Abstract
Key message
Three QTL for resistance to leaf blast were identified on chromosomes 1, 2, and 8 of the foxtail millet cultivar Yugu 5.
Abstract
Leaf blast disease of foxtail millet (Setaria italica) is caused by Pyricularia spp., can infect all the aboveground parts of plants, and is the most frequently observed blast disease in China. Lack of information on genetic control of disease resistance impedes developing leaf blast-resistant cultivars. An F6 recombinant inbred line (RIL) population from the cross Yugu 5 × Jigu 31 was phenotyped for its reactions to leaf blast in six field trials in the naturally diseased nurseries. An ultra-density genetic linkage map was constructed using 35,065 single nucleotide polymorphism (SNP) markers generated by sequencing of the RIL population. Three QTL, QLB-czas1, QLB-czas2, and QLB-cazas8, were detected in the genomic intervals of 276.6 kb, 1.62 Mb, and 1.75 Mb on chromosomes 1, 2, and 8 of Yugu 5, which explained 14–17% (2 environments), 9% (5 environments), and 12–20% (6 environments) of the phenotypic variations. Bulked segregant analysis (BSA) and RNA sequencing (BSR-Seq) method identified common SNPs that fell in the genomic region of QLB-czas8, providing additional evidence of localization of this QTL. Three and 19 predicted genes were annotated to be associated with disease resistance in the genomic intervals for QLB-czas2 and QLB-czas8. Due to their unique positions, these QTL appear to be new loci conferring resistance to leaf blast. The identification of these new resistance QTL will be useful in cultivar development and the study of the genetic control of blast resistance in foxtail millet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foxtail millet [Setaria italica (L.) Beauv.] has been cultivated as a staple food crop in China for thousands of years (Yang et al. 2012). It is well known for its adaptability to diverse agro-ecological conditions, especially in fields with poor nutrition and semi-dry environments (Dwivedi et al. 2012). This small grained cereal crop is also consumed for food and feed in other countries of East Asia, the Americans, Africa, and Europe (Singh et al. 2017a).
Among various diseases that occur in foxtail millet, blast disease poses a severe constraint on yield in many producing regions. In China, blast disease of foxtail millet was first reported in the 1930s (Lin 1948). Historically, epidemics of leaf blast had been reported throughout the major spring foxtail millet-growing regions (Liang et al. 1959; Yang 1962; Cao and Yan 1982). It is currently one of the major yield-limiting diseases in most spring and summer foxtail millet-producing areas in the northern part of China (Li et al. 2016; Ren et al. 2017; Nan et al. 2018). Although blast disease can infect different tissues of foxtail millet plants, the economic losses ultimately arise from the death of spikelets and even whole panicles, resulting in grain yield reductions. During the 1960s, yield losses of 3–50% caused by leaf blast were recorded in southeastern Shanxi province (Yang 1962). A range of 20–30% in yield reductions was later reported in infected fields in the major foxtail millet production regions of China (Yu 1978). When severe, the blast disease can cause up to a 30–40% loss of grain yield in other foxtail millet-producing regions of the world (Nagaraja et al. 2007).
Pyricularia oryzae Cavara was first reported from diseased foxtail millet plants (Kawakami 1902). However, Nishikado (1917) determined that P. setariae Nisikado was the cause of foxtail millet blast. Pyricularia species also incites blast disease in finger millet [Eleusine coracana (L.) Gaertn.] and pearl millet [Pennisetum glaucum (L.) R. Br.] (Viswanath and Seetharam 1989). Pyricularia setariae and P. grisea (Cooke) Sacc. were reported to cause leaf blast in foxtail millet in China (Liang et al. 1959; Li et al. 2020). Variation and differentiation of the physiological races of Pyricularia species from different foxtail millet production areas of the country have been described (Yan et al. 1985; Li et al. 2016; Ren et al. 2017). The use of some pesticides for controlling blast has resulted in resistance to fungicides (e.g., strobilurin fungicides) in the pathogen (Turaki et al. 2014; Castroagudín et al. 2015). This encouraged the use of genetic resistance for the management of diseases caused by Pyricularia spp.
Differences in the resistance of cultivars to millet blast were observed in early studies (Liang et al. 1959; Zhu 1964). From the 1980s to 2010s, thousands of foxtail millet cultivars and breeding lines were assessed for their resistance to leaf blast in greenhouse and/or field tests, and some highly resistant accessions were identified (Wang et al. 1985a, b; Wu 1985; Yan et al. 1988; Liu et al. 1990; Wei et al. 1999). Because of the increasing importance of leaf blast, tests of reaction to the disease have been mandated for the National Registration Trials for the commercial release of foxtail millet cultivars (Zhang et al. 2017). Assessment of 267 commercial cultivars of foxtail millet demonstrated that most were susceptible and only 9 (3.4%) and 33 (12.4%) were highly or moderately resistant (Dong et al. 2015). Among 888 foxtail millet accessions from different geographic regions in the world, only 14 accessions (1.6%) were highly resistant to leaf blast in a greenhouse test (Li et al. 2020). Genetic variation in resistance to leaf blast has also been observed among cultivars from the European and Asian countries (Nakayama et al. 2005). Several blast-resistant genotypes were identified in a core collection of 155 foxtail millet germplasm accessions from the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) gene bank at Patancheru, India (Sharma et al. 2014). Some Indian foxtail millet accessions have been tested for their reactions to leaf blast, resulting in the identification of several blast-resistant genotypes for breeding disease-resistant cultivars (D’Souza and Gaikwad 1984; Munirathnam et al. 2015; Rajesh et al. 2019).
Genetic dissection of resistance to leaf blast has been widely studied in rice (Oryza sativa L.), wheat (Triticum aestivum L.), finger millet, and pearl millet (Ramakrishnan et al. 2016; Devanna and Sharma 2018; Sanghani et al. 2018). A number of resistance genes conferring blast resistance have been characterized in rice and wheat (Wang et al. 2017; Devanna and Sharma 2018). However, only a few studies reported localization of genes or QTL for resistance to blast in the millet crops, such as finger millet (Reddy and Sivaramakrishnan 2017) and pearl millet (Sanghani et al. 2018). Similar studies have not reported for foxtail millet until the most recent genome-wide association study (GWAS) project was performed (Li et al. 2020). That study identified two genomic regions associated with leaf blast resistance on chromosomes 2 and 9. The Chinese summer foxtail millet cultivars Yugu 5 and Jigu 31 display contrasting responses to leaf blast (Dong et al. 2015). A recombinant inbred line (RIL) population was developed by crossing the leaf blast-resistant cultivar Yugu 5 with the susceptible cultivar Jigu 31. The objective of the present study was to identify the genetic loci conferring leaf blast resistance in Yugu 5 by means of genome re-sequencing and BSR-Seq approaches.
Materials and methods
Plant materials
Yugu 5 (pedigree: Yugu 1 × An 096) was crossed to Jigu 31 (pedigree: Jigu 19 × 1302-9) in the winter growing season of 2013 in Sanya, Hainan province. The single-seed descent method was used to develop an F6 RIL population consisting of 305 lines by alternatively selfing in the summer cropping seasons at Cangzhou, Hebei province, and in the winter cropping season in Sanya, Hainan province.Phenotyping was conducted with 305 lines whiles genotyping, linkage analysis and QTL mapping were carried out with 150 randomly selected lines. Only lines that showed uniform stand within a plot and did not have any missing data across the different trials were selected for the molecular work. The parents Yugu 5 and Jigu 31, as well as the susceptible control Jigu 19, were included in all the blast resistance assessments and genotyping analysis.
Field assessments for reactions to the natural infection of leaf blast
Reactions of the 305 RILs, together with the parents and the susceptible control, to leaf blast were assessed in field nurseries naturally infected by the leaf blast pathogen in Hebei province at two sites, Cangzhou (38°24′ N, 116°76′ E) in 2016 (2016CZ), 2017 (2017CZ), and 2018 (2018CZ), and Dongguang (37°76′ N, 116°60′ E) in 2017 (2017DG) during the summer cropping seasons. This set of plant materials were also phenotyped for the leaf blast resistance in Sanya, Hainan province (18°36′ N, 109°18′ E) in 2016 (2016SY) and 2017 (2017SY) during the winter cropping seasons. The causal fungi in Hebei and Hainan provinces were characterized as P. grisea (Li et al. 2016, 2020). The pathogen also was isolated from the diseased leaves of plants grown in Cangzhou and Sanya, which confirmed the identity of the fungus based on colony growth and spore morphology (data not shown). A randomized complete block design with two replicates was used in each trial to arrange the RILs, the parents, and the control cultivar. Each plot consisted of a single row 5 m in length with 0.74-m spacings between rows. At the 3-leaf stage, plants were manually thinned to 75 plants per row. At the grain filling stage when the disease symptoms were severe on the leaves of the susceptible control Jigu 19, disease reaction scores (DRS) of at least 10 plants per plot were rated on a 0–4 scale based on the size of lesions on leaf blades as described previously (Nakayama et al. 2005). Resistant and susceptible lines produced DRS 0–2 and 3–4, respectively.
High-throughput sequencing, SNP calling and genotyping
Genomic DNA was extracted from leaf tissues using the cetyltrimethylammonium bromide (CTAB) method, sheared into ~ 500 bp fragments with an S2/E210 Ultrasonicator (Covaris, Woburn, MA, USA), and end-repaired. The libraries for 150 randomly selected RILs and both parents were constructed for sequencing analysis on the Illumina HiSeq2500 (Illumina, Inc., San Diego, CA, USA). After removing the low-quality reads (quality score < 20e), clean reads were aligned to the S. italica reference genome assembly (https://www.ncbi.nlm.nih.gov/genome/?term=foxtail+millet) using the Burrows Burrows-Wheeler Wheeler Aligner (BWA) software (Li et al. 2009). Local realignment and base recalibration were conducted using the Genome Analysis Toolkit (GATK) v3.6 software (McKenna et al. 2010). A set of single nucleotide polymorphisms (SNPs) was produced by combining GATK and SAMtools (Li and Durbin 2009). The SNP loci between the parents and the RILs were identified using GATK with the default parameters. Polymorphic SNPs between the parents were used for bin calling. Genotypes of the RILs were determined on the basis of the SNP positions. The SNP datasets were merged using the “pileup” function of SAMtools software package (Li et al. 2009), and only biallelic SNPs were maintained. The SNPs that fell in the following categories were excluded from further analysis: (1) those with < 4 coverage in parents or < 15 SNPs within a scaffold and (2) those with an extreme segregation distortion (P < 0.01). Based on the parental SNPs, the variant call format (VCF) file was used for genotyping the RILs. Only genotype aa × bb was further used in this study (Hu et al. 2018).
Construction of bin and linkage maps and QTL analysis
The maps of the RILs were aligned, the genotypes were compared across a 3-kb interval, and adjacent intervals with identical genotypes across all RILs were combined into a recombination bin. A linkage map was established from the recombination bins that were used as genetic markers via software HighMap (Liu et al. 2014). Based on their positions in the foxtail millet genome, marker loci were assigned into linkage groups (LGs). The modified logarithm of odd (MLOD) scores between markers were used to confirm the effectiveness of markers for each LG, and those markers with MLOD < 5 were removed before ordering the bin markers using HighMap (Liu et al. 2014). The genotyping errors within LGs were corrected to ensure the establishment of the high-density and high-quality map. Distances between markers on the map were estimated using the Kosambi mapping function (Kosambi 1944).
The QTL for resistance to leaf blast were detected using the composite interval mapping (CIM) method in Windows QTL Cartographer 2.5 (Wang et al. 2010). QTL were called using the forward & backward regression method at the logarithm of odds (LOD) threshold of 3.0 based on 3000 replications at α = 0.05 and a walk speed of 1.0 cM. The determination coefficient (R2%) was calculated to indicate the percentage of the phenotypic variance explained by the effects of a specific QTL.
BSR-Seq analysis
Based on the phenotypes examined in all the trials, 30 resistant and 30 susceptible RILs that were consistent among all the 6 field tests were selected from the 305 RILs to form the resistant and susceptible bulked samples. Approximately equal-sized leaf segments from each representative plant were separately pooled for RNA-sequencing on the Illumina HiSeq 4000 platform (Illumina, Inc., San Diego, CA, USA) at the Beijing Northern Genome Research Technology (Beijing, China). Sequencing reads generated were subjected to quality control to remove adapter sequences and low-quality reads using the Trimmomatic v036 software with the default parameters (Bolger et al. 2014). High-quality reads were aligned to the S. italica reference genome assembly (https://www.ncbi.nlm.nih.gov/genome/?term=foxtail+millet) using STARv2.5.1b software with the mismatch rate of < 5% (Dobin et al. 2013). The uniquely mapped and confident alignments without PCR optical duplicates and spanning introns were used to identify SNP variants with “HaplotypeCaller” module in the GATK v3.6 software (McKenna et al. 2010). The SNP variants associated with disease resistance were determined with the criteria of P-values of the Fisher’s exact test (FET) < 1e-8 and allele frequency difference (AFD) > 0.6.
Physical mapping and gene annotation of the genomic intervals of the QTL identified
The sequences of the flanking markers of the blast resistance QTL identified were used as queries to search against the S. italica reference genome assembly (https://www.ncbi.nlm.nih.gov/genome/?term=foxtail+millet) to physically map the target QTL in the foxtail millet genome. The annotated genes in the genomic intervals between the flanking markers were retrieved. The expression patterns of the disease resistance-associated candidate genes in different foxtail millet tissues were analyzed by SIFGD (http://structuralbiology.cau.edu.cn/SIFGD/).
Statistical analysis
Analysis of variance (ANOVA) was performed for the DRS values obtained from each phenotyping trial. Pearson correlation coefficients for DRS among different trials were determined with PROC CORR. The Chi-squared (χ2) test was performed to test the goodness of fit for the observed separation of resistant and susceptible RILs from the expected separation ratios of 1:1 for single gene control or deviation from this ratio (2:1 or 3:1) for multiple gene control. All statistical analyses were performed in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Phenotypes of leaf blast resistance in the RIL population
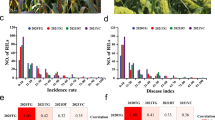
The susceptible control Jigu 19 had DRS 3 or 4 in all the trials, indicating that the disease pressure in the tested field environments was sufficient for evaluating disease reactions of the mapping population. Yugu 5 displayed a resistant phenotype with a DRS 0 at all sites, except for DRS 1 in 2017DG, while Jigu 31 always showed a susceptible phenotype with DRS 3 or 4 (Figs. 1a and b, Supplementary Table S1). Variation in the reactions to leaf blast was observed in 305 RILs (Supplementary Table S1). An ANOVA for DRS detected highly significant effects for the RILs in each trial (Supplementary Table S2). Significant effects were also observed for environments, indicating that the environment exerted an impact on the performance of leaf blast resistance. The segregation ratio of 1:1 for the resistant RILs (with DSR 0–2) to the susceptible RILs (with DSR 3–4) was observed for the trials 2016CZ, 2016SY, and 2017SY (χ21:1 = 0.24–2.22, P < 0.137–0.621) (Supplementary Table S2). However, this segregation ratio did not occur for the trials 2017CZ, 2018CZ, and 2017DG. Instead, a segregation ratio of 2:1 (resistant RILs versus susceptible RILs) was observed in those trials (χ21:1 = 0.01–3.39, P < 0.065–0.613). A ratio of 3 resistant RILs: 1 susceptible RILs was also observed for the trial 2017CZ (χ23:1 = 3.39, P < 0.065). This suggests the existence of more than one locus conferring resistance to leaf blast. Values of DRS for different environments were significantly correlated (r = 0.64–0.90, P < 0.0001) (Supplementary Table 3). This indicates a good agreement in the assessments of the RILs across different environments. The randomly selected 147 lines that were used for linkage analysis displayed similar performances in the ANOVA, segregation, and correlation analyses as the whole RIL population (Supplementary Tables 1, 2, and 3).
Re-sequencing of the parents Yugu 5 and Jigu 31
Sequencing analysis on an Illumina HiSeq platform generated 16.47 and 16.32 Gb of clean data for Yugu 5 and Jigu 31, with the average depths of about 35-fold. Using BWA, 110,071,460 and 109,003,750 reads from Yugu 5 and Jigu 31, respectively, were aligned to the foxtail millet reference genome assembly (Supplementary Table S4). Compared to the reference genome, the 2 cultivars produced 678,707 and 629,172 SNPs on different chromosomes (Fig. 2). The mean number of SNPs on each chromosome of Yugu 5 was 75,412, with a range of 34,862 on chromosome 9 to 124,286 on chromosome 3 (Supplementary Table S5). The mean number of SNPs on each chromosome of Jigu 31 was 69,908, ranging from 27,793 on chromosome 1 to 148,139 on chromosome 9.
Construction of genetic linkage map
Sequencing analysis was performed on the 150 randomly selected from the 305 RILs of cross Yugu 5 × Jigu 31. Three lines showed poor alignments with the foxtail millet reference genome, so they were excluded from further analysis. A total of 141.75 Gb of clean data were produced for the 147 RILs with the average depth of 2.17-fold (Supplementary Table S4); 6,311,904 clean reads were mapped to the reference genome. There were 886,183 SNPs between Yugu 5 and Jigu 31. Based on the variations in the sequences in Yugu 5 and Jigu 31 and the variant sequences among the RILs, 35,065 high-quality SNPs were used in the linkage analysis. A group of markers that were mapped to the same location constituted a bin or block. These SNPs were clustered in 2004 recombination bins, and they were incorporated in an ultra-dense genetic map using HighMap software. The molecular linkage map consisted of nine chromosomes with a range of 2155 (chromosome 4) to 6888 (chromosome 8) SNP markers and 160 (chromosome 7) to 370 (chromosome 1) bin markers. The map distance was 1806.77 cM with a density of 0.91 cM between adjacent bin markers (Supplementary Fig. 1 and Table S6).
Identification of QTL for resistance to leaf blast
Three QTL conferring resistance to leaf blast, designated QLB-czas1, QLB-czas2, and QLB-czas8, were identified in Yugu 5, respectively (Table 1). QLB-czas1 was detected on chromosome 1 in the marker interval of Block1537-Block1534 in 2018CZ and 2017SY (LOD = 4.8 and 5.6; R2 = 14 and 17%, respectively) (Fig. 3). QLB-czas2 was detected on chromosome 2 in the marker interval of Block5281-Block5592 in 5 of the 6 trials except for 2016SY, but the LOD values (ranging from 3.3 to 3.5) and the explained phenotypic variation (9%) were smaller than QLB-czas1 (Fig. 4). QLB-czas8 was identified on chromosome 8 in all the six trials (Fig. 5a). Peak LOD values ranging from 3.7 to 7.2 occurred at the marker interval of Block37312-Block37444 for all the environments, except for 2016SY in an interval of Block37312-Block37313 that still fell in the same genomic region as detected in other environments (Table 1). This QTL explained the blast resistant phenotypes from 12 to 20%, more effective against leaf blast than QLB-czas2.
Genetic linkage map of QLR-czas8 on chromosome 8 for leaf blast resistance in foxtail millet (a) and the positions of annotated disease resistance related genes in the target genomic region (b). The boxes indicate different classes of genes: green, disease resistance RPP13-like protein genes; blue: disease resistance response protein genes; orange: LRR receptor kinase family genes; red: LRR receptor-like serine/threonine-protein kinase family genes; and yellow: MLO-like protein 4-like gene. The differentially expressed genes associated with disease resistance are marked with asterisks (color figure online)
BSR-Seq analysis of the contrasting RNA bulks
RNA-Seq analysis generated 16,815,630 and 16,685,972 raw read pairs from the disease-resistant and disease-susceptible RNA bulks, respectively. After quality control, the disease-resistant RNA bulked sample was reduced to 16,812,502 high-quality read pairs and 10,865,246 (64.6%) of them were uniquely mapped to the S. italica reference genome assembly. The bulked RNA sample from the disease-susceptible RILs had 16,679,114 high-quality reads, of which 12,549,604 were uniquely mapped reads. Variant calling identified 87,032 SNPs and InDels between the two bulks, of which 40,760 of them met the criteria of SNP variants and were distributed on all chromosomes (Fig. 6a). The GATK software identified 518 candidate variants that were potentially associated with leaf blast resistance. These SNPs were anchored on all the chromosomes except for chromosome 5. The largest proportion of the candidate SNPs were located on chromosome 8 (315, 60.7%) (Fig. 6b). Forty-nine candidate SNP-containing sequences were anchored in the genomic region of 4,327,231–5,593,019, which fell in the genomic region of QLB-czas8 (highlighted in green in Fig. 6b). Chromosomes 1 and 2 had 31 and one candidate SNP, respectively, but none of them were anchored in the genomic regions of QLB-czas1 and QLB-czas2.
Distributions of SNPs on foxtail millet chromosomes (a) and the SNP variants on chromosome 8 generated by BSR-Seq analysis using the contrasting RNA bulks from the resistant and susceptible RILs (b). The numbers of polymorphic SNPs between the contrasting RNA bulks are shown in the brackets. The green dots indicate the common SNPs shared by the re-sequencing and BSR-Seq analyses (color figure online)
Effects of QTL on reactions to leaf blast infection in the RIL population of Yugu 5 × Jigu 31
The lines none, QLB-czas1, QLB-czas2, and QLB-czas8 and the allele combinations were identified from the RIL population of Yugu 5 × Jigu 31 based on the genotypes of the linked markers. Means of infection types of the six trials for each group of alleles are shown in Fig. 7. These QTL had an effect on reducing the infection types in reactions to leaf blast. The means of infection types for the QTL carrying lines were smaller than that of lines without any QTL. The effects of QLB-czas8 appeared to be greater than that of QLB-czas1 and QLB-czas2 as shown by smaller mean infection type. Interaction among alleles may exist since the combination of different alleles showed varied means of infection types. Since QLB-czas1 was detected in sites 2018CZ and 2017SY, mean of infection types for the lines carrying this allele (1.2 ± 1.2) was slightly smaller than that obtained from all the six trials (1.6 ± 1.2) (data not shown).
Physical mapping and analysis of the annotated genes in the candidate genomic intervals of the target QTL
The sequences of the flanking markers for the QTL identified on chromosomes 1, 2, and 8 were used to blast the foxtail millet reference genome. QLB-czas1 was physically located in a 276.6 kb genomic interval (26,283,986–26,560,597), which contained 16 predicted genes (Supplementary Table S7). None of the 14 genes with functional annotations was associated with disease resistance. The other 2 predicted genes were of unknown function. The physical location of QLB-czas2 was in a 1.62-Mb genomic interval (24,262,358–25,880,181) on chromosome 2. Among the 59 annotated genes in this genomic interval, genes SETIT_028727mg (encoding receptor-like protein kinase BRI1-like 3 like), SETIT_028674mg (putative disease resistance protein RGA4-like isoform X1), and SETIT_028986mg (probable LRR receptor-like serine/threonine-protein kinase At3g47570) were associated with disease resistance.
The target genomic region of QLB-czas8 spanned a 1.75-Mb (3,837,265–5,592,167) interval on chromosome 8. There were 133 annotated genes in this interval, which included 119 genes with annotated functions and 14 uncharacterized genes. Nineteen genes were predicted to be associated with disease resistance (Supplementary Table S8). Four, SETIT_026507mg, SETIT_027748mg, SETIT_028067mg, and SETIT_028102mg, were leucine-rich repeat (LRR) receptor kinase family genes. Six, SETIT_028378mg, SETIT_027508mg, SETIT_028294mg, SETIT_027332mg, SETIT_028142mg, and SETIT_025980mg, were LRR receptor-like serine/threonine-protein kinase family genes. Three, SETIT_026498mg, SETIT_025948mg, and SETIT_025924mg, were annotated as disease resistance RPP13-like protein genes. Five, SETIT_026915mg, SETIT_027383mg, SETIT_028306mg, SETIT_026914mg, and SETIT_026907mg, were disease resistance response protein 206-like genes. The remaining one SETIT_026238mg was an MLO-like protein 4-like gene. Some of these genes were located in different clusters in the reference genome regardless of their annotated functions (Fig. 5b).
The publicly accessed RNA-seq database was used to determine the expression patterns of the 3 and 19 disease resistance-associated genes on chromosomes 2 and 8 through SIFGD (http://structuralbiology.cau.edu.cn/SIFGD/). Two of the three annotated genes on chromosome 2 were expressed in the tassel, leaf, stem, and root tissues of foxtail millet, but none was highly expressed in the leaves (Supplementary Table S9). Among the 19 annotated genes on chromosome 8, 11 genes were observed in this database, and the other 8 genes were not observed. Four genes, SETIT_027508, SETIT_028067, SETIT_026915, and SETIT_027383, were highly expressed in the leaves. These genes were also highly expressed in other tissues. Another five genes, SETIT_028378, SETIT_027332, SETIT_026498, SETIT_025948, and SETIT_026914, were lightly expressed in leaves. Genes SETIT_028142 and SETIT_028306 were almost not expressed in leaves.
Discussion
In this study, the leaf blast resistance was characterized in foxtail millet cultivar Yugu 5. Three QTL were detected on chromosomes 1, 2, and 8 of that cultivar. QLB-czas8 was detected in all the 6 environments involved in the phenotypic evaluation, indicating that it is a stable locus for conferring leaf blast resistance. The identification of SNP loci in the same genomic interval on chromosome 8 by BSR-Seq analysis further evidenced the presence of QLB-czas8 in this genomic region. Since there was no genetic loci for leaf blast resistance on this chromosome, QLB-czas8 represents a new leaf blast resistance locus in foxtail millet. Similarly, QLB-czas1 is also a new locus because no gene for leaf blast resistance has been detected on chromosome 1; however, this QTL was only observed in 2 of the 6 environments. Another locus, QLB-czas2, was detected in five environments (except for environment 2016SY). The effects of this QTL in different environments were smaller than the other 2 loci. The location of QLB-czas2 was not compared with the previously reported genomic region associated with leaf blast resistance on chromosome 2, as the genomic information of that region was not available in the literature (Li et al. 2020). However, the annotated genes in the target genomic regions of the 2 QTL were different, implying that these QTL may not be identical even though they reside on the same chromosome.
Yugu 5 was released in Henan province in 1992 and has served as the control cultivar in the National Registration Trials from 2002 to 2007. It was commercially grown for almost three decades and still maintain its resistance to leaf blast. This was confirmed by periodically reported disease tests in fields and greenhouses across different years and locations (Ma et al. 2010; Dong et al. 2015). In the present study, Yugu 5 was resistant to leaf blast at the three locations in Hebei and Hainan provinces across three years 2016–2018. Additionally, we have noticed the leaf blast resistance of this cultivar in our field plots under natural infection conditions for many years (BH Tian, unpublished data). This provides further evidence of the effectiveness and stability of the QTL identified. The development of Yugu 5 involved Yugu 1 (Japan 60 Day × Tulong). Japan 60 Day and Yugu 1 have been frequently used as parents in breeding, leading to the release of many cultivars in the summer millet production regions, many of which were resistant to the blast disease (Wang et al. 1985a, b; Liu et al. 1996, 2006; Jiang et al. 2008). This suggests that the blast resistance in Yugu 5 was likely derived from Yugu 1. The widely use of those blast-resistant cultivars did not cause breakdown of their resistance, further offering evidence of the stability or durability of the QTL from this source.
Blast resistance in the millet crops, such as pearl millet and finger millet, was reported to be inherited in a qualitative or a quantitative mode (Gupta et al. 2012; Ramakrishnan et al. 2016; Singh et al. 2017b; Sanghani et al. 2018). Results of the phenotypes in different trials demonstrated that the resistant versus susceptible RILs of Yugu 5 × Jigu 31 segregated in a ratio of 1:1 in three environments of 2016CZ, 2016SY, and 2017SY, but in a ratio of 2:1 in the other three environments 2017CZ, 2018CZ, and 2017DG. This suggests that the resistance of Yugu 5 to leaf blast may be controlled by more than one locus, one of which is probably a major effective locus. In fact, the molecular mapping analysis using an ultra-density linkage map generated by re-sequencing of the RIL population identified three QTL on chromosomes 1, 2, and 8 that were responsible for the leaf blast resistance across different trials. When multiple QTL are detected, these QTL may not contribute equally to the phenotypic variation. So, BSA approach may detect a major QTL (Lin and Chen 2007). We performed BSA in combination with RNA-sequencing analysis and determined that chromosome 8 was most associated with leaf blast resistance. This suggests that QLB-czas8 is a major QTL, which is also supported by the greater effects of this QTL than the other two QTL on chromosomes 1 and 2.
In the target genomic regions of QLB-czas8, 19 annotated genes were predicted to encode proteins associated with disease resistance. Two LRR receptor-like serine/threonine-protein kinase family genes (i.e., SETIT_027508mg and SETIT_028067mg) and two disease resistance response protein 206-like genes (i.e., SETIT_026915 and SETIT_027383) were expressed in the leaves. These genes may be the candidates for QLB-czas8. None of the genes associated with disease resistance were homologous to the characterized R genes for the blast resistance from rice (data not shown), which indicates that an uncharacterized resistance gene may be responsible for the blast resistance conferred by QLB-czas8. Because there are many genes in the target genomic regions, a more saturated molecular map and a larger mapping population are required for finely mapping and final cloning the leaf blast-resistant QTL.
Summer millet cultivar Yugu 5 has been widely grown in northern China and commonly used as a parent in developing new cultivars. So, it represents an important genetic source of resistance to the millet blast disease. The identification of new QTL underlying the resistance to leaf blast will facilitate the use of Yugu 5 in the improvement of disease-resistant cultivars, as such information has been rarely reported in foxtail millet.
References
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Cao GM, Yan WY (1982) Study on control of the millet blast. Jilin Agric Sci 2:53–58
Castroagudín VL, Ceresini PC, de Oliveira SC, Reges JTA, Maciel JLN, Bonato ALV, Dorigan AF, McDonald BA (2015) Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 105:284–294
Devanna BN, Sharma TR (2018) Wheat blast disease management: cues from the advancements in molecular biology of rice-Magnaporthe pathosystem. J Plant Biochem Biotechnol 27:249–259
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Dong L, Quan JZ, Lu P, Ma JF, Li ZY, Bai H, Dong ZP (2015) Identification of main millet cultivars resistance to millet blast. China Plant Prot 35:33–37
D’Souza TF, Gaikwad AP (1984) Varietal resistance of setaria (Setaria italica) to blast caused by Pyricularia setariae. Indian Phytopathol 37:605–607
Dwivedi S, Upadhyaya H, Senthilvel S, Hash C, Fukunaga K, Diao X, Santra D, Baltensperger D, Prasad M (2012) Millets: genetic and genomic resources. Plant Breed Rev 35:247–375
Gupta SK, Sharma R, Rai KN, Thakur RP (2012) Inheritance of foliar blast resistance in pearl millet (Pennisetum glaucum). Plant Breed 131:217–219
Hu ZY, Deng GC, Mou HP, Xu YH, Chen L, Yang JH, Zhang MF (2018) A re-sequencing-based ultra-dense genetic map reveals a gummy stem blight resistance-associated gene in Cucumis melo. DNA Res 25:1–10
Jiang ZK, Liu JR, Wang SY, Yan HS, Liu HC, Liu RF, Li DH (2008) Analysis of pedigrees and genetic basis of main millet varieties in Henan province. Mod Agric Sci Technol 5:124–125
Kawakami T (1902) On the blast disease of rice. J Sapporo Agric Soc 3:1–3
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Li H, Durbin R (2009) Fast and accurate short read alignment with burrows wheeler transform. Bioinformatics 25:1754–1760
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map (SAM) format and SAMtools. Bioinformatics 25:2078–2079
Li ZJ, Jia GQ, Li XY, Li YC, Ma JF, Zhi H, Tang S, Zhang S, Chai Y, Li YD, Diao XM (2016) Determination of standard varieties for identifying physiological races of foxtail millet blast fungus. Sci Agric Sin 49:3308–3318
Li ZH, Jia GQ, LX Y, YC Li, Zhi H, Tang S, Ma JF, Zhang S, Li YD, Shang ZL, Diao XM (2020) Identification of blast-resistance loci through genome-wide association analysis in foxtail millet (Setaria italica (L.) Beauv.). J Integr Agric. https://doi.org/10.1016/S2095-3119(20)63196-3
Liang PY, Li YL, Shen LM (1959) Studies on millet blast caused by Pyricularia setariae Nishikado. Acta Phytopathol Sin 5:89–99
Lin L (1948) Host index of the parasitic fungi of Szechwan. China Plant Dis Rep 173(Suppl):1–38
Lin F, Chen XM (2007) Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor Appl Genet 114:1277–1287
Liu ZL, Cheng RH, Li XY (1996) Pedigree analysis and evaluation of the summer foxtail millet cultivars in northern China. Crops 5:24
Liu ZL, Cheng RH, Zhang FL, Shi ZG, Hou SL (2006) Millet variety in Boreali Sinica summer millets region and its pedigree evolution and analysis on genetic foundation. Acta Agric Boreali-Sin 21(Suppl):103–109
Liu H, Lin RF, Xu EN, Yang SW, Li ZL (1990) Identification of millet blast resistance to foxtail millet germplasm resources. Shaanxi Agric Sci 3:24–25
Liu DY, Ma CX, Hong WG, Huang L, Liu M, Liu H, Zeng HP, Deng DJ, Xin HG, Song J, Xu CH, Sun XW, Hou XL, Wang XW, Zheng HK (2014) Construction and analysis of high density linkage map using high-throughput sequencing data. PLoS ONE 9:e98855
Ma JF, Dong L, Li ZY, Zheng Z, Gan YJ, Dong ZP (2010) Yield comparison and germplasm screening of eleven disease resistance millet strains. J Hebei Agric Sci 14:119–122
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next generation DNA sequencing data. Genome Res 20:1297–1303
Munirathnam P, Venkataramanamma K, Anusha A (2015) Evaluation of foxtail millet genotypes for blast and rust diseases under field conditions. Curr Biot 9:263–268
Nagaraja A, Kumar J, Jain AK, Narasimhardu Y, Raghuchander T, Kumar B, Gowda BH (2007) Compendium of small millets diseases. Project Coordinator Cell, All India Coordinated Small Millets Improvement Project, UAS, GKVK Campus, Bengaluru. pp. 80
Nakayama H, Nagamine T, Hayashi N (2005) Genetic variation of blast resistance in foxtail millet (Setaria italica (L.) P. Beauv.) and its geographic distribution. Genet Resour Crop Evol 52:863–868
Nan CM, Li SG, Xia XY, Liu F, Pu NN, Liu M, Wang HJ, Liu EK, Xu LP (2018) Occurrence degree of diseases, insect pests and harm coming from birds in China’s main foxtail millet producing areas and prevention and treatment ideas. Agric Outlook 1:26–34
Nishikado Y (1917) Studies on rice blast fungus. Ber Ohara Inst 1:171–217
Rajesh M, Sudha A, Nirmalakumari A, Parasuraman P (2019) Identification of resistant sources for blast and rust in foxtail millet incited by Pyricularia setariae and Uromyces setariae-Italica. Int J Curr Microbiol App Sci 8:1796–1800
Ramakrishnan M, Antony Ceasar S, Duraipandiyan V, Vinod KK, Kalpana K, Al-Dhabi NA, Ignacinuthu S (2016) Tracing QTLs for leaf blast resistance and agronomic performance of finger millet (Eleusine coracana (L.) Gaertn.) genotypes through association mapping and in silico comparative genomics analyses. PLoS One 11:e0159264
Reddy INBL, Sivaramakrishnan S (2017) Identification of SSR markers which could differentiate blast disease resistance accessions in finger millet (Eleusine conracana (L.) Gaertn.). J Crop Sci Biotech 20:37–44
Ren SL, Bai H, Dong L, Dong ZP, Quan JZ, Li ZY, Xing JH (2017) Sequence analysis of rDNA-IGS of Magnaporthe oryzae isolates from different geographical origins in China. Acta Phytopathol Sin 47:305–312
Sanghani JM, Sanghani AO, Kothari VV, Raval SS, Kahodariya JH, Ramani HR, Vadher KJ, Gajera HP, Golakiya BA, Mandavia MK (2018) The SSR based linkage map construction and identification of QTLs for blast resistance in pearl millet. J Pharmacogn Phytochem 7:3057–3064
Sharma R, Girish AG, Upadhyaya HD, Humayun P, Babu TK, Rao VP, Thakur RP (2014) Identification of blast resistance in a core collection of foxtail millet germplasm. Plant Dis 98:519–524
Singh RK, Muthamilarasan M, Prasad M (2017) Foxtail millet: an introduction. In: Prasad M (ed) The foxtail millet genome. Springer, Cham, pp 1–9
Singh S, Sharma R, Pushpavathi B, Gupta SK, Durgarani ChV, Raj C (2017) Inheritance and allelic relationship among gene(s) for blast resistance in pearl millet [Pennisetum glaucum (L.) R. Br.]. Plant Breed 137:573–584
Turaki ZGS, Richard BI, Degri MM (2014) Assessment of the effect of fungicide and seed rate on the incidence of leaf blast (Magnaphorthe grisea), on the growth of foxtail millet (Setaria italica (L.) P. Beauv) in north-eastern Nigeria. Asian J Agric Rural Dev 4:142–148
Viswanath S, Seetharam A (1989) Diseases of small millets and their management in India. In: Seetharam A, Riley KW, Harinarayana G (eds) Small millets in Global Agriculture. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi, pp 237–253
Wang S, Basten JC, Zeng ZB (2010) Windows QTL cartographer 2.5. department of statistics. North Carolina State University, Raleigh
Wang YR, Chu JZ, Song YC, Xie SY, Yan WY, Jin LX, Liu H, Xie SR (1985) Screening tests of millet varieties for resistance to blast pathogen. Acta Phytophyl Sin 12:175–180
Wang BH, Ebbole DJ, Wang ZH (2017) The arms race between Magnaporthe oryzae and rice: diversity and interaction of Avr and R genes. J Integr Agric 16:2746–2760
Wang YR, Zhu JZ, Song YC, Xie SY, Yan WY, Jin LX, Liu H, Xie SR (1985) Screening tests of millet varieties for resistance to blast pathogen. Acta Phytophyl Sin 12:175–180
Wei L, Wang TC, Zhang GL (1999) A study on protein and fat contents and characterization of disease resistance in millet varieties. Acta Agric Boreali-Sin 14:1–5
Wu XL (1985) A preliminary study on the identification and screening of millet blast. Heilongjiang Agric Sci 1:46–50
Yan WY, Xie SY, Jin LX, Liu HJ, Hu JC (1985) A preliminary study on the physiological races of millet blast (Pyricularia setariae Nishikado). Sci Agric Sin 3:57–62
Yan WY, Xie SY, Liu HJ, Li NM (1988) Evaluation for the specialized resistance of millet germplasm resources and cultivars to millet blast in Jilin province. Jilin Agric Sci 1:6–10
Yang SW (1962) Investigation and study on foxtail millet blast. Shanxi Agric Sci 1:37–39
Yang XY, Wan ZW, Perry L, Lu HY, Wang Q, Zhao CH, Li J, Xie F, Yu JC, Cui TX, Wang T, Li MQ, Ge QS (2012) Early millet use in Northern China. Proc Natl Acad Sci USA 109:3726–3730
You Q, Zhang L, Yi X, Zhang Z, Xu W, Su Z (2015) SIFGD: Setaria italica functional genomics database. Mol Plant 8:967–970
Yu DF (1978) Millet diseases. Science Press, Beijing
Zhang AY, Guo EH, Diao XM, Fan HP, Li YH, Wang LX, Guo HL, Cheng LP, Wu YS (2017) Evaluation of foxtail millet cultivars developed in the middle and late-maturing spring-sowing region in Northwest China in 2005–2015. Sci Agric Sin 50:4486–4495
Zhu Q (1964) Some observations on the resistance of millet varieties to millet blast. Acta Phytophyl Sin 3:413
Acknowledgements
The authors thank Z. Wang of the China Agricultural University for his data analysis. The financial support provided by the Ministry of Agriculture of P.R. China (CARS-06-13.5-B1) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
HL and BT conceived and designed the study. BT developed the population. BT, LZ, YL, WW, and YZ conducted the field experiments. PW performed sequencing data analysis. HL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Hai-Chun Jing.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, B., Zhang, L., Liu, Y. et al. Identification of QTL for resistance to leaf blast in foxtail millet by genome re-sequencing analysis. Theor Appl Genet 134, 743–754 (2021). https://doi.org/10.1007/s00122-020-03730-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03730-w