze also 5-hydroxymethylation (5-hmC), which has been reported to be important in mammalian gene expression; 5-hmC was also detected in plants (Wang et al. 2015). The genomic DNAs of the control 16c, YEL and RED plants were first glucosylated using T4-BGT and treated with the restriction enzymes, HpaII and MspI. PCR was then performed with primer pair designed from the flanking region of the CCGG site in the 35S promoter. The results showed that the 35S promoter of 16c was not methylated, while slight DNA methylation was detected in YEL, and the 35S promoter of RED was significantly methylated (Fig. S6). 5-hmC on the 35S promoter was not detected in any of the samples. Therefore, the result of the experiment using the methylation-sensitive endonuclease well supports the results of our bisulfite sequencing.
DNA methylation state in RED16c pollen grains were separated into two patterns
Then we asked why the progressive demethylation of the 35S promoter occurred in the F1 hybrids, we considered that the DNA methylation status of gametes might provide a clue. We thus examined the methylation level of the 35S promoter in pollen grains by extracting DNA from RED16c pollen. Bisulfite sequencing of the plasmid clones revealed that the sequence patterns could be divided into two groups (Fig. 4). Some clones maintained CHH methylation but decreased CG methylation (group I, clones 1–6 in the plus strand and clones 1–5 in the minus strand). The other clones maintained the CG methylation but decreased the CHH methylation (group II, clones 7–10 in the plus strand and clones 6–10 in the minus strand) (Fig. 4). These results indicate that DNA methylation state was reconstituted in pollen grains.
Two methylation patterns in DNA isolated from RED16c pollen grains. The nucleotide position on the 35S promoter (transcription start site is set to + 1) is indicated above each strand. The red line shows subdomain A1. The DNA methylation state in the 35S promoter in pollens was investigated directly through bisulfite sequencing of DNA extracted from pollen grains. The clone sequences were of two types: those that maintained a high CHH methylation level (group I, clones 1–6 on the plus-strand and clones 1–5 on the minus-strand), and those that maintained CG methylation to some extent (group II, clones 7–10 on the plus-strand and clones 6–10 on the minus-strand)
Discussion
We here created an experimental system using transgenic plants; one parent has a fully methylated transgene, but the corresponding gene in the other plant is not methylated. Then, we induced artificial methylation of the 35S promoter in the N. benthamiana transgenic plant (16c) using our plant viral vector, creating RED16c whose 35S promoter was fully methylated. As our experimental plants grew, we observed a gradual increase in GFP expression with increasing demethylation of the 35S promoter in the F1 hybrids between RED16c and unmethylated 16c. We tried to correlate the phenotype of GFP with the methylation level of the 35S promoter and found that the phenotypes for both the plus- and minus-strands generally shifted from RED to ORN and from ORN to YEL as the methylation level decreased. However, only one exception was the transition of the ORN plants at 20 DAG (Fig. 3b) to 40 DAG (Fig. 3e). During this growth period, DNA methylation increased considerably, but it was not as high as the methylation level in RED. This increase in methylation is consistent with the results of the methylation analysis by Shen et al. (2012) using Arabidopsis plants. They observed an increase in global DNA methylation at an early growth of F1 plants crossed with parents with different methylation levels. Therefore, crossing parents with different levels of methylation may result in a temporary increase in methylation during the early growth stage, even if demethylation eventually progresses.
In the F1 hybrids, there was a drastic phenotype change from 40 to 65 DAG (Figs. 2b, S1) and a decrease in methylation in the entire promoter region (Figs. 3g, 3h, S5). DNA demethylation from 40 to 65 DAG may have had a threshold methylation level for the GFP restoration from the 35S promoter silencing. Taken together, these results indicate that methylation of the 35S promoter may be quickly eliminated in the hybrids as the plants grow when the two parents have extremely different methylation levels.
DNA methylation reprogramming is generally known to occur in gametes when plants produce offspring (Gehring 2019; Kawashima and Berger 2014). The methylation state in the gametes would naturally affect the methylation levels of F1 hybrids. As shown in Fig. 4, DNA methylation state in RED16c pollen grains were separated into two patterns. According to previous reports, compared to methylation in the somatic cells, CG methylation is generally maintained, but CHH methylation greatly decreases in the sperm nucleus of pollen grains (Calarco et al. 2012), whereas CG methylation is maintained in the vegetative nucleus during gametogenesis (Gehring 2019; Borges and Martienssen 2013). However, it has also been reported that CG methylation is reduced but CHH methylation maintained in LTR retrotransposons and intergenic regions in the vegetative cells (Calarco et al. 2012; Ibarra et al. 2012; Jullien et al. 2006, 2008, 2012). Considering that the 35S promoter is a transgene and thus a likely target of TGS in cells, methylation of the 35S promoter may be induced in a similar to the way it is induced in transposons. We therefore infer that the DNA methylation pattern of the 35S promoter in pollens had two types as the result of the mixture of the vegetative and sperm cell nuclei. In the female gametophyte, CHH methylation is maintained but CG methylation levels are comparable to the somatic methylation levels or partly reduced by hemimethylation (Ingouff et al. 2017). Therefore, the above knowledge on the DNA methylation in gametes agrees well with our observations as summarized in Fig. S7. We consider that CHH methylation was maintained in the egg and greatly reduced in the sperm, whereas CG methylation was partly reduced in the egg due to hemimethylation and maintained in the sperm. Consequently, fertilization of an egg and sperm will produce a situation where the methylation state is extremely different between alleles.
There is a phenomenon called passive DNA demethylation in which mainly CHH methylation levels progressively decrease as plants grow (Kawakatsu et al. 2017). In our F1 hybrids, we observed progressive DNA demethylation between 40 and 65 DAG, especially in the minus-strand of the 35S promoter (Fig. 3). There is a possibility that passive DNA demethylation may be involved in the mechanism for the progressive DNA demethylation. However, our observation cannot be simply explained by the passive DNA demethylation mechanism because we found that not only CHH but also CG and CHG sites were all drastically demethylated in the F1 hybrids, suggesting the existence of some unknown mechanism in which passive DNA demethylation may be partially involved. On the other hand, strand-biased (specific) DNA demethylation observed here was possibly explained by the strand-specific nature of DRM2 methylation (Zhong et al., 2014). The induction of strand-biased DNA methylation in plant DNA has been previously reported in detail in Arabidopsis (Luo and Preuss, 2003). Furthermore, strand-biased DNA methylation in plants is induced by RdDM and determined by the siRNAs specific to the target DNA sequence. That is, methylation occurs specifically on the strand guided by siRNAs for DRM2, de novo methyltransferase (Zhong et al., 2014). Our observation of strand-specific DNA demethylation may also be due to the fact that de novo DNA methylation by DRM2 did not occur after the supply of siRNAs was stopped in the next generation.
Reprogramming of the DNA methylation state in gametes, where CHH methylation in particular is eliminated in the sperm cell nuclei, is an important consideration for determining why the methylation level of the 35S promoter was very low in the F1 hybrid and why the RED phenotype eventually disappeared. In RED16c pollens, CHH methylation was greatly reduced, so that DNA methylation was barely maintained in the F1 hybrids (Fig. 5a). Consistently, when we analyzed the CHH methylation levels in the ORN types at 40 DAG, those on the plus- and minus-strands of the 35S promoter were both lower in 16c (egg) × RED16c (pollen) hybrid than in RED16c (egg) × 16c (pollen) hybrid (Fig. 5b), indicating that the DNA methylation state of the F1 hybrids is determined by the methylation status of the pollen and the egg cell during fertilization. Therefore, the difference in methylation between pollen and egg cells will affect that of the F1 plants. We thus believe that the F1 hybrids simply inherited the methylation state of the nuclear DNA of the egg and pollen, and the difference in methylation state between alleles may have triggered progressive demethylation in the F1 plants as the plants grow.
Schematic explanation of the gametes and zygotes when RED16c and 16c are crossed. a In the case of the cross between RED16c (egg) × 16c (pollen), TGS is initially maintained to some extent by the CHH methylation in the egg cell, but TGS is eventually eliminated due to low CG methylation. In the case of the cross between 16c (egg) × RED16c (pollen), 35S-TGS was eliminated immediately because there was only CG methylation in the sperm cell. b CHH methylation levels of the ORN type at 40 DAG. Methylation levels in both the 35S promoter plus- and minus-strands were lower in 16c (egg) × RED16c (pollen) than in RED16c (egg) × 16c (pollen). The asterisks indicate a statistical significance by two-tailed Fisher’s exact test (**P < 0.01). The number of the CHH sites used for the analysis were indicated below the x-axis
In our F1 hybrid, the cross of a fully methylated allele with an unmethylated counterpart induced demethylation of the corresponding allele in the next generation and in a progressive decrease in methylation level as the plant grew. This phenomenon of progressive demethylation in our F1 hybrids was a non-additive methylation that differed from both parents, and the expression of the demethylated genes would increase, which may be the driving force behind the hybrid vigor. Further analysis of the DNA methylation levels in seed embryos and endosperm of the F1 hybrids of 35S-TGS plants will provide more insight into the relationship between hybrid vigor and DNA methylation. To elucidate the mechanistic details of this hypothesized process, we will cross plants with the RED phenotype with plants with the ORN phenotype or cross two plants with the ORN phenotype. By using our 35S-TGS plants for crossing parents with differentially methylated 35S promoters, we may be able to predict the kind of methylation patterns that the F1 hybrids will have, depending on the combination of maternal and paternal parents with different methylation levels.
Availability of data and materials
All data generated and analyzed during this study are included in this published article.
References
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Benfey PN, Chua NH (1990) The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250:959–966
Borges F, Martienssen RA (2013) Establishing epigenetic variation during genome reprogramming. RNA Biol 10:490–494
Calarco JP, Borges F, Donoghue M, Ex FV, Jullien PE, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD, Martienssen RA (2012) Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 151:194–205
Cao X, Aufsatz W, Zilberman D, Mette MF, Huang MS, Matzke M, Jacobsen SE (2003) Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr Biol 13:2212–2217
Dalakouras A, Vlachostergios D (2021) Epigenetic approaches to crop breeding: current status and perspectives. J Exp Bot 28(72):5356–5371
Fujimoto R, Taylor JM, Shirasawa S, Peacock WJ, Dennis ES (2012) Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA 109:7109–7114
Fujimoto R, Uezono K, Ishikura S, Osabe K, Peacock WJ, Dennis ES (2018) Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breed Sci 68:145–158
Gehring M (2019) Epigenetic dynamics during flowering plant reproduction: evidence for reprogramming? New Phytol 224:91–96
Greaves IK, Bayon RG, Wang L, Zhu A, Liu PC, Groszmann M, Peacock WJ, Dennis ES (2015) Epigenetic changes in hybrids. Plant Physiol 168:1197–1205
Greaves IK, Eichten SR, Groszmann M, Wang A, Ying H, Peacock WJ, Dennis ES (2016) Twenty-four-nucleotide siRNAs produce heritable trans-chromosomal methylation in F1 Arabidopsis hybrids. Proc Natl Acad Sci USA 113:6895–6902
Greaves IK, Groszmann M, Wang A, Peacock WJ, Dennis ES (2014) Inheritance of trans chromosomal methylation patterns from Arabidopsis F1 hybrids. Proc Natl Acad Sci USA 111:2017–2022
Greaves IK, Groszmann M, Ying H, Taylor JM, James Peacock WJ, Dennis ES (2012) Trans chromosomal methylation in Arabidopsis hybrids. Proc Natl Acad Sci USA 109:3570–3575
Groszmann M, Greaves IK, Albert N, Fujimoto R, Helliwell CA, Dennis ES, Peacock WJ (2011a) Epigenetics in plants-vernalisation and hybrid vigour. Biochim Biophys Acta 1809:427–437
Groszmann M, Greaves IK, Albertyn ZI, Scofield GN, Peacock WJ, Dennis ES (2011b) Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc Natl Acad Sci USA 108:2617–2622
Groszmann M, Greaves IK, Fujimoto R, Peacock WJ, Dennis ES (2013) The role of epigenetics in hybrid vigour. Trends Genet 12:684–690
Ibarra CA, Feng X, Schoft VK, Hsieh TF, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, Rojas D, Fischer RL, Tamaru H, Zilberman D (2012) Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337:1360–1364
Ingouff M, Selles B, Michaud C, Vu TM, Berger F, Schorn AJ, Autran D, Durme MV, Nowack MK, Martienssen RA, Grimanelli D (2017) Live-cell analysis of DNA methylation during sexual reproduction in Arabidopsis reveals context and sex-specific dynamics controlled by noncanonical RdDM. Genes Dev 31:72–83
Jullien PE, Kinoshita T, Ohad N, Berger F (2006) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18:1360–1372
Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F (2008) Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol 6:e194
Jullien PE, Susaki D, Yelagandula R, Higashiyama T, Berger F (2012) DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr Biol 22:1825–1830
Kawakatsu T, Nery JR, Castanon R, Ecker JR (2017) Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol 18:171
Kawanabe T, Ishikura S, Miyaji N, Sasaki T, Wu LM, Itabashi E, Takada S, Shimizu M, Takasaki-Yasuda T, Osabe K, Peacock WJ, Dennis ES, Fujimoto R (2016) Role of DNA methylation in hybrid vigor in Arabidopsis thaliana. Proc Natl Acad Sci USA 113:6704–6711
Kawashima T, Berger F (2014) Epigenetic reprogramming in plant sexual reproduction. Nat Rev Genet 15:613–624
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Luo S, Preuss D (2003) Strand-biased DNA methylation associated with centromeric regions in Arabidopsis. Proc Natl Acad Sci USA 100:11133–11138
Matsunaga W, Shimura H, Shirakawa S, Isoda R, Inukai T, Matsumura T, Masuta C (2019) Transcriptional silencing of 35S driven-transgene is differentially determined depending on promoter methylation heterogeneity at specific cytosines in both plus- and minus-sense strands. BMC Plant Biol 19:24
Matzke MA, Kanno T, Matzke AJ (2015) RNA-Directed DNA Methylation: the evolution of a complex epigenetic pathway in flowering plants. Annu Rev Plant Biol 66:243–267
Otagaki S, Arai M, Takahashi A, Goto K, Hong JS, Masuta C (2006) Rapid induction of transcriptional and post-transcriptional gene silencing using a novel Cucumber mosaic virus vector. Plant Biotechnol 23:259–265
Shen H, He H, Li J, Chen W, Wang X, Guo L, Peng Z, He G, Zhong S, Qi Y, Terzaghi W, Deng XW (2012) Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell 24:875–892
Wang XL, Song SH, Wu YS, Li YL, Chen TT, Huang ZY, Liu S, Dunwell TL, Pfeifer GP, Dunwell JM, Wamaedeesa R, Ullah I, Wang Y, Hu SN (2015) Genome-wide mapping of 5-hydroxymethylcytosine in three rice cultivars reveals its preferential localization in transcriptionally silent transposable element genes. J Exp Bot 66:6651–6663
Zhang Q, Wang D, Lang Z, He L, Yang L, Zeng L, Li Y, Zhao C, Huang H, Zhang H, Zhang H, Zhu JK (2016) Methylation interactions in Arabidopsis hybrids require RNA-directed DNA methylation and are influenced by genetic variation. Proc Natl Acad Sci USA 113:4248–4256
Zhang Y, Harris CJ, Liu Q, Liu W, Ausin I, Long Y, Xiao L, Feng L, Chen X, Xie Y, Chen X, Zhan L, Feng S, Li JJ, Wang H, Zhai J, Jacobsen SE (2018) Large-scale comparative epigenomics reveals hierarchical regulation of non-CG methylation in Arabidopsis. Proc Natl Acad Sci USA 115:1069–1074
Zhong X, Du J, Hale CJ, Gallego-Bartolome J, Feng S, Vashisht AA, Chory J, Wohlschlegel JA, Patel DJ, Jacobsen SE (2014) Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 157:1050–1060
Zhou S, Xing M, Zhao Z, Gu Y, Xiao Y, Liu Q, Xu H (2021) DNA methylation modification in heterosis initiation through analyzing rice hybrid contemporary seeds. Crop J. https://doi.org/10.1016/j.cj.2020.12.003
Funding
This work was supported by the promotion services of the New Energy and Industrial Technology Development Organization (NEDO) (project code: P16009).
Author information
Authors and Affiliations
Contributions
CM and TI designed and coordinated the study. WM performed all analyses. All authors contributed to the analysis of results, manuscript revisions and approved the final publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Communicated by Kan Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsunaga, W., Inukai, T. & Masuta, C. Progressive DNA demethylation in epigenetic hybrids between parental plants with and without methylation of the transgene promoter. Theor Appl Genet 135, 883–893 (2022). https://doi.org/10.1007/s00122-021-04004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-04004-9