Abstract
Key message
A new gene Rph28 conferring resistance to barley leaf rust was discovered and fine-mapped on chromosome 5H from wild barley.
Abstract
Leaf rust is a highly destructive disease of barley caused by the fungal pathogen Puccinia hordei. Genetic resistance is considered to be the most effective, economical and eco-friendly approach to minimize losses caused by this disease. A study was undertaken to characterize and fine map a seedling resistance gene identified in a Hordeum vulgare ssp. spontaneum-derived barley line, HEB-04-101, that is broadly effective against a diverse set of Australian P. hordei pathotypes. Genetic analysis of an F3 population derived from a cross between HEB-04-101 and the H. vulgare cultivar Flagship (seedling susceptible) confirmed the presence of a single dominant gene for resistance in HEB-04-101. Selective genotyping was performed on representative plants from non-segregating homozygous resistant and homozygous susceptible F3 families using the targeted genotyping-by-sequencing (tGBS) assay. Putatively linked SNP markers with complete fixation were identified on the long arm of chromosome 5H spanning a physical interval between 622 and 669 Mb based on the 2017 Morex barley reference genome assembly. Several CAPS (cleaved amplified polymorphic sequences) markers were designed from the pseudomolecule sequence of the Morex assembly (v1.0 and v2.0), and 16 polymorphic markers were able to delineate the RphHEB locus to a 0.05 cM genetic interval spanning 98.6 kb. Based on its effectiveness and wild origin, RphHEB is distinct from all other designated Rph genes located on chromosome 5H and therefore the new locus symbol Rph28 is recommended for RphHEB in accordance with the rules and cataloguing system of barley gene nomenclature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barley leaf rust (BLR) is one of the most widespread and destructive foliar diseases of Hordeum spp. (Park et al. 2015). Caused by the biotrophic fungal pathogen Puccinia hordei Otth., leaf rust poses a significant threat to barley production causing yield reductions of up to 60% in susceptible cultivars, especially during severe epidemics (Cotterill et al. 1992). Genetic resistance is considered to be the most effective, economical and eco-friendly approach to minimize losses caused by BLR. Over the last 30 years, enormous efforts have been made to characterize and map genes conferring resistance to P. hordei in cultivated barley, resulting in the designation of 24 distinct all-stage resistance (ASR) and three adult plant resistance (APR) loci (Park et al. 2015; Kavanagh et al. 2017; Rothwell et al. 2020). Most of the 24 loci conferring ASR have been rendered ineffective due to rapid evolution of matching virulence in P. hordei populations, emphasizing the importance of ongoing efforts to both diversify and characterize new sources of resistance (Brooks et al. 2000; Park et al. 2015). The availability and utilization of diverse sources of effective resistance is crucial for successful barley breeding programmes (Singh et al. 2017). Therefore, it is essential to identify and map new resistance (R) genes and to develop breeder friendly molecular markers linked to these genes which would be helpful to identify and combine these genes through marker assisted selection.
Introgressing genes from various gene pools of Hordeum is an important approach to widen the genetic base of Hordeum vulgare (Vatter et al. 2018). Four genes (Rph10, Rph11, Rph13 and Rph15/Rph16) have been sourced from wild barley, H. vulgare ssp. spontaneum (Hvs), and a further four Rph genes (Rph17, Rph18, Rph22 and Rph26) have been sourced from bulbous barley, H. bulbosum; however, to date, none of these genes have been deployed in agriculture (Park et al. 2015). The wild progenitor, Hvs, has a higher level of genetic diversity for disease resistance genes compared to cultivated barley (Von Bothmer et al. 2003). A comparison of a sequenced wild barley genome (WB1) with the barley cultivar Morex revealed that WB1 carries more genes involved in resistance to various biotic and abiotic stresses (Liu et al. 2020), further highlighting the importance of the wild barley gene pool as a source of resistance.
Many different molecular marker systems have been employed for genetic mapping of Rph genes in barley, e.g. RAPD (random amplified polymorphic DNA), AFLP (amplified fragment length polymorphism), RFLP (restriction fragment length polymorphism) and SSR (simple sequence repeats) were used to map Rph2 (Borovkova et al. 1997; Franckowiak et al. 1997), Rph15 (Weerasena et al. 2004), Rph19 (Park and Karakousis 2002) and Rph21 (Sandhu et al. 2012), respectively. In addition to conventional molecular markers, NGS (next-generation sequencing) technologies such as DArT (Diversity arrays technology) and GBS (genotyping by sequencing) now provide high throughput and cost-effective genotyping platforms that facilitate fast detection and application of SNPs (single nucleotide polymorphisms) in barley (Darrier et al. 2019). DArT has been employed successfully in mapping several Rph genes including Rph14, Rph20 and Rph23 (Golegaonkar et al. 2009; Hickey et al. 2011; Singh et al. 2015). Furthermore, a complexity reduction GBS method- DArT-Seq platform was also successfully used to map Rph9.am, Rph24, Rph25 and Rph27 (Dracatos et al. 2014; Ziems et al. 2017; Kavanagh et al. 2017; Rothwell et al. 2020).
The NGS technologies usually identify the target interval at Megabase (Mb) levels (Kayam et al. 2017) and then chromosomal regions can be further saturated with markers of choice to fine map the target locus. Among the different molecular marker types used for fine mapping traits of interest in plants, co-dominant markers e.g. insertion-deletion and SNP [KASP (kompetitive allele-specific PCR) or CAPS (cleaved amplified polymorphic sequences)] are preferred due to their abundance and ability to distinguish homozygous from heterozygous marker loci (Liu et al. 2016). CAPS markers have been effectively employed in barley where high levels of polymorphism are available (Shavrukov 2014, 2016). Recently, Fazlikhani et al. (2019) performed high resolution mapping of the leaf rust resistance locus RphMBR1012 using a combination of SSRs, insertion/deletion polymorphisms (InDels) and SNPs (KASP and CAPS marker systems). The recent availability of a reference genome assembly for barley cultivar Morex (Mascher et al. 2017) through increased access to the gene space has consequently improved the efficiency of molecular marker development for fine mapping studies. For instance, the Rph13 locus (originally derived from Hvs) in barley accession PI 531849 was recently fine-mapped to the long arm of chromosome 3H using the Morex genome as a road map, despite its previously reported possible linkage with Rph9 on 5H (Jost et al. 2020).
In order to diversify the genetic base of barley leaf rust resistance and utilize potential of available barley reference genome assembly and NGS, the present study was conducted to characterize BLR resistance in the Hvs derived barley line HEB-04-101 (referred to as RphHEB) identified from the wild barley nested association mapping population HEB-25 (Maurer et al. 2015). The barley line HEB-04-101 was used in this study because it is resistant to predominant Australian P. hordei pathotypes. The second objective of this study was to fine map RphHEB locus and develop CAPS markers and identify possible candidate genes.
Materials and methods
Plant materials and pathogen isolates
Maurer et al. (2015) developed a Nested Association Mapping (NAM) population for barley known as HEB-25 (HEB = Halle Exotic Barley) by crossing the European cultivar Barke (H. vulgare) with 24 highly divergent Hvs and one agriocrithon accession, with the aim of increasing genetic diversity. The resulting F1s from each cross combination were then backcrossed with Barke as a female parent. BC1 plants for each cross were selfed three times to produce 1420 BC1S3 (equivalent to BC1F3:4) lines. These HEB lines were introduced to Australia for detailed phenotypic evaluation of rust resistance and seed is currently maintained at the University of Adelaide, Australia. Initial rust testing of the HEB population at Plant Breeding Institute Cobbitty (PBIC) with P. hordei pathotype 5457 P+ identified over 100 lines with variable resistant infection type (IT) responses (D. Singh, unpublished). One of these lines (HEB-04-101) was used as a resistant parent and crossed to the seedling susceptible Australian barley cultivar Flagship for inheritance and mapping studies.
Development of mapping population
An F3 mapping population (n = 125) was developed by crossing HEB-04-101 and Australian barley cultivar Flagship (seedling susceptible to pathotype 5457 P+) for genetic analysis, characterization and mapping of the resistance in HEB-04-101 (tentatively designated as RphHEB). F1 seed was harvested, threshed and sown to raise F2 seed. Approximately 150 seeds from F2 were space planted at the Horse Unit field site of PBIC, and the individual F2 plants were harvested to generate F3 families. The seeds were threshed and stored in dehumidified rooms under controlled temperature until further testing and sowing.
Sowings and inoculations
Twenty to 25 seeds from each F3 family, including parents, were sown in 90-mm-diameter pots containing Grange Horticultural® soil premix (comprised of 80% 0–8 mm composted pine bark, 10% 0–3 mm composted pine bark, 10% propagating sand, 1 kg/m3 gypsum, 1 kg/m3 superphosphate, 0.25 kg/m3 potassium nitrate, 0.25 kg/m3 nitroform and 1.5 kg/m3 magrilime). Seed of 23 P. hordei differentials (Park et al. 2015) was also sown as clumps as a control. All pots were fertilized with Aquasol (@) 25 g/10 L of water) at the time of sowing as well as one day before inoculation. The seedlings were raised in disease-free rooms maintained at 18–20 °C. Ten days old seedlings with fully expanded first leaves were inoculated with P. hordei pathotype 5457 P+. A suspension was prepared by adding 10 mg urediniospores to 10 ml of light mineral oil (Isopar L® Univar, Ingleburn, NSW, Australia) for 200 pots. The mixture was then homogenously atomized over the seedlings with a mist atomizer. Following inoculation, seedlings were incubated at temperature 20 ± 5 °C in a dark chamber for 24 h in which an ultrasonic humidifier created 100% humidity. After 24 h of incubation, seedlings were moved to microclimate rooms maintained at 22–24 °C with natural light and automatic drip irrigation (5 min cycle, 4 times a day).
Phenotyping and genetic analysis of mapping population
Disease assessments were made 10–12 days post-inoculation using a ‘0–4’ infection type (IT) scale as outlined by Park and Karakousis (2002). ITs of “0”, “; (fleck)”, “1” and “2” were used to indicate the resistant response, while ITs of 3+ and above were used to indicate susceptible host response. Variation in the IT patterns was indicated by using the symbols + (more than average for the class), − (less than average for the class), C (chlorosis) and N (necrosis). F3 lines were scored as homozygous susceptible (HS) or homozygous resistant (HR) when all the seedlings (approximately 25) of individual family produced susceptible or resistant ITs, respectively. Lines were scored as segregating (Seg) when both resistant and susceptible plants were detected within an individual F3 family. For pooled analysis, resistant and susceptible plants from segregating F3 families were counted and recorded. Chi-squared (χ2) analysis was performed to determine the goodness of fit of observed ratios to expected ratios. P values were calculated from χ2 values using the online calculator “Quickcalcs” (GraphPad Software Inc, USA).
Targeted genotyping-by-sequencing (tGBS) genotyping
Genomic DNA was extracted from a single leaf of individual plants selected from F3 lines classified as HR and HS, including the parents, using the CTAB (cetyl trimethylammonium bromide) protocol as described by Fulton et al. (1995). All samples were quantified using a spectrophotometer (Nanodrop™, Biolab, Melbourne, VIC, Australia). DNA quality was assessed on a 0.8% agarose gel, and all samples were diluted to 100 ng/µl. Twenty-five HR and 25 HS lines and the parents were genotyped using the tGBS service provided by Agriculture Victoria Research, Bundoora, Australia. For tGBS analysis, samples were analysed using a custom bioinformatics pipeline. In brief, this pipeline processes sample reads from the tGBS assay to generate genotype calls for polymorphic loci. First, the sample read data are used to build an allele-specific reference (ASR) and then allelism among the ASR sequences is determined from their alignment to the Barley Morex IBSC reference genome assembly. Markers were classified as putatively linked when genotype calls were at least 70% fixed across samples within one or more of the phenotypic classes (HR and HS).
Development of molecular markers and conversion of identified SNPs to CAPS
Fifty-seven CAPS markers (Supplementary Table 1) were designed using Primer 3 Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) within the physical region (47 Mb, identified through tGBS) harbouring RphHEB, based on the Morex reference v1.0 and v2.0. DNA from the parents HEB-04-101 and Flagship was PCR amplified using CAPS oligonucleotides in a 50 µl reaction volume containing 20 µl genomic DNA (10 ng/µl), 10 µl MyFi Buffer (Bioline), 0.5 µl MyFi polymerase (Bioline), 10 µl (1.5 µM) each of forward and reverse primers and 9.5 µl double-distilled water. All reactions were conducted in a 96 well-plate in an automated thermocycler (Bio-Rad T100) with an initial denaturation step of 95 °C at 10 min, followed by 30 cycles at 94 °C for 20 s, 60 °C for 30 s and 72 °C for 30 s with final extension at 72 °C for 10 min. Parental PCR products were then purified using Agencourt AMPure protocol-“000601v024” (Agencourt Bioscience Corporation) and sent to AGRF (Australian Genome Research Facility) for Sanger sequencing. To determine polymorphisms between the parents, the resulting parental sequences were analysed for the presence of SNPs using Sequencher 5.1 software (Gene Codes, Ann Arbor, MI, United States). Identified SNPs were converted into CAPS markers and further subjected to dCAPS (Derived Cleaved Amplified Polymorphic Sequences) Finder 2.0 (http://helix.wustl.edu/dcaps/) to identify restriction endonuclease sites (Neff et al. 2002). Restriction mapper version 3.0 (http://www.restrictionmapper.org/) was used to analyse the results from dCAPS finder. Of 57 designed CAPS markers, 16 were polymorphic (Table 1) and were used for fine mapping of RphHEB.
Marker genotyping, linkage analysis and map construction
Twenty to twenty-five plants from each of 125 F3 families from HEB-04-101/Flagship mapping population were genotyped with 16 polymorphic markers (Supplementary Table 2) that were evenly distributed within the 47 Mb target region [based on Morex reference v1.0 and v2.0 (Mascher et al. 2017; Mascher 2019)] identified on the long-arm chromosome 5HL using tGBS data. PCR reactions were performed using 10 µl reaction volumes, and all other conditions were the same as described above. PCR products were digested for three hours using temperatures specific for the respective restriction enzymes used for each of the interrogated SNP variants according to manufacturer’s instructions (New England Biolabs, Australia). Digested PCR products were loaded to a 2% agarose gel with 1 × TAE buffer and then subjected to electrophoresis for 60 min @ 120 V. After electrophoresis, gels loaded with products were visualized under UV light using Gel Doc IT imaging System (Model M-26, Bioimaging Systems, CA, USA). Genetic map was constructed using software Voorrips (2002) and physical map using the software Pretzel (https://plantinformatics.io/; Keeble-Gagnere et al. 2019).
Results
Phenotyping and genetic analysis of RphHEB/Flagship population
Parent HEB-04-101 produced a low to intermediate IT ('0;' to ';1 + CN') during rust tests at the seedling stage in response to five P. hordei pathotypes (200 P-, 220 P+ +13, 253 P−, 5653 P+ and 5457 P+). In contrast, the Flagship parent was susceptible (IT ‘3+’) in response to all five pathotypes (Table 2; Fig. 1). Seedlings of the HEB-04-101/Flagship F3 population (n = 125) segregated for a single gene when inoculated with P. hordei pathotype 5457 P+. Three distinct phenotypic classes (HR, Seg and HS) were observed among F3 families, and Chi-squared analysis was best fit to a 1:2:1 (HR/Seg/HS) segregation ratio expected for monogenic inheritance (χ2 = 0.98, P > 0.6; Table 3). Further pooled analysis based on recording resistant (R) and susceptible (S) individuals within 68 segregating lines revealed segregation of 736 resistant (R) plants and 238 susceptible (S) plants conforming to the goodness of fit for a single gene (3R:1S) ratio (736R:238S, χ2 = 0.16, P = 0.68) and demonstrating the dominant nature of RphHEB.
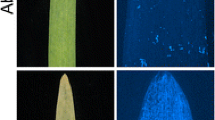
Phenotypic response of F3 families derived from HEB-04-101 × Flagship when infected with P. hordei pathotype 5457 P+ (PBI culture # 612). The susceptible parent Flagship (a) shows large uredinia compared to resistant parent HEB-04-101 (b) which shows chlorosis and necrosis with restricted uredinia. c, d and e represent lines from segregating population with various resistant infection types, while f represents a susceptible line from segregating population with large sporulating uredinia
Targeted genotyping by sequencing (tGBS)
Targeted genotyping-by-sequencing analysis was performed on 25 HR and 25 HS lines. A total of 119 putatively linked alleles were found on the long arm of chromosome 5H (Table 4). Of these, 23 had a predominance of the resistant parent genotype in the resistant progeny lines and a predominance of the susceptible parent genotype in the susceptible progeny lines, while additional 43 and 53 markers were prevalent for the resistant genotype only, or susceptible genotype only, respectively. Based on the Morex (v1.0) barley reference assembly, the distribution of putatively linked markers positioned the RphHEB locus within a 47 Mb physical interval (622–669 Mb) on the long arm of chromosome 5H (Fig. 2).
Putatively linked markers on chromosome 5H identified through targeted genotyping by sequencing in v1 and v2 Morex genome assembly. Blue highlighted region indicated linked region from 622 to 669 Mbp in v1. Diagram generated using the software Pretzel (https://plantinformatics.io/; Keeble-Gagnere et al. 2019)
Fine mapping of RphHEB
In total, 57 CAPS derived from four rounds of marker design were developed within a physical interval of 47 Mb (based on tGBS data and Morex v1) on the long arm of chromosome 5H. Sixteen polymorphic markers were used for recombination-based fine mapping of the RphHEB locus. We genotyped single representative plants from each F3 family from 125 individuals with these polymorphic markers (Fig. 3). Once the genotype of 125 families was determined, the progeny of the same samples were rust tested at the F4 generation, and data were used to fine map RphHEB.
Gel image showing digested PCR products in F3 mapping population using enzyme AciI. From L–R (1 = easy ladder Bioline, 2–18 = lines from F3 population, 19 = Flagship and 20 = HEB-04-101. After restriction digestion, susceptible parent Flagship (# 19) produced 2 bands of ∼ 250 bp and 550 bp, resistant parent HEB-04-101 (# 20) produced a product size of ∼ 900 bp. Well # 2, 6 and 16 show heterozygous lines carrying alleles from both parents
In the first round, 16 markers were developed, and four polymorphic markers were used to genotype the entire F3 mapping population. Two flanking markers, M1 (636.11 Mb) and M16 (646.30 Mb), were identified that placed RphHEB at a genetic distance of 6.8 and 4.8 cM, respectively, corresponding to a physical interval of 10.20 Mb. In a second round, 21 further markers were developed between M1 and M16 to further narrow the RphHEB interval; eight polymorphic markers were used to genotype 125 F3 families, which identified seven informative recombinants (Table 5) and allowed the identification of new flanking markers: M5 (640.33 Mb) and M10 (640.71 Mb) delimiting RphHEB to 380 kb. Development of further 14 markers in a third round and subsequent genotyping of the mapping population with polymorphic markers enabled RphHEB to be mapped between M7 (640.56 Mb) and M12 (640.69 Mb), restricting the physical window to 137 kb.
Based on a newly available iteration of the v1.0 2017 Morex genome assembly (referred to as v2.0), we were able to accurately predict the physical interval of the RphHEB locus. A 137 kb (640,562,732–640,699,963) interval between the flanking markers (M7 and M12) as determined based on Morex v1.0 corresponded to 217 kb (573,253,857–573,471,458) in v2.0. The quality of genome assemblies within this region on chromosome 5HL was determined for both Morex v1.0 and v2.0 using the genomic similarity search tool YASS (https://bioinfo.lifl.fr/yass/yass.php) and visualized using a dot plot analysis. The analysis revealed a putative 310 kb inversion from 640.59 to 640.90 Mb (Fig. 4). When the region between M7 and M12 was investigated for high confidence (HC) genes, only two HC genes were detected in Morex v1.0, while 10 HC genes were found in v2.0. Therefore, Morex v2.0 was used as a road map to design more markers between M7 and M12. Finally, six markers (between M7 and M12) within the 217 kb region were developed and used to genotype three critical recombinants (1749, 1819 and 1859) (Table 5). This genotypic analysis led to the finalization of two closely linked markers M8 (573.30 Mb) and M9 (573.39 Mb) that positioned RphHEB to a genetic distance of 0.4 and 0.8 cM in the F3 and narrowed the physical interval to 98.6 kb (Fig. 5).
Physical and genetic map for Rph28 based on Morex genome assembly v1 and v2. a Barley chromosome 5HL showing physical window of 47 Mb (622–669) in Morex genome assembly v1 based on tGBS markers. Figure illustrates that a region 634–642 Mb of v1 corresponds to 569–574 Mb in Morex assembly v2. b Detail representation of 640 Mb (v1) and 573 Mb (v2) linked regions and Rph28 fine mapping based on 16 polymorphic markers. Figure shows Rph28 is fine-mapped in a physical region of 98.6 kb between M8 (0.4 cM) and M9 (0.8 cM) at 573.30 and 573.39 Mb, respectively. Five HC genes including two disease resistant genes highlighted in purple are shown between flanking markers. Diagram generated using the software Pretzel (Keeble-Gagnere et al. 2019) and Mapchart 2.32 (Voorrips 2002)
In order to further increase the map resolution at the RphHEB locus, M8 and M9 were used as flanking markers to genotype a further 970 F2 plants derived from a cross between Flagship and HEB-04-101 and three recombinants were identified. Progeny testing and phenotyping of these three recombinants with pt. 5457 P+ allowed mapping of RphHEB at a genetic distance of 0.05 cM between M8 and M9 in the F2. The physical window remained the same (98.6 kb) because 14 more markers (10 designed within gene r2.5HG0437510 and four within r2.5HG0437530) between M8 and M9 amplified DNA of Morex but did not amplify DNA from HEB-04-101.
Gene annotation
We compared and analysed CDS HC genes in the 98.6 kb region between M8 and M9 in Morex v2.0 2019 (https://webblast.ipk-gatersleben.de/barley_ibsc/). Gene annotation revealed the presence of five HC genes based on the v2.0 2019 Morex reference genome (Table 6). Among these five predicted genes, two (r2.5HG0437510 and r2.5HG0437530) are predicted to encode NLR (nucleotide binding-site and leucine-rich repeat) immune receptors and were annotated as resistance-like proteins (https://doi.ipk-gatersleben.de/DOI/83e8e186-dc4b-47f7-a820-28ad37cb176b/d1067eba-1d08-42e2-85ec-66bfd5112cd8/2). Fourteen more CAPS markers were designed within the sequence of these two NLR gene candidates. PCR amplification was attempted using these 14 markers on HEB-04-101 DNA, using Morex as a control. All primer pairs successfully amplified products of expected size using Morex genomic DNA; however, none of the primers amplified products using the resistant parent (HEB-04-101). Similarly, an attempt was made to amplify PCR products from several other wild barley leaf rust resistant lines, HEB-05-053, HEB-03-055 and HEB-04-106 (Davinder Singh, unpublished). PCR amplification was unsuccessful on all HEB lines, suggesting possible sequence variation or the absence of these resistance genes in wild barley relative to the Morex reference. To explain the apparent sequence variation between the HEB lines and Morex at the RphHEB locus, the entire gene sequence (retrieved from IPK) from the two NLR genes in the target region was used as a query and compared to the homologous region of the recently sequenced wild barley reference genome-WB1 (Liu et al. 2020) using BLASTn tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?BLAST_SPEC=blast2seqandLINK_LOC=align2seqandPAGE_TYPE=BlastSearch). Deletion of 912 bp was detected in one of the resistance genes (r2.5HG0437510) in Morex relative to WB1, suggesting the possibility of an insertion/deletion in RphHEB that may have led to amplification failure of markers. It is also possible that the variation detected between WB1 and Morex to the NLR genes is completely different to variation at the RphHEB locus.
Discussion
Several previous studies (Russell et al. 2004; Jakob et al. 2014; Wang et al. 2015) have demonstrated that Hvs has a greater genetic diversity than cultivated barley H. vulgare because of its long co-evolution with various pathogens in nature. At the same time, genetic diversity of cultivated barley has been reduced over time as a consequence of domestication, selective breeding, or both (Badr and El-Shazly 2012). This highlights the need to explore untapped diversity in wild barley progenitors to broaden the genetic diversity in cultivated barley. This is especially so in the case of resistance to biotic stress, given that several studies have established that wild barley is a rich source of resistance genes to rust pathogens (Moseman et al. 1990; Fetch et al. 2003; Steffenson et al. 2007).
Maurer et al. (2015) developed a NAM population- ‘Halle Exotic Barley 25’ (HEB-25) from initial crosses between the spring barley elite cultivar Barke (H. vulgare) and 25 highly divergent exotic barley accessions (24 wild barley accessions of Hvs and one Tibetan H. vulgare ssp. agriocrithon accession). The HEB-25 population was introduced from Germany to Australia to assess its value in barley breeding. Preliminary rust screening of 1420 HEB lines identified over 100 that were highly resistant in both the greenhouse and the field. The present study reports on the characterization and fine mapping of a new seedling Rph gene (tentatively designated as RphHEB) in one of these lines, HEB-04-101, which was shown to be effective to a wide array of Australian P. hordei pathotypes. Using a selective genotyping approach, RphHEB was localized to the telomeric region of the long arm of chromosome 5H within an interval of 47 Mb.
Of all the Rph genes identified to date, Rph2 (Borovkova et al. 1997; Franckowiak et al. 1997) and Rph20 (an adult plant resistance gene reported by Hickey et al. 2011) are located on chromosome 5HS, while the alleles Rph9, Rph9.am, Rph12 (Borovkova et al. 1998; Dracatos et al. 2014) and Rph25 (Kavanagh et al. 2017) are located on chromosome 5HL. The pathotype used in this study (5457 P+) is virulent on all five alleles located on chromosome 5H that confer seedling resistance to P. hordei (viz. Rph2, Rph9/Rph9.am/Rph12 and Rph25), demonstrating that the leaf rust resistance in HEB-04-101 is distinct from them. Previous genome wide association studies by Vatter et al. (2018) identified a QTL for leaf rust resistance (QPh.5H-1) in the same HEB-25 population on chromosome 5HL; a single, highly significant SNP marker- i_SCRI_RS_212784 positioned QPh.5H-1 at a physical location of 534,723,802 bp based on the Morex v1.0 assembly. The physical position of QPh.5H-1 does not overlap with the 47 Mb physical region for the RphHEB locus identified in the present study, indicating that Qph.5H-1 and RphHEB are distinct. While Jin et al. (1996) reported a very distant linkage (30.4 ± 4.5%) between Rph13 in PI531849 and Rph9 (on chromosome 5H) in Hor2596, two very recent studies confirmed that Rph13 actually maps to the long arm of chromosome 3H and not 5H (Jost et al. 2020; Martin et al. 2020).
Morex references v1.0 and v2.0 were used to design markers in a 47 Mb region identified through a next-generation sequencing approach. The results of this study revealed the usefulness of the Morex reference for molecular marker development and fine mapping of resistance genes. Recombination-based mapping approach was used to localize RphHEB to the telomeric region of chromosome 5HL. A high number of recombination events was observed in the region carrying RphHEB, which facilitated narrowing the interval to 98.6 kb in a population size of 125 individuals. Although F2 population was screened with the flanking markers M8 and M9 and three recombinants were detected, the target interval could not be reduced further as the markers developed between M8 and M9 did not amplify using DNA from the RphHEB parent. Despite the availability of the Hvs WB1 sequence (Liu et al. 2020), a lack of sequence information for the resistant parent HEB-04-101 precluded narrowing the region further.
In the present study, the final interval of 98.6 kb carried five high confidence candidate genes of which r2.5HG0437470 and r2.5HG0437480 were annotated as zinc finger proteins and a third gene r2.5HG0437490 was predicted to encode an actin depolymerizing factor (ADF). The remaining two genes-r2.5HG0437510 and r2.5HG0437530 annotated in the interval belong to the NLR gene family, which is the largest class of resistance genes characterized in plants so far (Grant et al. 1998; Jones et al. 2016). Most rust resistance genes isolated from cereals to date belong to the NLR family, including genes Rph1 (Dracatos et al. 2019) and Rph15 (Chen et al. 2020) in barley, and Lr21 (Huang et al. 2003) and Sr33 (Periyannan et al. 2013) in wheat. The two NLR genes identified in our target interval are the most likely candidates to underlie the leaf rust resistance in HEB-04–101. A third gene encoding for ADF is also a possible candidate for RphHEB. ADFs are encoded by genes that play a crucial role in defence related mechanisms in plants (Huang et al. 2020), for example the stem rust resistance gene Rpg4 (resistance to Puccinia graminis) in barley encodes an ADF protein (Kleinhofs et al. 2009).
Unsuccessful PCR amplification of HEB-04-101 with 14 markers (developed between M8 and M9) within two NLR genes suggests a possible variation between sequence of these resistance genes in Morex and wild barley as the same markers within these high confidence genes were successfully amplified on Morex DNA. Several previous studies suggest that repeated sequences within NLR genes can give rise to structural variations that lead to the evolution of new resistance genes (Hulbert 1997; Ellis and Jones 1998; Tamborski and Krasileva 2020). On comparing sequences from the two NLR genes in the target region with the recently sequenced wild barley reference genome-WB1, a sequence variation (912 bp deletion) was detected in gene r2.5HG0437510.1, suggesting the possibility of sequence variation at RphHEB locus that may have contributed to the amplification failure of the NLR gene markers on the resistant parent. This led to the conclusion that the RphHEB locus may not be present in Morex. It is also possible that genes other than those that were annotated in the interval of the resistant parent -HEB-04-101, are present. A lack of sequence information for HEB-04-101 and variation between the wild barley and Morex reference genomes impeded further marker development in the 98.6 kb interval. The future availability of sequence information for HEB-04-101 resistant accession will play an important role in identifying the gene conferring RphHEB mediated resistance. Various rapid cloning strategies such as MutChromSeq (Sandnchez-Martin et al. 2016) which has been recently used to clone Rph1 (Dracatos et al. 2019) and MutRenSeq (Steuernagel et al. 2017) can be employed to fully understand the nature and structure of the RphHEB locus.
Various Rph genes have been identified and characterized from wild relatives of barley, but very few have been effectively utilized in breeding programmes due to the problem of linkage drag (Summers and Brown 2013). Introgressing RphHEB in barley breeding programmes will be more efficient as the donor line HEB-04-101 has been crossed with the barley cultivar Barke during the development of the HEB-25 population (Vatter et al. 2018). Furthermore, to develop the mapping population used to characterize the RphHEB locus, the Australian barley cultivar Flagship was intentionally used as a leaf rust susceptible parent. Flagship is an early- to mid-season Australian malting variety carrying the APR gene Rph20, which is also resistant to several other barley diseases such as cereal cyst nematode, spot- and net-form of blotch, and scald. Several lines have been isolated from the mapping population carrying both the RphHEB and Rph20 resistance genes in the Flagship background. It is likely that several of these lines will have reduced linkage drag from the wild progenitor donor of the RphHEB resistance and will therefore have tremendous potential for increased diversification of Australian and global barley breeding programmes. Although RphHEB is widely effective to Australian pathotypes of P. hordei, its effectiveness to populations of P. hordei outside of Australia is unknown. It will be therefore very useful in future to test RphHEB stocks generated in this study with an array of global P. hordei pathotypes to predict value of this gene in resistance breeding at a continental scale.
In conclusion, this study characterized a new gene (RphHEB) conferring seedling resistance to leaf rust, mapped it to the long arm of chromosome 5H using targeted genotype by sequencing approach and demonstrated its distinctiveness from all other Rph genes mapped to the same chromosome. Using the recently available Morex reference genome and a recombination-based mapping approach, RphHEB locus was fine-mapped to a 98.6 kb physical interval and in the process closely linked CAPS marker M9 was identified that has potential for marker assisted selection (MAS) of the gene. Although M9 is the marker closely linked to RphHEB, yet it is not a co-segregating marker and hence may provide false positive or negative results. Marker M9 was validated on 80 Australian barley cultivars, and based on M9-marker genotyping, RphHEB was present in 12 cultivars out of 80 (data not presented). As these cultivars are susceptible and unlikely carry RphHEB, it is concluded that M9 is likely producing some false positives and the marker has about 85 percent accuracy for MAS. We are currently developing a chemically induced mutant population to identify knockout mutants to functionally verify the candidates identified in the present study and facilitate the cloning of the RphHEB. As RphHEB is a new and distinct leaf rust resistance locus, the gene symbol Rph28 is recommended for RphHEB in accordance with the rules and cataloguing system of barley gene nomenclature.
References
Badr A, El-Shazly H (2012) Molecular approaches to origin, ancestry and domestication history of crop plants: Barley and clover as examples. J Genet Eng Biotechnol 10:1–12
Borovkova IG, Jin Y, Steffenson BJ (1998) Chromosomal location and genetic relationship of leaf rust resistance genes Rph9 and Rph12 in barley. Phytopathology 88:76–80
Borovkova IG, Steffenson BJ, Jin Y, Kilian A, Kleinhofs A, Blake TK (1997) Identification and mapping of a leaf rust resistance gene in barley line Q21861. Genome 40:236–241
Brooks WS, Griffey CA, Steffenson BJ, Vivar HE (2000) Genes governing resistance to Puccinia hordei in thirteen spring barley accessions. Phytopathology 90:1131–1136
Chen C, Clark B, Martin MJ, Matny ON, Steffenson BJ, Franckowiak JD, Mascher M, Singh D, Perovic D, Richardson T, Periyannan S, Lagudah ES, Park RF, Dracatos PM (2020) Ancient BED-domain-containing immune receptor from wild barley confers widely effective resistance to leaf rust. BioRxiv.
Cotterill PJ, Rees RG, Platz GJ, Dill-Macky R (1992) Effects of leaf rust on selected Australian barleys. Aust J Exp Agric 32:747–751
Darrier B, Russell J, Milner SG, Hedley PE, Shaw PD, Macaulay M, Ramsay LD, Halpin C, Mascher M, Fleury DL, Langridge P (2019) A comparison of mainstream genotyping platforms for the evaluation and use of barley genetic resources. Front Plant Sci 10:544
Dracatos PM, Bartos J, Elmansour H, Singh D, Karafiatova M, Zhang P, Steuernagel B, Svačina R, Cobbin JC, Clark B, Hoxha S, Khatkar MS, Dolezel J, Wulff BB, Park RF (2019) The coiled-coil NLR Rph1, confers leaf rust resistance in barley cultivar Sudan. Plant Physiol 179:1362–1372
Dracatos PM, Khatkar MS, Singh D, Park RF (2014) Genetic mapping of a new race specific resistance allele effective to Puccinia hordei at the Rph9/Rph12 locus on chromosome 5HL in barley. BMC Plant Biol 14:1598
Ellis J, Jones D (1998) Structure and function of proteins controlling strain-specific pathogen resistance in plants. Curr Opin Plant Biol 1:288–293
Fazlikhani L, Keilwagen J, Kopahnke D, Deising H, Ordon F, Perovic D (2019) High resolution mapping of RphMBR1012 conferring resistance to Puccinia hordei in barley (Hordeum vulgare L.). Front Plant Sci 10:640
Fetch TG Jr, Steffenson BJ, Nevo E (2003) Diversity and sources of multiple disease resistance in Hordeum spontaneum. Plant Dis 87:1439–1448
Franckowiak JD, Jin Y, Steffenson BJ (1997) Recommended allele symbols for leaf rust resistance genes in barley. Barley Genetics Newsletter (USA).
Fulton TM, Chunwongse J, Tanksley SD (1995) Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol Report 13:207–209
Golegaonkar PG, Singh D, Park RF (2009) Evaluation of seedling and adult plant resistance to Puccinia hordei in barley. Euphytica 166:183–197
Grant MR, McDowell JM, Sharpe AG, de Torres ZM, Lydiate DJ, Dangl JL (1998) Independent deletions of a pathogen-resistance gene in Brassica and Arabidopsis. Proc Natl Acad Sci 95:15843–15848
Hickey LT, Lawson W, Platz GJ, Dieters M, Arief VN, German S, Fletcher S, Park RF, Singh D, Pereyra S, Franckowiak J (2011) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123:55–68
Huang J, Sun W, Ren J, Yang R, Fan J, Li Y, Wang X, Joseph S, Deng W, Zhai L (2020) Genome-wide identification and characterization of actin-depolymerizing factor (ADF) family genes and expression analysis of responses to various stresses in Zea mays L.. Int J Mol Sci 21:1751
Hulbert SH (1997) Structure and evolution of the rp1 complex conferring rust resistance in maize. Annu Rev Phytopathol 35:293–310
Huang L, Brooks SA, Li W, Fellers JP, Trick HN, Gill BS (2003) Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 164:655–664
Jakob SS, Rodder D, Engler JO, Shaaf S, Ozkan H, Blattner FR, Kilian B (2014) Evolutionary history of wild barley (Hordeum vulgare subsp. spontaneum) analyzed using multilocus sequence data and paleodistribution modeling. Genome Biol Evol 6:685–702
Jin Y, Cui GH, Steffenson BJ, Franckowiak JD (1996) New leaf rust resistance genes in barley and their allelic and linkage relationships with other Rph genes. Phytopathology 86:887–890
Jones JD, Vance RE, Dangl JL (2016) Intracellular innate immune surveillance devices in plants and animals. Science 354:6316
Jost M, Singh D, Lagudah E, Park RF, Dracatos P (2020) Fine mapping of leaf rust resistance gene Rph13 from wild barley. Theor Appl Genet 133:1887–1895
Kavanagh PJ, Singh D, Bansal UK, Park RF (2017) Inheritance and characterization of the new and rare gene Rph25 conferring seedling resistance in Hordeum vulgare against Puccinia hordei. Plant Breed 136:908–912
Kayam G, Brand Y, Faigenboim-Doron A, Patil A, Hedvat I, Hovav R (2017) Fine mapping the branching habit trait in cultivated peanut by combining bulked segregant analysis and high-throughput sequencing. Front Plant Sci 8:467
Keeble-Gagnere G, Isdale D, Suchecki RL, Kruger A, Lomas K, Carroll D, Li S, Whan A, Hayden M, Tibbits J (2019) Integrating past, present and future wheat research with Pretzel. doi:https://doi.org/10.1101/517953. https://plantinformatics.io/.
Kleinhofs A, Brueggeman R, Nirmala J, Zhang L, Mirlohi A, Druka A, Rostoks N, Steffenson BJ (2009) Barley stem rust resistance genes: structure and function. Plant Genome 2:109–120
Liu M, Li Y, Ma Y, Zhao Q, Stiller J, Feng Q, Tian Q, Liu D, Han B, Liu C (2020) The draft genome of a wild barley genotype reveals its enrichment in genes related to biotic and abiotic stresses compared to cultivated barley. Plant Biotechnol J 18:443–456
Liu WC, Liu ZD, Huang C, Lu MH, Liu J, Yang QP (2016) Statistics and analysis of crop yield losses caused by main diseases and insect pests in recent 10 years. Plant Prot 42:1–9
Martin MJ, Chicaiza O, Caffarel JC, Sallam AH, Druka A, Waugh R, Ordon F, Kopahnke D, Keilwagen J, Perovic D, Fetch TG Jr, Jin Y, Franckowiak JD, Steffenson BJ (2020) Development of barley introgression lines carrying the leaf rust resistance genes Rph1 to Rph15. Crop Sci 60:282–302
Mascher M (2019) Pseudomolecules and annotation of the second version of the reference genome sequence assembly of barley cv. Morex [Morex V2]. doi:https://doi.org/10.5447/IPK/2019/8.
Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V, Dockter C, Hedley PE, Russell J, Bayer M (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544:427–433
Maurer A, Draba V, Jiang Y, Schnaithmann F, Sharma R, Schumann E, Kilian B, Reif JC, Pillen K (2015) Modelling the genetic architecture of flowering time control in barley through nested association mapping. BMC Genomics 16:1–12
Moseman JG, Nevo E, El-Morshidy MA (1990) Reactions of Hordeum spontaneum to infection with two cultures of Puccinia hordei from Israel and United States. Euphytica 49:169–175
Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18:613–615
Park RF, Golegaonkar PG, Derevnina L, Sandhu KS, Karaoglu H, Elmansour HM, Dracatos PM, Singh D (2015) Leaf rust of cultivated barley: pathology and control. Annu Rev Phytopathol 53:565–589
Park RF, Karakousis A (2002) Characterization and mapping of gene Rph19 conferring resistance to Puccinia hordei in the cultivar ‘Reka 1’and several Australian barleys. Plant Breed 121:232–236
Periyannan S, Moore J, Ayliffe M, Bansal U, Wang X, Huang L, Deal K, Luo M, Kong X, Bariana H, Mago RM, McIntosh R, Dodds P, Dvorak J, Lagudah E, (2013) The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science 341:786–788
Rothwell CT, Singh D, Dracatos PM, Park RF (2020) Inheritance and characterization of Rph27: a third race-specific resistance gene in the barley cultivar Quinn. Phytopathology 110:1067–1073
Russell J, Booth A, Fuller J, Harrower B, Hedley P, Machray G, Powell W (2004) A comparison of sequence-based polymorphism and haplotype content in transcribed and anonymous regions of the barley genome. Genome 47:389–398
Sandnchez-Martin J, Steuernagel B, Ghosh S, Herren G, Hurni S, Adamski N, Vrána J, Kubalakova M, Krattinger SG, Wicker T, Dolezel J, Keller B, Wulff BH (2016) Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol 17:221
Sandhu KS, Forrest KL, Kong S, Bansal UK, Singh D, Hayden MJ, Park RF (2012) Inheritance and molecular mapping of a gene conferring seedling resistance against Puccinia hordei in the barley cultivar Ricardo. Theor Appl Genet 125:1403–1411
Shavrukov Y (2014) Why are the development and application of CAPS markers so different in bread wheat compared to barley. Cleaved Amplified Polymorphic Sequences (CAPS) Markers. Plant Biol, 211–232.
Shavrukov Y (2016) Comparison of SNP and CAPS markers application in genetic research in wheat and barley. BMC Plant Biol 16:11
Singh D, Dracatos PM, Derevnina L, Zhou M, Park RF (2015) Rph23: a new designated additive adult plant resistance gene to leaf rust in barley on chromosome 7H. Plant Breed 134:62–69
Singh D, Dracatos PM, Loughman R, Park RF (2017) Genetic mapping of resistance to Puccinia hordei in three barley doubled-haploid populations. Euphytica 213:16
Steffenson BJ, Olivera P, Roy JK, Jin Y, Smith KP, Muehlbauer GJ (2007) A walk on the wild side: mining wild wheat and barley collections for rust resistance genes. Aust J Agric Res 58:532–544
Steuernagel B, Witek K, Jones JD, Wulff BB (2017) MutRenSeq: a method for rapid cloning of plant disease resistance genes. In: Wheat rust diseases. Methods in molecular biology, vol 1659. Humana Press, New York, pp 215–229. https://doi.org/10.1007/978-1-4939-7249-4_19
Summers RW, Brown JKM (2013) Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant Pathol 62:115–121
Tamborski J, Krasileva KV (2020) Evolution of plant NLRs: from natural history to precise modifications. Annu Rev Plant Biol 71:355–378
Vatter T, Maurer A, Perovic D, Kopahnke D, Pillen K, Ordon F (2018) Identification of QTL conferring resistance to stripe rust (Puccinia striiformis f. sP. hordei) and leaf rust (Puccinia hordei) in barley using nested association mapping (NAM). PLoS ONE 13:1
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Heredity 93:77–78
Von Bothmer R, Sato K, Knüpffer H, van Hintum T (2003) Barley diversity-an introduction. Divers Barley 1:3–8
Wang Y, Ren X, Sun D, Sun G (2015) Origin of worldwide cultivated barley revealed by NAM-1 gene and grain protein content. Front Plant Sci 6:803
Weerasena JS, Steffenson BJ, Falk AB (2004) Conversion of an amplified fragment length polymorphism marker into a co-dominant marker in the mapping of the Rph15 gene conferring resistance to barley leaf rust, Puccinia hordei Otth. Theor Appl Genet 108:712–719
Ziems LA, Hickey LT, Platz GJ, Franckowiak JD, Dracatos PM, Singh D, Park RF (2017) Characterization of Rph24: a gene conferring adult plant resistance to Puccinia hordei in barley. Phytopathology 107:834–841
Acknowledgements
This research was partially supported by the Grain Research Development Corporation (GRDC). Authors would also like to thank Mr Matthew Williams, Mr Gary Standen and Ms Bethany Clark for their technical support.
Author information
Authors and Affiliations
Contributions
MM led the studies and DS designed the studies. DS, PD and RFP supervised the studies. TM, AP, AM and KP provided critical material/germplasm. MM, PD, TK, KF and MP performed recombination and/or tGBS analysis. MM and DS performed phenotyping and data analysis. MM and DS wrote the MS, and all authors contributed to the MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Kevin Smith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehnaz, M., Dracatos, P., Pham, A. et al. Discovery and fine mapping of Rph28: a new gene conferring resistance to Puccinia hordei from wild barley. Theor Appl Genet 134, 2167–2179 (2021). https://doi.org/10.1007/s00122-021-03814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03814-1