Abstract
Key message
Soybean acyl-ACP thioesterase gene family have been characterized; GmFATA1A mutants were discovered to confer high oleic acid, while GmFATB mutants presented low palmitic and high oleic acid seed content.
Abstract
Soybean oil stability and quality are primarily determined by the relative proportions of saturated versus unsaturated fatty acids. Commodity soybean typically contains 11% palmitic acid, as the primary saturated fatty acids. Reducing palmitic acid content is the principal approach to minimize the levels of saturated fatty acids in soybean. Though high palmitic acid enhances oxidative stability of soybean oil, it is negatively correlated with oil and oleic acid content and can cause coronary heart diseases for humans. For plants, acyl–acyl carrier protein (ACP) thioesterases (TEs) are a group of enzymes to hydrolyze acyl group and release free fatty acid from plastid. Among them, GmFATB1A has become the main target to genetically reduce the palmitic acid content in soybean. However, the role of members in soybean acyl-ACP thioesterase gene family is largely unknown. In this study, we characterized two classes of TEs, GmFATA, and GmFATB in soybean. We also denominated two GmFATA members and discovered six additional members that belong to GmFATB gene family through phylogenetic, syntenic, and in silico analysis. Using TILLING-by-Sequencing+, we identified an allelic series of mutations in five soybean acyl-ACP thioesterase genes, including GmFATA1A, GmFATB1A, GmFATB1B, GmFATB2A, and GmFATB2B. Additionally, we discovered mutations at GmFATA1A to confer high oleic acid (up to 34.5%) content, while mutations at GmFATB presented low palmitic acid (as low as 5.6%) and high oleic acid (up to 36.5%) phenotypes. The obtained soybean mutants with altered fatty acid content can be used in soybean breeding program for improving soybean oil composition traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean oil is an important edible resource of vegetable oil that makes up 53% in the U.S. vegetable oil consumption in 2017 (American Soybean Association 2018). As the predominant saturated fatty acid, palmitic acid (16:0) typically accounts for 11% in conventional soybean oil (Fehr 2007). Although elevated palmitic acid content improves oxidative stability of soybean oil, it also causes the decrease in oleic acid and oil contents (Stoltzfus et al. 2000). On the contrary, reducing palmitic acid content has been reported to reduce the risks of developing cardiovascular diseases for humans (Hu et al. 1997). To produce edible oil with < 7% total saturates required by U.S. Food and Drug Administration, several soybean lines with reduced palmitic acid phenotype have been identified as potential genetic resources for developing low palmitic acid cultivars (Rebetzke et al. 1998). In fatty acid biosynthetic pathway, 16:0-ACP fatty acid thioesterase (FATB) is the major target to genetically reduce the level of palmitic acid in soybean seeds.
Plant acyl–acyl carrier protein (ACP) thioesterase (TE), an enzyme terminates plastidial fatty acid biosynthesis, catalyzed acyl-ACP thioester bond hydrolysis to release free fatty acids and ACP. The substrate specificity of individual TEs is essential for the chain length of fatty acids exported from the plastid (Hills 1999; Voelker 1996). In the online database named ThYme, there are 25 TE families, from which Family TE14 include bacterial and plant acyl-ACP TEs (Cantu et al. 2010). Based on amino acid sequence alignment and substrate specificity, the plant TEs have been categorized into two classes, FATA and FATB (Voelker et al. 1997). The FATA class primarily hydrolyze 18:1-ACP with minor activity toward saturated acyl-ACP substrates, while FATB class shows preference for acyl-ACP with saturated fatty acyl chains (Dormann et al. 1995; Salas and Ohlrogge 2002). They both contain two helix/multi-stranded sheet motifs (hotdog domains), in which residues in the N-terminal domain were found to affect substrate specificity of enzymes and highly conserved residues in the C-terminal domain involved in catalysis (Mayer and Shanklin 2005). Two thioesterases maintain the saturated/unsaturated balance of membrane fatty acids for normal plant growth under critical conditions (Bonaventure et al. 2003).
As an allotetraploid crop species, soybean possesses a highly duplicated genome that ~ 75% of its genes are present with multiple copies. Two whole-genome duplication events have occurred in soybean genome, including one shared by legume species 59 million years ago and another glycine-specific one around 13 million year ago. The number of genes involved in acyl lipid biosynthesis in soybean is almost doubled compared to Arabidopsis (Schmutz et al. 2010). The gene families involved in fatty acid synthesis are generally much larger in soybean, such as omega-6 fatty acid desaturase (FAD2) with seven members (Lakhssassi et al. 2021). Such genetic redundancy drastically increases the complexity of genetic basis behind agronomical important traits but provide an invaluable resource for breeding desired phenotypes.
From mutagenized soybean lines, five quantitative trait loci (QTLs) have been associated with low palmitic acid phenotype, including fap1 in C1726, fap* in ELLP2, fap3 in A22, sop1 in J3, and fapnc in N79-2077-12 (Burton et al. 1994; Cardinal et al. 2014; De Vries et al. 2011; Rahman et al. 1996; Stijšin et al. 1998). With an exception of fapnc allelic with fap3, fap1, fap3, and fap* are independent alleles conferring low palmitic acid content (Primomo 2000; Schnebly et al. 1994). At fap1, a disrupted splicing mutation in a 3-ketoacyl-ACP synthase enzyme III (GmKASIIIA) has been associated with reduced palmitic acid phenotype (Cardinal et al. 2014). At fap3, a single nucleotide polymorphism (SNP) has caused loss-of-function for GmFATB1A (De Vries et al. 2011). Fapnc represents the second allele of GmFATB1A, in which a deletion is responsible for low palmitic acid phenotype (Cardinal et al. 2007). Thapa et al. (2016) have identified two additional alleles of GmFATB1A from soybean mutant lines with 30% reduction in palmitic acid content. More recently, a 254-kb genomic deletion, including the GmFATB1A gene, has been reported to result in reduced palmitic acid content in soybean seeds (Bachleda et al. 2016; Goettel et al. 2016). Alternatively, downregulation of GmFATB gene expression can reduce palmitic acid content in soybean seeds (Buhr et al. 2002; Wilson et al. 2001). In Arabidopsis, a FATB knockout mutant has shown not only the low saturated fatty acid content, but also slow seedling growth and low-viable seed development (Bonaventure et al. 2003). However, no reports on FATB soybean mutants with negative impact on soybean growth and seed quality have been published so far.
TILLING (Targeting Induced Local Lesions IN Genomes) has been developed to screen-induced mutations from a chemical mutagenized population in early 2000s (McCallum et al. 2000). It combines traditional chemical mutagenesis with a high-throughput mutation screening method. Ethylmethane sulfonate (EMS) is widely used as the most common chemical mutagen to randomly create point mutations in plant genome (Anderson et al. 2018; Kandoth et al. 2017; Koornneef et al. 1982; Lakhssassi et al. 2020a). A large number of TILLING populations have been developed in a variety of plant species, such as barley, legume, maize, rice, sorghum, and wheat (Till et al. 2009). Using reverse genetic methods like TILLING, scholars have been studying the gene function for economically important traits in soybean, such as disease resistance and seed oil composition traits. Two missense mutations in the GmSHMT08 gene are identified in soybean cv. ‘Forrest’ mutant populations and result in alternation of SCN-resistant phenotype (Liu et al. 2012). Three missense mutations in individual soybean lines were detected in the GmFAD2-1A, and one of them leads to high oleic acid and low linoleic acid contents in the seed oil (Dierking and Bilyeu 2009). However, the complex traits resulted from duplicated soybean genome dramatically lower the efficiency of mutation screening in soybean. Using gel-based TILLING, a recent study shows that no mutations were found in either GmFAD2-1A or GmFAD2-1B from 2,000 EMS-mutagenized soybean lines, but five mutants in either of targeted genes were identified using forward phenotypic screening, which was followed by targeted sequencing analysis (Lakhssassi et al. 2017). The adoption of exome capture sequencing enabled the high-throughput screening for hidden mutations in multiple homologous wheat genes controlling one trait (Krasileva et al. 2017). More recently, we developed a versatile TILLING-by-Sequencing+ technology and discovered novel genes associated with improved seed stearic acid content in soybeans (Lakhssassi et al. 2020b).
In the current study, we characterized the soybean acyl-ACP thioesterase gene family through a comprehensive analysis of phylogeny, gene structure and expression, synteny, and conserved domain variations and identified six additional members belonging to GmFATB gene family. Using TILLING-by-Sequencing+, we discovered for the first time that EMS-induced mutations in GmFATA1A resulted in high oleic acid content in soybean seed. Mutations at four GmFATB members also are associated with low palmitic acid and high oleic acid contents. These GmFAT mutants are the valuable sources to breed new soybean cultivars with low saturated and high monounsaturated fatty acid contents.
Materials and methods
Identification of FATA and FATB from soybean and other plant species
The putative soybean acyl-ACP thioesterase genes were identified by BLASTP searches against soybean reference genome (Glycine max, Wm82.a2.v1) at Phytozome (v12.1) using Arbidopisis thaliana acyl-ACP thioesterase protein sequences as queries (https://phytozome.jgi.doe.gov). Using the same approach, the putative acyl-ACP thioesterases were identified from reference genome of Phaseolus vulgaris (v2.1), Medicago truncatula (Mt4.0v1), Brassica rapa FPsc (v1.3), Oryza sativa (v7_JGI), Lotus japonicas genome assembly build 3.0 (http://www.kazusa.or.jp/lotus/), Elaeis guineensis assembly EG5 (https://www.ncbi.nlm.nih.gov/genome/2669), and Cocos nucifera assembly ASM812446v1 (https://www.ncbi.nlm.nih.gov/genome/?term=Cocos+nucifera). The total of 50 identified protein sequences with accession numbers were included in this study.
Phylogenetic analysis
Multiple sequence alignments of the full-length acyl-ACP thioesterase protein sequences from nine plant species were performed with MUltiple Sequence Comparison by Log-Expectation (MUSCLE). An unrooted phylogenetic tree was then constructed by maximum likelihood (ML) method in MEGA X using Jones-Taylor-Thornton Gamma Distributed (JTT + G) model for all FAT genes and JTT + G + I model with Invariant Sites (I) for soybean FAT genes (Hall 2013; Kumar et al. 2018).
Gene structure, expression profiling, and conserved domain analysis
The genomic and coding sequences of soybean acyl-ACP thioesterase genes retrieved from Phytozome v12.1 were aligned to generate the gene exon–intron structure diagram using the Gene Structure Display Server (Hu et al. 2015). To analyze the tissue-specific expression of soybean acyl-ACP thioesterase genes, normalized transcript data in six different tissues were downloaded from Soybase (https://www.soybase.org/soyseq/). The expressions profiling was visualized through heatmap using Heatmapper (Babicki et al. 2016). Followed by multiple sequence alignment between FATA and FATB in soybean and A. thaliana, the residues for substrate specifying have been proposed based on the criteria described by Jing et al. (2018). Catalytic residues in conserved motifs of soybean acyl-ACP thioesterases were identified from NCBI Conserved Domain Database (CDD) (https://www.ncbi.nlm.nih.gov/cdd).
Chromosomal localization and syntenic analysis
The locations of soybean acyl-ACP thioesterase genes and their corresponding chromosomes were drawn based on soybean genome annotation a2.v1 on SoyBase. Syntenic analysis was performed using soybean acyl-ACP thioesterase genes as locus identifier in plant genome duplication database (PGDD) (Lee et al. 2012). Nonsynonymous (Ka) versus synonymous substitution (Ks) rates were calculated based on their values retrieved from PGDD. For gene pairs whose information are not available at PGDD, PAL2NAL program was used to estimate Ka and Ks (Suyama et al. 2006). Given the Ks values and a rate of 6.1 × 10−9 substitutions per site per year, the divergence time (T) was equal to Ks/(2 × 6.1 × 10−9) × 10−6 Mya for each gene pair (Chen et al. 2014).
Development of EMS-mutagenized soybean populations
EMS mutagenesis was performed as described in the past (Meksem et al. 2008). The soybean cv. Forrest and PI88788 seeds were used to generate M2 population in the greenhouse at SIUC Horticulture Research Center (HRC). Forrest is a Peking type and PI88788 is a PI88788 type in SCN resistance with the major loci being the Rhg4 + rhg1-a and the rhg1-b, respectively. A total of 4032 M2 lines were advanced to M3 generations by single-seed descent in the field between 2012 and 2015. M3 seeds from each mutant line were harvested, thrashed, and stored at − 20 °C.
Mutation detection and validation
The mutations in five soybean acyl-ACP thioesterase genes were detected using TILLING-by-Sequencing+ method. A subset of mutations at GmFATA1A, GmFATB1A, GmFATB1B, GmFATB2A, and GmFATB2B were confirmed by Sanger sequencing. PCR primers were designed to amplify the fragments covering the exons of three soybean acyl-ACP thioesterase genes using Primer3 (Koressaar and Remm 2007). The PCR program was set up with 30 cycles of amplification at 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 1 min. The PCR products were then purified using QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA). The purified samples were sent for sequencing at GENEWIZ (https://www.genewiz.com/). The putative mutations were identified by alignment sampled sequences to reference using Unipro UGENE (Okonechnikov et al. 2012).
Fatty acid analysis of seeds from GmFAT mutants
Five major fatty acids’ content was measured from selected M2/M3 lines according to the two-step methylation procedure (Kramer et al. 1997). At least three seeds per line were crushed in 16 mm × 200 mm tube with Teflon-lined screw cap individually. 2 mL sodium methoxide was added into tube followed by 50 °C incubation for 10 min. After cooling for 5 min, the samples were mixed with 3 mL of 5% (v/v) methanolic HCl, incubated at 80 °C for 10 min, and cooled for 7 min. Each tube was then added with 7.5 mL of 6% (w/v) potassium carbonate and 1 mL of hexane and centrifuged at 1200 g for 5 min. The upper layer was transferred to vials, from which the individual fatty acid content was determined as a percentage of the total fatty acids of soybean seed by gas chromatography. A Shimadzu GC-2010 (Columbia, MD) gas chromatograph fitted with a flame ionization detector was equipped with a Supelco 60-m SP-2560-fused silica capillary famewax column (0.25 mm i.d. × 0.25 μm film thickness). The standard fatty acids were run first to create calibration reference.
Results
Identification of plant acyl-ACP thioesterase gene family members in soybean
Four FATB genes have previously been identified in soybean, from which GmFATB1A is associated with reducing palmitic acid content (Cardinal et al. 2007). To identify the putative members of TE family in soybean, a BLASTP search against the soybean genome database (Wm82.a2.v1) was performed by using A. thaliana TE protein sequences as queries. Combined with soybean TEs from Family TE14 in the ThYme database, a total of 12 TEs have been found in soybean genome, including ten GmFATB and two GmFATA. Based on nomenclature proposed previously, additional six GmFATB genes are denominated as GmFATB3A (Glyma.04G197400), GmFATB3B (Glyma.06G168100), GmFATB4A (Glyma.04G197500), GmFATB4B (Glyma06g17625), GmFATB5A (Glyma.10G268200), GmFATB5B (Glyma.20G122900), as well as two GmFATA genes, GmFATA1A (Glyma.18G167300) and GmFATA1B (Glyma.08G349200) (Table 1).
Amino acid sequence alignment has shown that two genes in each subfamily of GmFATB and GmFATA share high similarity, such as GmFATB1A/FmFATB1B (96%), GmFATB3A/GmFATB3B (94%), and GmFATA1A/GmFATA1B (93%). The coding DNA sequence (CDS) lengths of the GmFATB range from 1140 to 1269 bp with an average of 1203 bp while that of GmFATA averages 1140 bp. The sizes and predicted molecular weight of GmFATB1 and GmFATB2 subfamilies are larger than 400 amino acids and 45.8 kDa, respectively. GmFATB1A, GmFATB1B, and GmFATB2B show acidic isoelectric point (pI) values, whereas the rest of soybean TEs presented basic pI values (Table 1).
Phylogenetic analysis of plant acyl-ACP thioesterase gene family
13 FATA and 25 FATB proteins from other three legumes, two dicot species, and three monocot species have been identified through BLAST searches using A. thaliana TE protein sequences. A maximum likelihood (ML) tree was constructed with 50 protein sequences to elucidate the phylogenetic relationships among TEs from nine plant species (Fig. 1). As expected, two distinct clusters are formed to separate 15 FATA members from 35 FATB ones. In FATB cluster, all 35 FATB members could be classified into four subgroups. In subgroup I, GmFATB1A, GmFATB1B, GmFATB2A, and GmFATB2B are grouped together with AtFATB, BrFATB, and eight FATB members from other three legume species. Subgroup II contains all FATB members from three monocot species except one OsFATB. There are seven FATB members in subgroup III, including GmFATB3A, GmFATB3B, GmFATB4A, and GmFATB4B. And subgroup IV has GmFATB5A, GmFATB5B, and three FATB members from two legumes and one monocot species (Fig. 1). On the other hand, FATA members from all legume species are clustered apart from ones in monocot species. However, AtFATA are grouped with BrFATA in two different branches. The phylogenetic analysis also shows a close evolutionary relationship within each of six soybean TEs gene pairs with ≥ 88% reliability (Fig. 1).
Phylogenetic tree of acyl-ACP thioesterases gene family from nine plant species. The protein sequences of all acyl-ACP thioesterases were subjected to a MUSCLE multiple alignment and phylogenetic tree was constructed by maximum likelihood (ML) method using Mega X. The two members of soybean GmFATA gene subfamily were labeled with filled squares. The previously identified four members of soybean GmFATB1/2 subfamilies were marked with filled circles, while the newly identified members of GmFATB3/4/5 subfamilies in this study were labeled with empty circles The name and abbreviation of plant species used for the analysis are Arabidopsis thaliana (At); Glycine max (Gm); Phaseolus vulgaris (Pv); Medicago truncatula (Mt); Lotus japonicas (Lj); Brassica rapa (Br); Cocos nucifera (Cn); Elaeis guineensis (Eg); Oriza sativa (Os)
Gene structure and expression profiling of soybean acyl-ACP thioesterase genes
Given the two whole-genome duplication events, the soybean TE gene family consists of 12 members, which is four times more than those in Arabidopsis and double compared to the number of TEs in common bean, palm, and rice. Compared to an average of 5860 bp for GmFATA, the gene lengths of GmFATB1 and GmFATB2 subfamilies are more than 4195 bp while that of GmFATB3, GmFATB4, and GmFATB5 subfamilies are 3143 bp on average (Table 1). The GmFATB2A has the longest gene length among soybean TEs due to its extended 3’-UTR region. The gene structures of GmFATB are highly conserved with six exons for all ten members; on the contrary, GmFATA1A and GmFATA1B have seven and eight exons, respectively (Fig. 2).
Phylogenetic relationships and gene structures of GmFATA and GmFATB. The protein sequences of all soybean acyl-ACP thioesterases were subjected to a MUSCLE alignment, and phylogenetic gene tree was constructed using Mega X. The structures of 12 soybean acyl-ACP thioesterase genes were plotted with yellow boxes representing exons (coding DNA sequence, CDS), black lines illustrating introns, and blue boxes indicating 5′-UTR and 3′-UTR regions. The size of gene structures could be measured by the scale in the unit of base pair (bp) at the bottom. The gene structure was drawn using the Gene Structure Display Server
GmFATB1A, GmFATB1B, and GmFATB2A show relatively high expression in soybean seeds while the transcripts of two GmFATB2 genes were abundant in soybean flowers. GmFATA1A and GmFATA1B were also highly expressed in soybean seeds. Two GmFATB1 genes expressed relatively high levels in soybean root and nodule. Additionally, the expression of GmFATB1, GmFATB2, and GmFATA exhibits similar patterns in leaves and pod. The expression of GmFATB3A, GmFATB3B, and GmFATB5A is recorded as 0 in most of tested tissues and no RNA-seq data are available for GmFATB4A, GmFATB4B, and GmFATB5B in Soybase (Figure S1).
Chromosomal distribution and gene duplication
Based on the physical locations, 12 soybean TE genes are unevenly distributed on eight soybean chromosomes (Fig. 3). Chromosome 4 and 6 contain three GmFATB genes each while only one GmFATB gene each is present on chromosomes 5, 10, 17, and 20. Two GmFATA genes are located at chromosome 8 and 18, respectively. Among the GmFATB subfamilies, GmFATB1 and GmFATB5 are evenly distributed on four chromosomes. Nevertheless, the other three subfamilies, GmFATB2, GmFATB3, and GmFATB4, are concentrated on two chromosomes with three on each (Fig. 3).
Chromosomal locations and duplications of soybean acyl-ACP thioesterase genes. Each chromosome number is indicated above bar by Roman number and the scale (on the left) is in mega base (Mb). The size of chromosome and gene locations are based on soybean genome annotation a2.v1 on SoyBase. Each pair of segmental duplication in GmFATA and GmFATB subfamilies is connected by red and blue lines, respectively. The tandem duplicated genes are shown in rectangle box
The duplication analyses have shown that all soybean TE genes are located within eight duplicated blocks (Table 2). The gene pair, GmFATB1A and GmFATB1B, belongs to a large duplicated segment containing 62 anchor genes, while GmFATB3A/GmFATB4B and GmFATB5A/GmFAT5B are presented in huge syntenic regions with 711 and 884 anchor genes, respectively (Figure S2). Two gene pairs, GmFATB3A/GmFATB4A and GmFATB3B/GmFATB4B, are regarded as the outcomes of tandem duplication events due to their tight physical distance of less than 7 kb (without any genes in between). The ratio of nonsynonymous to synonymous substitutions (Ka/Ks) was calculated for each gene pair to determine the types of natural selection acting on coding sequences. The Ka/Ks of soybean TEs gene pairs is less than 0.5, which suggests that the evolution of soybean TEs is under purifying selection (Juretic et al. 2005; Li et al. 1981). The duplication of eight gene pairs is estimated to have occurred between 7.38 and 76.23 Mya based on 6.161029 synonymous mutations per synonymous site per year for soybean (Table 2).
Conserved domain variations among plant TEs
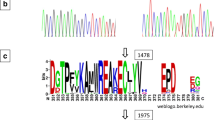
The protein sequences of 15 soybean and Arabidopsis thaliana TEs, four FATA and eleven FATB, were aligned to compare residues within two conserved hotdog domains. A residue that is conserved within one plant TE class but differs between FATA and FATB classes may contribute to the difference in substrate specificity (Jing et al. 2018). Based on these criteria, a total of 13 residues were selected, from which A194G, T208V, and D276E have previously been reported as specificity determining positions (Mayer and Shanklin 2007). Additionally, seven residues are found as completely different between FATA and FATB classes in hotdog domain I, including K150R, N163D, V178T, H212Q, I213V, R236K, and K246R. In hotdog domain II, another three residues, T347K, D362E, and D372E, meet the same criteria (Table 3). Among the ten newly identified residues, three residues, V178T, H212Q, and T347K, present non-conservative difference in amino acid between FATA and FATB, while the rest of seven residues contain conservative changes. In addition, three conserved catalytic residues, N340, H342, and C377, may form a papain-like catalytic triad across the FATA and FATB classes (Mayer and Shanklin 2005). From Conserved Domain Database (CDD) at National Center for Biotechnology Information (NCBI), seven active sites of plant TEs have been revealed in hotdog domain I. Among them, two residues, T208V and R236K, overlap with ones identified as substrate specifying residues, while the rest are highly conserved between FATA and FATB classes except two mismatches at positions 237 and 238 (Figure S3).
Identification of new alleles of GmFAT to improve fatty acid composition in soybean seed
Five soybean acyl-ACP thioesterase genes, GmFATA1A, GmFATB1A/1B, and GmFATB2A/2B, have been included in screening mutations through TILLING-by-Sequencing+. The estimated mutation density of these five genes is 1/232 kb using the formula as the total number of mutations divided by the total number of base pairs (amplicon size x individuals screened) (Table 4) (Cooper et al. 2008). Among the 280 identified mutations in these five GmFAT genes, the typical EMS-type mutations are the majority of base changes with 45.7% in G to A and 37.1% in C to T, while the other types of mutations only took up 17.1% (Table 4). In the coding regions of these five GmFAT genes, a total of 118 amino acid changes are detected, from which 71.2% are missense mutations, 26.3% are silent mutations, and 2.5% are nonsense mutations. Nonsense mutations are found in GmFATA1A and GmFATB2A/2B genes, whereas no nonsense mutations are present in GmFATB1A/1B genes (Table 4).
A subset of GmFAT mutants has been confirmed by Sanger sequencing, and their novel alleles have been associated with altered fatty acid profiles. Six missense mutations (S37F, A55T, T146I, A231V, G277E, and V310I) are identified from GmFATA1A mutants, in which two mutants, F243 and F393, present > 30% high oleic acid content. Another four mutants, F636, F1305, F740, and F1188, display moderately high oleic acid content (> 24%) compared to Forrest wild type (18.0%) (Table 5). Five GmFATB1A mutants (F1040, F1129, F1539, F1200, and F1166) carry the missense mutations, P18L, G128R, R138H, G223E, and A371T, respectively. The palmitic acid content of these five mutants ranges from 8.9 to 10.7%, but an increase in oleic acid content (23.9–34.0%) is also found in GmFATB1A mutants (Table 5). Likewise, five missense mutations (P118S, G128E, A174T, D284N, and R348K) are detected at GmFATB1B, from which one mutant (F3) shows a decreased palmitic acid content by 51.7% when compared to Forrest wild type. All five GmFATB1B mutants also present an elevated oleic acid content up to 33.4% (Table 5). Another five missense mutations, P16L, A153T, A373T, R385Q, and G395D, are identified at GmFATB2A, from which four show a reduction in palmitic acid content. Two missense and one nonsense mutations are discovered at GmFATB2B. All GmFATB2A and GmFATB2B mutants show an increase in oleic acid content (Table 5).
Discussion
Among type II fatty acid synthases (FAS), the plant TE is a major contributing factor in determining the carbon chain length of fatty acids through their substrate specificity. The large number of soybean TE genes implied genome expansion of the soybean compared to counterparts in other plant species. Previous studies have identified four unique GmFATB genes, from which mutations at GmFATB1A result in low palmitic acid content in soybean seed (Cardinal et al. 2007). In this study, we performed a genome-wide search for soybean TE genes with the aid of Phytozome and ThYme databases. Additional six GmFATB genes have been identified and named according to previously proposed nomenclature, as well as two GmFATA genes (Table 1). The total number of TE genes is four times higher in soybean compared to A. thaliana.
Here, we conducted an overall phylogenetic analysis of plant TEs gene families from nine plant species using maximum likelihood (ML) method (Fig. 1). Interestingly, GmFATB1 and GmFATB2, GmFATB3 and GmFATB4, and GmFATB5 subfamilies are in three different subgroups under FATB cluster, respectively. For plant species with high palmitic acid levels, such as coconut and palm, their FATBs appear to evolve independently from dicot species. Although two FATA and one FATB genes are presented in Arabidopsis genome, members from FATA class are generally much fewer than ones from FATB class in other higher plants. Two GmFATA are grouped with FATAs in other three legume species but apart from ones in other dicot species (Fig. 1).
The gene structures are similar within GmFATB1/GmFATB2 subfamilies, GmFATB3/GmFATB4/GmFATB5 subfamilies, and GmFATA. Although the GmFATA have the longer gene lengths due to the extended intron length, the coding sequence lengths of GmFATA are generally shorter than those of GmFATB. The gene lengths of GmFATB1 and GmFATB2 subfamilies are longer than those of GmFATB3, GmFATB4, and GmFATB5 subfamilies, as are their coding sequences (Table 1). With the advent of intron gain/loss events, all GmFATB lose at least one intron when they evolve divergently from GmFATA (Fig. 2).
The expression profiling data reveal various expression patterns of eight soybean TE genes in six soybean tissues. The similar expression patterns point to functional redundancy during soybean evolution, which could lead to neofunctionalization and subfunctionalization within soybean TE gene family (Figure S1). As expected, the high transcript level of GmFATB1 subfamilies, GmFATB2A, and GmFATA has been detected in soybean seeds, which indicated that these genes play a major role in releasing free fatty acids to cytosol. Thus, they should be the main targets to genetically modify fatty acids composition in soybean seed. For the newly identified GmFATB members, the very low expression level of GmFATB3A, GmFATB3B, and GmFATB5A in all six tissues suggests that their functions need to be explored further (Figure S1).
The distribution of 12 soybean TE genes has been shown to be on eight chromosomes (Fig. 3). Chromosomes 4 and 6 contain the largest number of TE genes (3), whereas chromosomes 5, 8, 10, 17, 18, and 20 only have one TE gene on each. The majority of soybean TE genes are found toward the chromosome ends, suggesting potential inter-chromosomal crossovers due to the high genetic recombination rates. Plant species acquired novel traits and adapted to various environments through gene duplication (Bowers et al. 2003; Eckardt 2004). There are three main gene duplication patterns, including segmental duplication, tandem duplication, and transposition (Kong et al. 2007). Our syntenic analysis shows that soybean TE gene family expands through both segmental and tandem duplications (Table 2). It is also well known that two whole-genome duplication events have occurred in soybean genome, including one shared by legume species 59 million years ago and another glycine-specific one around 13 million year ago (Schmutz et al. 2010,2014; Young and Bharti 2012). The duplication time of soybean TE gene pairs is estimated to match with either of these two time periods. GmFATB1B/GmFATB2A, GmFATB1B/GmFATB2B, GmFATB3A/GmFATB4A, GmFATB3A/GmFATB4B, and GmFATB3B/GmFATB4B are formed between 45.90 and 76.23 Mya while the duplication of GmFATB1A/GmFATB1B, GmFATB5A/GmFATB5B, and GmFATA1A/GmFATA1B has occurred between 7.38 and 8.20 Mya (Table 2).
Within two hotdog domains, 10 newly identified residues that are completely different between GmFATA and GmFATB could be the candidate positions to determine the differences in substrate specificity of TEs in soybean. Compared to other seven residues, three residues (V178T, H212Q, and T347K) may play a more important role in substrate specifying due to their non-conservative difference in amino acid (Table 3). In the current study, one GmFATA1A mutant (F740) has been identified to possess non-conservative amino acid changes at a previously reported specificity determining position (T208V) and confer an increased level of oleic acid in soybean seed (Tables 3 and 5) (Mayer and Shanklin 2007).
The mutations at GmFATB1A have been repeatedly associated with low palmitic acid phenotype in soybean. The expression level of GmFATA and GmFATB may have significant impact on soybean seed fatty acid composition, however, future studies are required to elucidate the role of soybean acyl-ACP thioesterases in controlling seed oleic acid content (Byfield and Upchurch 2007). This is the first time to discover that the novel alleles of GmFATA1A confer an elevated oleic acid content in soybean seeds. The increase in oleic acid content in GmFATA1A mutants, F243 and F393, is comparable to the high oleic acid content in either GmFAD2-1A or GmFAD2-1B mutants with the same genetic background (Table 5) (Lakhssassi et al. 2017). Meanwhile, the novel alleles of GmFATB1A, GmFATB1B, GmFATB2A, GmFATB2B are identified to confer low palmitic acid content in soybean seeds. Interestingly, these GmFATB mutants also present an elevated oleic acid content, which is consistent with the significant increase in oleic acid content from other previously reported GmFATB1A mutants (Table 5) (Bachleda et al. 2016; Cardinal et al. 2007). Zhou et al. (2019) indicate that a negative correlation exists between palmitic acid and oleic contents in both natural and mutagenized soybean populations. The identified GmFAT mutants are the new sources of seed high oleic acid and low palmitic acid contents for soybean breeding.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
American Soybean Association (2018) A Reference guide to soybean facts and figures
Anderson J, Lakhssassi N, Kantartzi SK, Meksem K (2018) Nonhypothesis analysis of a mutagenic soybean (Glycine max[L.]) population for protein and fatty-acid composition. J Am Oil Chem Soc 95:461–471
Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS (2016) Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 44:W147–W153
Bachleda N, Pham A, Li Z (2016) Identifying FATB1a deletion that causes reduced palmitic acid content in soybean N87–2122-4 to develop a functional marker for marker-assisted selection. Mol Breed 36:45
Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15:1020–1033
Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422:433–438
Buhr T, Sato S, Ebrahim F, Xing A, Zhou Y, Mathiesen M, Schweiger B, Kinney A, Staswick P, Clemente T (2002) Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J 30:155–163
Burton J, Wilson R, Brim C (1994) Registration of N79-2077-12 and N87-2122-4, two soybean germplasm lines with reduced palmitic acid in seed oil. Crop Sci 34:313
Byfield GE, Upchurch RG (2007) Effect of temperature on delta-9 stearoyl-ACP and microsomal omega-6 desaturase gene expression and fatty acid content in developing soybean seeds. Crop Sci 47:1698–1704
Cantu DC, Chen Y, Lemons ML, Reilly PJ (2010) ThYme: a database for thioester-active enzymes. Nucleic Acids Res 39:D342–D346
Cardinal AJ, Burton JW, Camacho-Roger AM, Yang JH, Wilson RF, Dewey RE (2007) Molecular analysis of soybean lines with low palmitic acid content in the seed oil. Crop Sci 47:304–310
Cardinal AJ, Whetten R, Wang S, Auclair J, Hyten D, Cregan P, Bachlava E, Gillman J, Ramirez M, Dewey R (2014) Mapping the low palmitate fap1 mutation and validation of its effects in soybean oil and agronomic traits in three soybean populations. Theor Appl Genet 127:97–111
Chen X, Chen Z, Zhao H, Zhao Y, Cheng B, Xiang Y (2014) Genome-wide analysis of soybean HD-Zip gene family and expression profiling under salinity and drought treatments. PLoS ONE 9:e87156
Cooper JL, Till BJ, Laport RG, Darlow MC, Kleffner JM, Jamai A, El-Mellouki T, Liu S, Ritchie R, Nielsen N (2008) TILLING to detect induced mutations in soybean. BMC Plant Biol 8:9
De Vries BD, Fehr WR, Welke GA, Dewey RE (2011) Molecular characterization of the mutant fap3(A22) allele for reduced palmitate concentration in soybean. Crop Sci 51:1611–1616
Dierking EC, Bilyeu KD (2009) New sources of soybean seed meal and oil composition traits identified through TILLING. BMC Plant Biol 9:89
Dormann P, Voelker TA, Ohlrogge JB (1995) Cloning and expression in Escherichia coli of a novel thioesterase from Arabidopsis thaliana specific for long-chain acyl–acyl carrier proteins. Arch Biochem Biophys 316:612–618
Eckardt NA (2004) Two genomes are better than one: widespread paleopolyploidy in plants and evolutionary effects. Plant Cell 16:1647–1649
Fehr WR (2007) Breeding for modified fatty acid composition in soybean. Crop Sci 47:S72–S87
Goettel W, Ramirez M, Upchurch RG, Y-qC An (2016) Identification and characterization of large DNA deletions affecting oil quality traits in soybean seeds through transcriptome sequencing analysis. Theor Appl Genet 129:1577–1593
Hall BG (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30:1229–1235
Hills MJ (1999) Improving oil functionality by tuning catalysis of thioesterase. Trends Plant Sci 4:421–422
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC (1997) Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 337:1491–1499
Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31:1296–1297
Jing F, Zhao L, Yandeau-Nelson MD, Nikolau BJ (2018) Two distinct domains contribute to the substrate acyl chain length selectivity of plant acyl-ACP thioesterase. Nat Commun 9:1–10
Juretic N, Hoen DR, Huynh ML, Harrison PM, Bureau TE (2005) The evolutionary fate of MULE-mediated duplications of host gene fragments in rice. Genome Res 15:1292–1297
Kandoth PK, Liu S, Prenger E, Ludwig A, Lakhssassi N, Heinz R, Zhou Z, Howland A, Gunther J, Eidson S, Dhroso A, LaFayette P, Tucker D, Johnson S, Anderson J, Alaswad A, Cianzio SR, Parrott WA, Korkin D, Meksem K, Mitchum MG (2017) Systematic mutagenesis of serine hydroxymethyltransferase reveals an essential role in nematode resistance. Plant Physiol 175:1370–1380
Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, DePamphilis CW (2007) Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J 50:873–885
Koornneef M, Dellaert L, Van der Veen J (1982) EMS-and relation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutat Res Fundam Mol Mech Mutagen 93:109–123
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291
Kramer JK, Fellner V, Dugan ME, Sauer FD, Mossoba MM, Yurawecz MP (1997) Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 32:1219–1228
Krasileva KV, Vasquez-Gross HA, Howell T, Bailey P, Paraiso F, Clissold L, Simmonds J, Ramirez-Gonzalez RH, Wang X, Borrill P (2017) Uncovering hidden variation in polyploid wheat. Proc Natl Acad Sci 114:E913–E921
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lakhssassi N, Zhou Z, Liu S, Colantonio V, AbuGhazaleh A, Meksem K (2017) Characterization of the FAD2 gene family in soybean reveals the limitations of gel-based TILLING in genes with high copy number. Front Plant Sci 8:324
Lakhssassi N, Piya S, Knizia D, El Baze A, Cullen MA, Meksem J, Lakhssassi A, Hewezi T, Meksem K (2020a) Mutations at the serine hydroxymethyltransferase impact its interaction with a soluble nsf attachment protein and a pathogenesis-related protein in soybean. Vaccines (basel) 8:349
Lakhssassi N, Zhou Z, Liu S, Piya S, Cullen MA, El Baze A, Knizia D, Patil GB, Badad O, Embaby MG, Meksem J, Lakhssassi A, AbuGhazaleh A, Hewezi T, Meksem K (2020b) Soybean TILLING-by-Sequencing+ reveals the role of novel GmSACPD members in the unsaturated fatty acid biosynthesis while maintaining healthy nodules. J Exp Bot 71:6969–6987
Lakhssassi N, Lopes-Caitar VS, Knizia D, Cullen MA, Badad O, El Baze A, Zhou Z, Embaby MG, Meksem J, Lakhssassi A, Chen P, AbuGhazaleh A, Vuong TD, Nguyen HT, Hewezi T, Meksem K (2021) TILLING-by-sequencing+ reveals the role of novel fatty acid desaturases (GmFAD2-2s) in increasing soybean seed oleic acid content. Cells 10:1245
Lee T-H, Tang H, Wang X, Paterson AH (2012) PGDD: a database of gene and genome duplication in plants. Nucleic Acids Res 41:D1152–D1158
Li W-H, Gojobori T, Nei M (1981) Pseudogenes as a paradigm of neutral evolution. Nature 292:237–239
Liu S, Kandoth PK, Warren SD, Yeckel G, Heinz R, Alden J, Yang C, Jamai A, El-Mellouki T, Juvale PS (2012) A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 492:256–260
Mayer KM, Shanklin J (2005) A structural model of the plant acyl-acyl carrier protein thioesterase FatB comprises two helix/4-stranded sheet domains, the N-terminal domain containing residues that affect specificity and the C-terminal domain containing catalytic residues. J Biol Chem 280:3621–3627
Mayer KM, Shanklin J (2007) Identification of amino acid residues involved in substrate specificity of plant acyl-ACP thioesterases using a bioinformatics-guided approach. BMC Plant Biol 7:1
McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeted screening for induced mutations. Nat Biotechnol 18:455
Meksem K, Liu S, Liu XH, Jamai A, Mitchum MG, Bendahmane A, El-Mellouki T (2008) TILLING: a reverse genetics and a functional genomics tool in soybean. In: Kahl G, Meksem K (eds) The handbook of plant functional genomics: concepts and protocols. Wiley, Weinheim, pp 251–265
Okonechnikov K, Golosova O, Fursov M, Team U (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28:1166–1167
Primomo V (2000) Inheritance and stability of palmitic acid alleles in soybeans (Glycine max L Merr.). Master's thesis Univ of Guelph, Guelph, ON, Canada
Rahman SM, Takagi Y, Kinoshita T (1996) Genetic analysis of palmitic acid contents using two soybean mutants, J3 and J10. Jpn J Breed 46:343–347
Rebetzke GJ, Burton JW, Carter TE Jr, Wilson RF (1998) Genetic variation for modifiers controlling reduced saturated fatty acid content in soybean. Crop Sci 38:303–308
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, Jenkins J, Shu S, Song Q, Chavarro C (2014) A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet 46:707
Schnebly SR, Fehr WR, Welke GA, Hammond EG, Duvick DN (1994) Inheritance of reduced and elevated palmitate in mutant lines of soybean. Crop Sci 34:829–833
Stijšin D, Ablett GR, Luzzi BM, Tanner JW (1998) Use of gene substitution values to quantify partial dominance in low palmitic acid soybean. Crop Sci 38:1437–1441
Stoltzfus DL, Fehr WR, Welke GA (2000) Relationship of elevated palmitate to soybean seed traits. Crop Sci 40:52–54
Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:W609–W612
Thapa R, Carrero-Colón M, Hudson KA (2016) New alleles of FATB1A to reduce palmitic acid levels in soybean. Crop Sci 56:1076–1080
Till B, Afza R, Bado S, Huynh O, Jankowicz-Cieslak J, Matijevic M, Mba C (2009) Global TILLING projects. In: Induced plant mutations in the genomics era Food and Agriculture Organization of the United Nations, Rome. pp 237–239
Voelker T (1996) Plant acyl-ACP thioesterases: chain-length determining enzymes in plant fatty acid biosynthesis. In: Setlow JK (ed) Genetic engineering. Springer, Berlin, pp 111–133
Voelker TA, Jones A, Cranmer AM, Davies HM, Knutzon DS (1997) Broad-range and binary-range acyl–acyl-carrier-protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol 114:669–677
Wilson R, Marquardt T, Novitzky W, Burton J, Wilcox J, Kinney A, Dewey R (2001) Metabolic mechanisms associated with alleles governing the 16:0 concentration of soybean oil. J Am Oil Chem Soc 78:335–340
Young ND, Bharti AK (2012) Genome-enabled insights into legume biology. Annu Rev Plant Biol 63:283–305
Zhou Z, Lakhssassi N, Cullen MA, El Baz A, Vuong TD, Nguyen HT, Meksem K (2019) Assessment of phenotypic variations and correlation among seed composition traits in mutagenized soybean populations. Genes 10:975
Funding
This research was supported in part from the United Soybean Board, project USB-2020-162-0127 to K.M. and N.L.
Author information
Authors and Affiliations
Contributions
ZZ wrote the manuscript draft, made the corresponding figures and tables, performed phylogenetic, gene structure, expression profiling, chromosome and syntenic, and in silico analysis. NL proofread, edited the manuscript, and directed the work together with KM. ZZ, NL, and SL developed the EMS-mutagenized populations. ZZ, NL, DK, MC, and OB did TILLNG-by-Sequencing+ analysis and identified GmFAT mutations. ZZ, MC, AEB, MGE, TDV, and HTN performed fatty acid phenotyping and edited the manuscript. KM conceived and designed the experiments, supervised the work, and edited the manuscript. All authors edited, reviewed, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Z., Lakhssassi, N., Knizia, D. et al. Genome-wide identification and analysis of soybean acyl-ACP thioesterase gene family reveals the role of GmFAT to improve fatty acid composition in soybean seed. Theor Appl Genet 134, 3611–3623 (2021). https://doi.org/10.1007/s00122-021-03917-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03917-9