Abstract
Key message
The foxglove aphid resistance gene Raso2 from PI 366121 was fine-mapped to 77 Kb region, and one candidate gene was identified.
Abstract
The foxglove aphid (FA: Aulacorthum solani Kaltenbach) is an important insect pest that causes serious yield losses in soybean. The FA resistance gene Raso2 from wild soybean PI 366121 was previously mapped to a 13 cM interval on soybean chromosome 7. However, fine-mapping of Raso2 was needed to improve the effectiveness of marker-assisted selection (MAS) and to eventually clone it. The objectives of this study were to fine-map Raso2 from PI 366121 using Axiom® 180 K SoyaSNP array, to confirm the resistance and inheritance of Raso2 in a different background, and to identify candidate gene(s). The 105 F4:8 recombinant inbred lines were used to fine-map the gene and to test antibiosis and antixenosis of Raso2 to FA. These efforts resulted in the mapping of Raso2 on 1 cM interval which corresponds to 77 Kb containing eight annotated genes based on the Williams 82 reference genome assembly (Wm82.a2.v1). Interestingly, all nonsynonymous substitutions were in Glyma.07g077700 which encodes the disease resistance protein containing LRR domain and expression of the gene in PI 366121 was significantly higher than that in Williams 82. In addition, distinct SNPs within Glyma.07g077700 that can distinguish PI 366121 and diverse FA-susceptible soybeans were identified. We also confirmed that Raso2 presented the resistance to FA and the Mendelian inheritance for single dominant gene in a different background. The results of this study would provide fundamental information on MAS for development of FA-resistant cultivars as well as functional study and cloning of the candidate gene in soybean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the major Hemipteran pests in soybean, foxglove aphid (FA, Aulacorthum solani Kaltenbach) is a highly polyphagous insect. It has a wide range of host plants on which it can feed 540 identified plant species from 33 orders and 82 families across the world (Jandricic et al. 2010). The FA can overwinter as eggs in the holocyclic or as nymphs/adults in the anholocyclic on various host plants (Leather 1992; Turl 1983; Lee et al. 2002). It can damage the plants it feeds upon by sucking plant sap and depriving the plant of nutrients, and by transmitting viruses. High FA populations directly reduce soybean yield when their feeding causes stunting, leaf distortion, and severe leaf yellowing. An additional threat posed by the FA is its ability to transmit about 40 kinds of viruses to soybean (Blackman and Eastop 2000). As a major insect pest of soybean in Korea (Kim et al. 1991) and Japan, yield loss of 90% was reported in Japan in 2000 (Nagano et al. 2001). In spite of significant potential of yield losses by FA, relatively little research on the genetic basis of resistance to the insect and breeding efforts for developing FA-resistant cultivars in soybean have been conducted.
Soybean [Glycine max (L.) Merr] is one of the most important crops for food and feed sources across the world. Wild soybean (Glycine soja Siebold & Zucc) has been known as a progenitor species of soybean (Glycine max). Wild soybeans have been considered as a gene bank for soybean breeding programs because it contains positive genetic variations on seed compositions (Pham et al. 2010; Jun et al. 2008; Zhang et al. 2016) and tolerances to abiotic stresses (Ji et al. 2010; Kao et al. 2006; Li et al. 2017). Wild soybean has been also used as sources of resistances to biotic stresses including insect pests in soybean. For example, Rhg1 from PI 88788 for SCN resistance, Raso2 from PI 366121 for FA resistance (Lee et al. 2015a), and two quantitative trait loci (QTLs) from G. soja 85–32 for soybean aphid (SA; Aphis glycines Matsumura) resistance (Zhang et al. 2017a) were derived from wild soybeans. Because it has been reported that cultivated soybean has narrow genetic base, continuous supply of genetic diversity/variations in target traits would be fundamental component for successful breeding programs in the future. However, genetic diversity from wild species could often result in a barrier to progress due to their unfavorable agronomic characteristics such as small less seed yield/size, pod shattering, and hard seed (Smalley et al. 2004).
Host plant resistance can provide an effective and economical way to control insect pests. In addition, host plant resistance is regarded as the most important component of an integrated pest management program for insect control (Harrewijn and Minks 1989). Three kinds of host plant resistance to insect pests have been reported (Painter 1951; Kogan and Ortman 1978), and antibiosis and antixenosis have been reported as resistant responses to FA in soybean (Lee et al. 2015a; Koh et al. 2018). Antibiosis is the ability of host plants to reduce the survival, growth, or reproduction of insects and is often caused by the production of toxic chemicals or the secondary metabolites by the plants (Kim et al. 2008). Antixenosis is the ability of host plants to repel insects. Therefore, feeding or oviposition on the plants could be reduced. The third type of host plant resistance is tolerance. Although it does not associated with ability to produce of toxic chemicals or to repel insects, genotypes with tolerance do not present a significant yield loss by equal levels of colonization that occur on susceptible genotypes.
Till now, several insect resistance genes have been mapped on soybean chromosome 7 (https://www.soybase.org). In the case of SA, a single dominant resistance gene Rag1 from Jackson (PI 548657) and Dowling (PI 548663) were mapped on chromosome 7 (Li et al. 2007; Kim et al. 2010a, b). One SA resistance gene in PI 587732 (Kim et al. 2014) and one QTL in PI 567541B (Zhang et al. 2009) were also mapped on chromosome 7. In the case of FA, no resistance gene or QTL has been mapped on chromosome 7, while Ohnishi et al. (2012) mapped Raso1 from Adams (PI 548502) on chromosome 3. For other insect pests, two QTLs in Himeshirazu (PI 594177) providing antibiosis type of resistance to common cutworm (CCW; Spodoptera litura Fabricius) were mapped on chromosome 7. One was mapped between Satt220 and Satt175 and another was positioned between Satt567 and Satt463 (Komatsu et al. 2005). Oki et al. (2012) suggested that the antixenosis in Himeshirazu to CCW controlled by previously identified QTLs might be associated with pubescence characteristics. Rector et al. (2000) and Narvel et al. (2001) reported that a QTL in PI 229358 with antibiosis to corn earworm was flanked by Satt220 and Satt463 on chromosome 7.
The dominant FA resistance gene Raso2 from PI 366121 was previously mapped to a 13 cM interval by Lee et al. (2015a). However, fine-mapping of Raso2 would be needed to improve the effectiveness of marker-assisted selection (MAS) and to eventually clone it. Therefore, the objectives of this study were (1) to fine-map FA resistance gene Raso2 from PI 366121 with 180 K Axiom® SoyaSNP assay, (2) to identify and evaluate expression levels of candidate gene(s), (3) to validate resistance and inheritance of Raso2 in a different background, and (4) to investigate sequence variations of the candidate gene(s) for Raso2 in diverse soybean germplasm.
Materials and methods
Plant materials
One recombinant inbred line (RIL) and one F3 population were used in the present study. The first population of 105 F4:8 RILs from the cross between Williams 82 and PI 366121 which was previously used to map Raso2 (Lee et al. 2015a) was used to fine-map Raso2. PI 366121 is a maturity group (MG) IV wild soybean accession originating from Fukusima, Japan (USDA-ARS Germplasm Resource Information Network, http://www.ars-grin.gpv/npgs/; accessed 31 Oct. 2020). It has been reported that PI 366121 presented resistance to FA (Lee et al. 2015a; Koh et al. 2018), a purple flower color, and black colors of pod, seed coat, and hilum. Williams 82 (PI 518671) is a MG III soybean cultivar (Bernard and Cremeens 1988) that is susceptible to SA (Kim et al. 2008) and FA (Lee et al. 2015a). Williams 82 was selected for the parent of the first population due to its susceptibility to FA and it has been used a source of reference genome for soybean (Schmutz et al. 2010).
The second population was used to validate a resistance and an inheritance of Raso2 to FA in a different background. The population consisted of 41 F3 individuals derived from a cross between PI 483463 and PI 366121. PI 483463 is a FA-susceptible wild soybean accession which was originally collected from Shanxi Sheng, China (USDA-ARS Germplasm Resource Information Network, accessed 31 Oct. 2020). We hypothesized that the resistance to FA in the second population would be controlled by Raso2 and presents the Mendelian inheritance for a single gene in the F3 generation.
Evaluation of foxglove aphid resistance
The FA was collected at the Crop Environmental Research Division of National Institute of Crop Science, Suwon, Korea during the summer of 2008 by collecting aphids from nearby soybean fields. The FA was maintained on a continuous supply of plants of FA-susceptible Korean soybean germplasm (Sowon) (Park et al. 2000) in a growth chamber at 22–25 ºC, 15/9 day/night photoperiod at 370 µmol m−2 s−1 photosynthetically active radiation irradiation, and 60–80% of relative humidity.
Both the antixenosis (choice test) and antibiosis (nonchoice test) to FA were evaluated as described by Lee et al. (2015a). The tests were conducted in a growth chamber under the conditions as described above. For both tests, four adult FAs were placed on the upper side leaf of each plant at the V1 growth stage (Fehr et al. 1971) with a paint brush. For the tests, resistance was evaluated by scoring plants with primary leaf damage (PLD) and total plant damage (TPD). Jandricic et al. (2010) have reported that the number of FAs was not highly correlated with the total plant damage grade unlike soybean aphids. Thus, PLD and TPD were used for our previous and current studies. The PLD and TPD were graded by assigning scores 1 (no damage to the inoculated leaf), 2 (< 5% damage), 3 (5–30% damage), 5 (31–50% damage), 7 (51–70% damage), or 9 (> 70% damage) (Lee et al. 2015a). For antixenosis test, RILs and the parents of the first population were planted in 10 × 5 trays (550L × 270 W × 120H mm) with Sowon in the center column. The plants were arranged in a complete randomized design (CRD) with three replications. Resistance was evaluated at fourteen days after infestation by scoring plants with the PLD and TPD. For antibiosis test, RILs and the parents were planted in the same tray without Sowon. After infestation, the plants were isolated with a 120-mesh cage to restrict aphid movement among plants. Seven days after inoculation, the PLD and TPD were scored for each plant.
To validate resistance to FA and inheritance of Raso2 in the second population, antibiosis was tested as described above. Five plants from each line were inoculated with five adult FAs, and then, lines were determined as homozygous resistant (when all plants have scores less than 2), heterozygous (when plants have scores between 2 and 3), or homozygous susceptible (when all plants have scores greater than 3) to FA based on responses of the five plants of each line.
DNA extraction and SNP genotyping
Genomic DNA from each line and the parents in the first population was extracted using young trifoliate leaves with the CTAB (hexadecyltrimethylammonium bromide) method described by Porebski et al. (1997) with the following modifications: an incubation time of 90 min, re-suspension of the DNA pellet in 500 μL 1 × TE, and no RNase A treatment. All DNA was firstly quantified by ND-1000 Spectrophotometer and diluted to 100 ng µL−1 for further study. For the SNP the genotyping, Axiom® 180 K (180,961) SoyaSNP array (Affymetrix, CA, USA) was used (Lee et al. 2015b). Genomic DNA from the lines and parents were hybridized to Affymetrix GeneTitan array system and then scanned with GeneTitan® Scanner (Affymetrix, CA, USA) according to the manufacturer’s protocol. SNP genotype analysis was conducted based on Axiom® Genotyping Solution Data Analysis User Guide (http://www.affymetrix.com). Of the 180,961 SNPs, 28,752 high-quality SNPs with the following parameters: (1) missing value < 10% and (2) minor allele frequency (MAF) < 0.001 were used to fine-genetic map Raso2.
Construction of Genetic Linkage Map and QTL analysis
A linkage mapping of the first population was conducted using QTL IciMapping software (version 4.1) with the following parameters: (1) a logarithm of odds (LOD) of 3.0 to group markers into linkage groups, (2) a ordering algorithm of nnTwoOpt for tour construction and two-opt for tour improvement, and (3) rippling by sum of adjacent recombination fractions (Li et al. 2008).
Associations between the two phenotypes, PLD and TPD, and SNP markers were first tested by a single-factor analysis of variance using IciMapping software with 3.0 of LOD threshold. Multiple-regression analysis with all significant markers on single linkage group was conducted using PROC REG function in SAS 9.3 (SAS Institute, NC, USA) with α = 0.05 to determine the total phenotypic variance explained (R2) by QTL. In addition, the inclusive composite interval mapping (ICIM) was performed using a 1.0 cM walk speed and 3.0 of LOD threshold (Li et al. 2008).
Evaluation of expression level of the candidate gene
To evaluate expression levels of the candidate gene, ten FAs were first inoculated to the trifoliate of Williams82 and PI 366121, parents of the first population. And then leaf samples were collected after 0, 24, and 48 h after inoculation with three biological replications. Total RNA was isolated using Trizol reagent (Invitrogen, CA, USA), 10 μg of the total RNA was purified via magnetic beads (Thermo Fischer Scientific, USA) and cDNA was synthesized using a reverse transcription reaction (EcoDry cDNA Synthesis Premix) following the manufacturer’s instructions (Takara Bio Inc., Japan). The expression levels were determined by quantitative real-time polymerase chain reaction (qRT-PCR) using an ABI StepOnePlus system (Applied Biosystems, CA, USA). All experiments were performed with three replications and the results were analyzed using StepOne software V2.1 (Applied Biosystems, CA, USA). Primers for target gene were designed using Primer3.0 version 4.1.0 (http://primer3.ut.ee/). Primer sequences for the target candidate gene (Glyma.07g077700) were 5'GTTTGACTCTTAGCTCGTTACCAA3' for forward and 5′CAAGGTTTGCTGAACGACAT3′ for reverse. Primers for housekeeping gene (Cons6; Glyma.12G051100) were 5′AGATAGGGAAATGGTGCAGGT3′ for forward and 5′CTAATGGCAATTGCAGCTCTC3′ for reverse (Libault et al. 2008). T-test in SAS 9.3 (SAS Institute, NC, USA) was conducted to compare relative expressions of the gene between Williams 82 and PI 366121.
Sequence comparison of the candidate gene between PI 366121 and diverse soybean germplasm
To confirm sequence variations within the candidate gene identified from the first population, sequences of eight exon regions in the Glyma.07g077700 between PI 366121 and 26 parental soybean germplasms for the Korean soybean nested association mapping (NAM) populations were compared (Kim et al. 2021). Out of 26 germplasm, one Korean soybean cultivar, Cheongja, presents resistance to Korean FA biotype but resistant gene(s) in the germplasm has not been identified. Other 25 germplasm were susceptible to Korean FA biotype (Kang Lab, unpublished data).
Results
To narrow down the genetic interval containing the Raso2 from PI 366121, Axiom® 180 K SoyaSNP array was employed to 105 F4:8 RIL from the first population. Among the high-quality SNPs, 28,752 SNPs after eliminating multi-collinearity and/or redundant markers were finally used for construction of genetic linkage map. The high-density genetic linkage map of 105 RILs consisted of 20 chromosomes which spanned about 4300 cM. Compared to the previous study using 414 SNP markers from GoldenGate® assay (Lee et al. 2015a), average distance between SNPs in the present study was about 0.15 cM increased as 45 times than the previous study (Fig. 1; Table 1).
Genetic linkage maps of 105 F4:8 recombinant inbred lines from Williams 82 and PI 366121. a Genetic linkage map using the GoldenGate® assay (Lee et al. 2015a), b genetic linkage map using the 180 K Axiom® SoyaSNP assay (current study)
The QTL analysis revealed that the major QTL on chromosome 7 was highly associated with the tested four phenotypes (PLD and TPD from the choice and nonchoice test). The QTL was positioned in the interval previously identified by Lee et al. (2015a) and was mapped between two SNP markers, AX-90462843 and AX-90334585 (Figs. 2, 3). Therefore, it could be assured that the mapped QTL in the present study was Raso2. This effort resulted in narrowing the genetic interval containing the gene from 13 to 1 cM in genetic length using the same population. Based on the ICIM, Raso2 accounted for 35.3 and 28.5% of the phenotypic variations for PLD and TPD in the choice test, respectively (Table 2). In the nonchoice test, 21.5 and 20.2% of variances for PLD and TPD was explained by Raso2, respectively (Table 2). Additive effect of Raso2 region ranged from 0.9 to 1.5 according to the test and traits (Table 2).
Inclusive composite interval mapping for primary leaf damage (PLD) and total plant damage (TPD) in the choice and nonchoice test in the first population. BARC markers from GoldenGate® assay (Lee et al. 2015a) and AX markers from Axiom® 180 k SoyaSNP array (present study) on soybean chromosome 7
Based on the Williams 82 genome assembly (Wm82.a2.v1), Raso2 was located within 76 Kb region defined by the above SNP markers and the current gene annotation of the region predicted the presence of eight gene models (Fig. 3). Of these genes, six annotated gene models encode putative protein with function (Table 3). Through re-sequencings of exon regions in the eight annotated genes, sixteen SNPs between Williams 82 and PI 366121 were identified from three annotated genes (Table 3). Two, two, and twelve SNPs between Williams 82 and PI 366121 were detected in Glyma.07g077000, Glyma.07g077400, and Glyma.07g077700, respectively. Among the SNPs, ten SNPs were nonsynonymous substitutions and all of them were located within one gene, Glyma.07g077700, which is putative R gene encodes the disease resistance protein (PTHR23155) containing leucine-rich repeat (LRR) domain (Table 3).
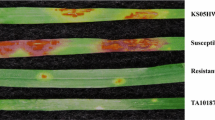
To validate the Raso2 resistance to FA and inheritance of the gene in a different background, 41 F3 families derived from PI 366121 and PI 483,463 were tested with FAs. The population was segregated as 12 (homozygous resistant):21 (heterozygous; segregating):8 (homozygous susceptible) based on the responses of the five plants from each line and the ratio was fit to the Mendelian inheritance for a single dominant gene, 1:2:1 (χ2 = 0.805, P = 0.67, Table 4).
A relative expression level of the candidate gene between Williams 82 and PI 366121 was analyzed by qRT-PCR (Fig. 4). No statistical difference in the expression levels of Glyma.07g077700 between Williams 82 and PI 366121 was detected at 0 and 24 h after inoculation. However, the expression level of the gene was dramatically increased 48 h after inoculation in PI 366121 and the expression level between Williams 82 and PI 366121 was significantly different (Fig. 4; P < 0.0001).
Expression level of Glyma.07g077700 in Williams 82 and PI 366121. Ten foxglove aphids were inoculated and the relative expressions of the gene were determined 0, 24, and 48 h after the inoculation (n = 3). Bar (I) represents a standard deviation. *Represents a significant difference in two expression levels (P < 0.0001)
To confirm the sequence variations within the candidate gene, Glyma.07g077700, identified from the first population, exon regions of the candidate gene were re-sequenced and compared between PI 366121 and 26 soybean germplasm. Several FA-susceptible germplasms had the same alleles as PI 366121 in the SNP 3–1, 3–9, 3–10, 3–11, and 3–12 (Table 5). Therefore, it could be assumed that the allele substitutions in above SNPs might be not associated with the FA resistance from PI 366121. In remaining SNPs, all susceptible germplasm had a different allele from PI 366121. The SNPs 3–2, 3–3, 3–4, 3–5, 3–6, 3–7, and 3–8 located between 7,093,145 and 7,096,564 bp on soybean chromosome 7 presented a distinct allele between the FA-resistant source and the FA-susceptible germplasm. All FA-susceptible germplasm had the different alleles from PI 366121 at the seven loci. Expected amino acid replacements by the SNPs were listed in Table 5. However, the SNP 3–2 was synonymous type and could not cause an amino acid replacement. Therefore, six SNPs within exon 5 only presented unique variation for PI 366121 and amino acid replacements. Based on the SNPs, Cheongja had the same alleles as other FA-susceptible germplasm. Therefore, it is likely that resistant gene(s) from PI 366121 and Cheongja are different.
Discussion
The FA resistance gene Raso2 from PI 366121 was fine-mapped on chromosome 7 in this study. By the high-density genetic linkage map using the 180 K Axiom® SoyaSNP assay, the Raso2 interval was narrow down from 13 cM (2.2 Mbp) with 275 annotated genes to 1 cM (76 Kb) with eight annotated gene models. Ohnishi et al. (2012) previously mapped the Raso1 from Adams on chromosome 3. Kim et al. (2010a, b) previously fine-mapped the SA resistance gene Rag1 from Dowling on chromosome 7 and one SA resistance gene in PI 587,732 was also mapped on chromosome 7 (Kim et al. 2014). Firstly, we confirmed that Raso1 from Adams and Raso2 from PI 366121 were different FA resistance genes because two genes were mapped on different chromosome as well as Adams presented susceptible reaction to the Korean FA biotype (Lee et al. 2015a). In the case of SA resistance genes mapped on chromosome 7, physical positions of the SA resistance genes and Raso2 were quite different. The SA resistance genes from Dowling and PI 587,732 were mapped on 43 Mb regions, while Raso2 from PI 366121 was positioned on 7 Mb regions on chromosome 7 (https://soybase.org/). Other reported insect resistance genes were also physically far from Raso2. Considered quite distances in the physical locations on the Williams 82 genome assembly (Wm82.a2.v1), it is likely that the Raso2 is different gene from previously reported insect resistance genes although they were mapped on the same chromosome.
Our mapping efforts were greatly accelerated by the availability of the high-density SNP array and the public sequence of the soybean genome. Lee et al. (2015a) previously mapped Raso2 region using the GoldenGate® assay containing 1,536 SNP loci but only 414 SNPs were actually used to map the gene. In the present study, the 180 K Axiom® SoyaSNP array was used to genotype the same population and 28,752 SNPs were used to fine-map Raso2 region. The average distance between SNP markers mapped in the present study was approximately 0.15 cM as increased 45 times than the previous study (6.8 cM) and they sufficiently covered all genomic regions of soybean (Fig. 1, Table 1). In addition to high-density SNP markers, the use of precisely tested phenotypic data from the choice and nonchoice tests could also play pivotal role in fine-mapping the gene and evaluating effects of the gene to the tested phenotypes. High-depth re-sequencing information on PI 366121 and diverse soybean germplasm also play important role in identifying sequence variations within the candidate gene which might be associated with the resistance to FA. Therefore, for successful fine-mapping identifying candidate gene(s) of target gene, use of accurately evaluated diverse phenotypes, high-density genetic map, and diverse soybean germplasm would be essential.
Based on the Williams 82 genome assembly (Wm82.a2.v1) which was the FA-susceptible parent in the first population, the current gene annotation of the 76 Kb region containing the Raso2 predicts the presence of eight annotated genes. Of these genes, Glyma.07g077700 is the only gene encoding disease resistance protein with leucine-rich repeat (LRR) domain. Glyma.07g077700 is a homolog gene of AT4G26090.1 in Arabidopsis which is a member of the RPS2 gene family encoding a nucleotide-binding domain shared with APAF1, R gene products and CED4 (NB-ARC) protein that confers to disease resistance (https://soybase.org/, https://www.arabidopsis.org/). Based on the legume information system (https://legumeinfo.org/), the gene has significant homology with Vradi0292s00010 encoding disease resistance protein [coiled-coil (CC)–nucleotide-binding site (NBS)–LRR class] in mung bean (Vigna radiate). The majority of cloned resistance genes are members of the NBS-LRR gene family. Cloned NBS-LRR genes that confer aphid resistances include the Mi for resistance to potato aphid (Macrosiphum euphorbiae) in tomato (Lycopersion esculentum Mill.) (Rossi et al. 1998) and the Vat for resistance to A. gossypii in melon (Cucumis melo L.) (Dogimont et al. 2009). In the case of soybean aphid resistance, Rag1 (Kim et al. 2010a) and Rag2 (Kim et al. 2010b), Rag6 and Rag3c (Zhang et al. 2017b) were fine-mapped to a NBS-LRR cluster region, respectively. In Medicago truncatula, resistance genes to bluegreen aphid (Acyrthosiphon kondoi S.) and pea aphid (Acyrthosiphon pisum H.) were also mapped in a NBS-LRR cluster on different chromosomes, respectively (Klingler et al. 2005; Kamphuis et al. 2016). Resistance genes to green peach aphid (Myzus persicae S.) in pepper (Capsicum spp.) and rosy apple aphid (Dysaphis plantaginea P.) in apple (Malus x domestica) were fine-mapped a locus containing NBS-LRR genes (Sun et al. 2020; Pagliarani et al. 2016). Most R proteins contain leucine-rich repeats (LRRs), a central NBS, and a variable amino-terminal domain (Takken et al. 2006). The LRRs are mainly involved in recognition, whereas the amino-terminal domain determines signaling specificity. It has been reported that the NBS forms part of a nucleotide-binding (NB)–ARC domain that presumably functions as a molecular switch. The distinctly expression of Glyma.07g077700 in PI 366121 at 48 h after FA inoculation might indicate that the high expression of the gene was associated with the resistance to FA. Further studies would be needed to ensure the relationship between the FA resistance and expression levels of Glyma.07g077700 in diverse soybean germplasm or transgenic plants.
The high-resolution genetic map and SNP markers identified in the present study will facilitate MAS of Raso2 and pyramiding of Raso2 with other target genes on chromosome 7 because of their very close proximity to the gene. In addition, the identification of the precise physical position of Raso2 region and the candidate gene (Glyma.07g077700) on the soybean chromosome 7 could greatly facilitate the cloning and functional characterization of the gene. The cloning of Raso2 might improve understanding of FA defense mechanism in soybean as well as other host species of FA. This information could be applied to compare the function of this gene to other aphid resistance genes such as Raso1, Rag1, Rag2, or cloned insect resistance genes in other species. We are conducting ongoing studies to clone Raso2. Transformation of Glyma.07g077700 from PI 366121 to Willams82 or other transformation recipient lines as well as genome editing of Glyma.07g077700 in Williams 82 according to the SNP information within the candidate gene might be needed to investigate functions of the candidate gene and interaction of the gene with other chromosome regions.
References
Bernard RL, Cremeens CR (1988) registration of Williams 82 soybean. Crop Sci 28:1027–1028
Blackman RL, Eastop VF (2000) Aphids on the world’s crops: an identification and information guide, 2nd edn. Wiley, Chichester
Dogimont C, Bendahmane A, Pitrat M, Burget-Bigeard E, Hagen L, Le Menn A, Pauquet J, Rousselle P, Caboche M, Chovelon V (2009) Gene resistant to Aphis gossypii. US Patent 7,576,264, 18 Aug 2009
Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of development description for soybean, Glycine max (L.) Merrill. Crop Sci 11:929–931
Harrewijn P, Minks AK (1989) Integrated aphid management: general aspecrs. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies, and control, vol 2C. Elsevier, Amsterdam, pp 267–272
Jandricic SE, Wraight SP, Bennett KC, Sanderson JP (2010) Developmental times and life table statistics of Aulacorthum solani (Hemiptera: Aphididae) at six constant temperatures, with recommendations on the application of temperature-dependent development models. Environ Entomol 39:1631–1642
Ji W, Zhu Y, Li Y, Yang L, Zhao X, Cai H, Bai X (2010) Over-expression of a glutathione S-transferase gene, GsGST, from wild soybean (Glycine soja) enhances drought and salt tolerance in transgenic tobacco. Biotech Lett 32(8):1173–1179
Jun TH, Van K, Kim MY, Lee SH, Walker DR (2008) Association analysis using SSR markers to find QTL for seed protein content in soybean. Euphytica 162(2):179–191
Kamphuis LG, Guo S-M, Gao L-L, Singh KB (2016) Genetic mapping of a major resistance gene to pea aphid (Acyrthosipon pisum) in the model legume Medicago truncatula. Int J Mol Sci 17(8):1224
Kao WY, Tsai TT, Tsai HC, Shih CN (2006) Response of three Glycine species to salt stress. Environ Exp Bot 56(1):120–125
Kim DH, Lee GH, Park JH, Hwang CY (1991) Occurrence aspects and ecological characteristics of foxglove aphid, Aulacorthum solani, Kaltenbach (Homoptera: Aphididae) in soybean. Res Rept RDA 33:28–32
Kim KS, Bellendir S, Hudson KA, Hill CB, Hartman GL, Hyten DL, Hudson MW, Diers BW (2010a) fine mapping the soybean aphid resistance gene Rag1 in soybean. Theor Appl Genet 120:1063–1071
Kim KS, Hill CB, Hartman GL, Hyten DL, Hudson ME, Diers BW (2010b) Fine mapping of the soybean aphid–resistance gene Rag2 in soybean PI 200538. Theor Appl Genet 121:599–610
Kim KS, Chirumamilla A, Hill CB, Hartman GL, Diers BW (2014) Identification and molecular mapping of two soybean aphid resistance genes in soybean PI 587732. Theor Appl Genet 127:1251–1259
Kim KS, Hill CB, Hartman GL, Mian MAR, Diers BW (2008) Discovery of soybean aphid biotypes. Crop Sci 48:923–928
Kim MS, Lozano R, Kim JH, Bae DN, Kim ST, Park JH, Choi MS, Kim J, Ok HV, Park SK, Gore MA, Moon JK, Jeong SC (2021) The patterns of deleterious mutations during the domestication of soybean. Nat Commun 12:97. https://doi.org/10.1038/s41467-020-20337-3
Klingler J, Creasy R, Gao LL, Nair RM, Calix AS, Jacob HS, Edwards OR, Singh KB (2005) Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol 137:1445–1455
Kogan M, Ortman EE (1978) Antixenosis: A new twrm proposed to define Painter’s “nonpreference” modality of resistance. Bull Entomol Soc Am 24:175–176
Koh HM, Park S et al (2018) Resistance sources for the foxglove aphid in soybean. J Crop Sci 63(3):257–264
Komatsu K, Okuda S, Takahashi M, Matsunaga R, Nakazawa Y (2005) QTL mapping of antibiosis resistance to common cutworm (Spodoptera litura Fabricius) in Soybean. Crop Sci 45(5):2044–2048
Leather SR (1992) Aspects of aphid overwintering (Homoptera: Aphidinea: Aphididae). Entomol Gen 17:101–113
Lee JS, Yoo MH, Jung JK, Bilyeu KD, Lee JD, Kang ST (2015a) Detection of novel QTLs for foxglove aphid resistance in soybean. Theor Appl Genet 128:1481–1488
Lee S, Holman J, Havelka J (2002) illustrated catalogue of aphididae in the Korean Peninsula. Part I. Subfamily Aphidinae (Hemiptera: Sternorrhyncha); KRIBB & CIS: Chuncheon, Korea, pp 106–108
Lee YG, Jeong N, Kim JH, Lee K, Kim KH, Pirani A et al (2015b) Development, validation and genetic analysis of a large soybean SNP genotyping array. Plant J 81:625–636
Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M, Clough SJ, Stacey G (2008) Identification of four soybean reference genes for gene expression normalization. Plant Genome 1:44–54
Li MW, Xin D, Gao Y, Li KP, Fan K, Muñoz NB et al (2017) Using genomic information to improve soybean adaptability to climate change. J Exp Bot 68(8):1823–1834
Li H, Ribaut JM, Li Z, Wang J (2008) Inclusive composite interval mapping (ICIM) for digenic epistasis of quantitative traits in biparental population. Theor Appl Genet 116:243–260
Li Y, Hill CB, Carlson S, Diers BW, Hartman GL (2007) Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Mol Breeding 19:25–34
Nagano T, Umetsu Y, Hoshi N, Kidokoro T (2001) Outbreak of the foxglove aphid, Aulacorthum solani (Kaltenbach), in soybean fields of Miyagi Prefecture. II. Effect on soybean yield. Annual Report of the Society of Plant Protection of North Japan 52:168–171
Narvel JM, Walker DR, Rector BG, All JN, Parrott WA, Boerma HR (2001) A retrospective DNA marker assessment of the development of insect resistant Soybean. Crop Sci 41(6):1931–1939
Oki N, Komatsu K, Sayama T, Ishimoto M, Takahashi M, Takahashi M (2012) Genetic analysis of antixenosis resistance to the common cutworm (Spodoptera litura Fabricius) and its relationship with pubescence characteristics in soybean (Glycine max (L.) Merr.). Breed Sci 61(5):608–617
Pagliarani G, Dapena E, Miñarro M et al (2016) Fine mapping of the rosy apple aphid resistance Dp-fl locus on Linkage Group 8 of the apple cultivar ‘Florina.’ Tree Genet Genom 12:56
Painter RH (1951) Insect resistance in crop plants. Macmillan, New York
Park KY, Yun HT, Moon JK, Ku JH, Hwang JJ, Lee SH, Seung YK, Ryu YH, Chung WK, Lee YH, Kin SD, Chung MN (2000) A new soybean cultivar for sprout with good storability and disease resistance, ‘Sowonkong.’ Korean J Breed 32(3):298–299
Pham AT, Lee JD, Shannon JG, Bilyeu KD (2010) Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol 10(1):195
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Rector BG, All JN, Parrott WA, Boerma HR (2000) Quantitative trait loci for antibiosis resistance to corn earworm in soybean. Crop Sci 40(1):233–238
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 95:9750–9754
Smalley MD, Fehr WR, Cianzio SR, Han F, Sebastian SA, Streit LG (2004) Quantitative trait loci for soybean seed yield in elite and plant introduction germplasm. Crop Sci 44:436–442
Sun M, Voorrips RE, van’t Westende W, et al (2020) Aphid resistance in Capsicum maps to a locus containing LRR-RLK gene analogues. Theor Appl Genet 133:227–237
Takken FLW, Albrecht M, Tameling WIL (2006) Resistance proteins: molecular switches of plant defense. Curr Opin Plant Biol 9:383–390
Turl LAD (1983) The effect of winter weather on the survival of aphid populations on weeds in Scotland. EPPO Bull 13:139–143
Zhang G, Gu C, Wang D (2009) Molecular mapping of soybean aphid resistance genes in PI 567541B. Theor Appl Gen 118(3):473–482
Zhang J, Yang D, Li M, Shi L (2016) Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS ONE 11(7):e059622
Zhang S, Zhang Z, Bales C et al (2017a) Mapping novel aphid resistance QTL from wild soybean, Glycine soja 85–32. Theor Appl Genet 130:1941–1952
Zhang S, Zhang Z, Wen Z et al (2017b) Fine mapping of the soybean aphid-resistance genes Rag6 and Rag3c from Glycine soja 85–32. Theor Appl Genet 130:2601–2615
Acknowledgements
This research was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ01319201), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
KSK and JMK contributed equally to this research. KSK outlined, wrote the first draft of the manuscript, revised, and finalized the manuscript; JMK performed evaluation of FA resistance, analyzed the data, and wrote M&M, figures, and tables in the first manuscript; JJ, IS, and SP performed experiments; SCJ and JSL analyzed the genotypic data; JDL provided the second soybean population; JKJ provided the Foxglove aphid; BKH performed the gene expression study; and SK acquired funding and supervised the study and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Evans Lagudah.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, KS., Kim, JM., Jung, J. et al. Fine-mapping and candidate gene analysis for the foxglove aphid resistance gene Raso2 from wild soybean PI 366121. Theor Appl Genet 134, 2687–2698 (2021). https://doi.org/10.1007/s00122-021-03853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03853-8