Abstract
Key message
A novel, stably expressed, and plant height-independent QTL for spike extension length on 5AS was identified and validated in different populations using a newly developed and tightly linked KASP marker.
Abstract
As an important component of plant height (PH), spike extension length (SEL) plays a significant role in formation of an ideotype in wheat. Despite the fact that numerous loci for SEL in wheat have been reported, our knowledge on PH-independent loci remains to be limited. In this study, two recombinant inbred line (RIL) populations genotyped using the Wheat55K SNP were used to detect quantitative trait loci (QTL) controlling SEL across six environments. A total of 30 QTL for SEL were detected in these two RIL populations, and four of them, i.e., QSEL.sicau-2CN-4D, QSEL.sicau-2SY-4B.2, QSEL.sicau-2SY-4D.1, and QSEL.sicau-2CN-5A, were stably expressed. Genetic and conditional QTL analysis showed that the first three were significantly associated with PH, while the last one, QSEL.sicau-2CN-5A, is independent of PH. Comparison of genetic and physical maps suggested that only QSEL.sicau-2CN-5A located on chromosome arm 5AS is likely a novel QTL. A Kompetitive Allele-Specific PCR (KASP) marker, KASP-AX-110413733, tightly linked to this novel QTL was developed to successfully confirm its effect in three different genetic populations. Further, in the interval where QSEL.sicau-2CN-5A was located on ‘Chinese Spring’ wheat reference genome, three promising genes mainly expressed in wheat stem were predicated and they all encode the cytochrome P450 that was demonstrated to be closely associated with SEL elongation in rice. In addition, significant correlations between SEL and PH, spikelet number per spike, and thousand-grain weight were also detected. Altogether, our results broaden our understanding on genetic basis of SEL and will be useful for marker-based selection of lines with different SELs and fine mapping the novel and PH-independent QTL QSEL.sicau-2CN-5A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An ideotype in wheat has greater potential in yield increase given its advantages with improved photosynthesis, reduced lodging, and enhanced ability of allocation of assimilation to grain (Yao and Zhu 1998; Berry et al. 2007; Cui et al. 2011; Semenov et al. 2013). Spike extension length (SEL), part of plant height (PH), is the distance from the base of the panicle to the petiole of the flag leaf (Yu et al. 2014) and plays an important role in formation of an ideotype (Zhang et al. 2017). The longer SEL can increase airflow in panicle and decrease air humidity, and thus reducing Fusarium Head Blight (FHB) (Ma et al. 2017). However, a higher PH usually increased with the addition of SEL may lead to the bending or falling of the panicles thus resulting in yield and quality decline (Bridgemohan and Bridgemohan 2014). Therefore, only a suitable SEL can lead to the high yield of wheat.
Numerous studies associated with SEL have been reported in rice. For example, the overexpression of CYP94C2b may increase the number of internode elongated cells (Kurotani et al. 2015). The cytochrome P450 monooxygenase regulated the accumulation of gibberellins (GAs) to affect the elongation of SEL (Zhu et al. 2006; Zhang et al. 2008b). However, in wheat, few studies have been reported on SEL (Ma et al. 2006; Qiao et al. 2007; Ku et al. 2015; Zhang et al. 2018; Ji et al. 2019). For example, the QTL for SEL on the chromosome 5D increased SEL by 0.16 mm (Tian et al. 2015). Five stable QTL have important effects on the development of plant height components (Zhang et al. 2017). In addition, six new QTL for controlling SEL were identified using genome-wide association analysis (Li et al. 2019b).
SEL is closely associated with the development of inflorescence, and it is an important organ for studying the transportation system in wheat (Reynolds et al. 2009; Dixon et al. 2020). However, many QTL controlled SEL were significantly affected by PH, making it difficult to precisely dissect the effect of SEL on wheat yield in breeding (Law et al. 1978; Hoogendoorn et al. 1990). Furthermore, our knowledge on the genetic basis and regulation mechanism about SEL in wheat is largely unknown. Thus, it is essential to detect and identify QTL for SEL that is not affected by PH.
In addition, it is widely reported that the multivariable conditional analysis for analyzing the contributions of component traits to a complex trait was used to detect whether a given QTL is affected by other traits (Cui et al. 2011; Yu et al. 2014; Tao et al. 2019). In this study, we thus used this method to identify possible QTL for SEL that is not dependent on PH. Our results revealed four stable QTL controlling SEL identified in two recombinant inbred line (RIL) populations based on the genetic maps constructed using the Wheat55K SNP array. A new Kompetitive Allele-Specific PCR (KASP) marker was developed to further validate a novel QTL that was independent of PH in three populations. Possible candidate genes associated with the novel QTL were predicated as well. These results laid a foundation for molecular marker-based selection of wheat lines with different SELs and cloning the novel and stable QTL.
Materials and methods
Plant materials
Five populations were used in this study. They were 20828/Chuannong 16 (CN16; 2CN, 199 F6 RILs including two parents) (Liu et al. 2018), 20828/SY95-71 (2SY, 128 F7 RILs including two parents) (Liu et al. 2020), S849-8/CN16 (SCN, 217 F5 lines including two parents), MTL7/CN16 (M7C, 128 F3 lines including two parents), and MZ-5/CN16 (MC, 116 F3 lines including two parents). 20828, SY95-71, S849-8, MTL7, and MZ-5 are advanced wheat lines that have been used in wheat breeding. CN16 is a commercial variety (Ma et al. 2019a). 2CN and 2SY RIL populations were used for QTL mapping and the other three were used for validating the identified PH-independent QTL.
Phenotypic evaluation
The 2CN and 2SY populations were grown in six different environments for phenotypic evaluation: Wenjiang (103° 51ʹ E, 30° 43ʹ N) in 2018 and 2019 (2018WJ, and 2019WJ), Chongzhou (103° 38ʹ E, 30° 32ʹ N) in 2018 and 2019 (2018CZ, and 2019CZ), Ya’an (103° 0ʹ E, 29° 58ʹ N) in 2018 and 2019 (2018YA and 2019YA) of Sichuan Province in China. SCN, M7C, and MC populations were grown in Chongzhou in 2020. A random block design was used for all of the trials. Each line was planted in a single 1.5 m row with 0.3 m between rows, and the sowing density was 15 seeds per row with 0.1 m between plants within a row (Liu et al. 2018). All field trials were well irrigated and managed in accordance with local standard practices (Ma et al. 2020).
At least three representative and disease-free plants in each line were selected to measure the PH, SL, spikelet number per spike (SNS), thousand-grain weight (TGW), and SEL according to a previous study (Tao et al. 2019). Among these traits, data of PH (Li et al. 2019a; Liu et al. 2020), SL (Li et al. 2019a), SNS (Ma et al. 2019a; Liu et al. 2020), and TGW (Ma et al. 2019c, 2020; Liu et al. 2020) from 2CN and 2SY were retrieved from our previous studies (Table S1).
Genetic map construction and QTL analysis
Two genetic linkage maps constructed using the Wheat55K SNP array were used in this study. In the 2CN population, the markers were distributed in 34 linkage groups with an average interval of 0.84 cM between adjacent markers (Liu et al. 2018; Ma et al. 2019b). In the 2SY population, the markers were distributed in 38 linkage groups and the genetic map covered a total genetic distance of 4,273.03 cM with mean, minimum, and maximum marker densities of 1.69, 0.70, and 5.33 cM/marker, respectively (Liu et al. 2020). We used IciMapping 4.1 based on Biparental Populations (BIP) module with the inclusive composite interval mapping (ICIM, https://www.isbreeding.net/software/?type=detail&id%20=18) for QTL detection in 2CN and 2SY populations. The minimal logarithm of odds (LOD) score to accept the presence of a QTL was set at 2.5 (Lin et al. 1996). QTL was named based on the International Rules of Genetic Nomenclature (https://wheat.pw.usda.gov/ggpages/wgc/98/Intro.htm). ‘Sicau’ represents Sichuan Agricultural University.
Conditional QTL analysis
Conditional QTL analysis was usually to interpret the relationship between complex traits and components (Cui et al. 2011). In conditional QTL analysis, the LOD value was decreased or increased significantly but was greater than 2.5 indicating that the QTL was partially, but not adequately, correlated with other traits; the LOD value was reduced to less than 2.5 meaning that the QTL was thoroughly contributed by other traits (Zhu 1995; Cui et al. 2011). The conditional phenotypic values (SEL|PH) were obtained by QGAStation2.0.exe software (Cui et al. 2011). IciMapping 4.1 was also used to identify the conditional QTL with conditional phenotypic values.
Physical intervals of the novel QTL and comparison with previously reported QTL
The physical location of the novel QTL’s flanking markers on T. aestivum cv. Chinese Spring or CS [International Wheat Genome Sequencing Consortium (IWGSC) RefSeq v1.0] was obtained from our previous studies (Liu et al. 2018; Ma et al. 2019b).
The probe sequences of flanking markers for each previously reported QTL or genes related to SEL on 4B, 4D and 5A were used for BLASTn against (E-value of 1e-5) the genome assemblies of CS [(IWGSC) RefSeq v1.0; https://urgi.versailles.inra.fr/download/iwgsc/] (IWGSC 2018) to get their physical locations. The UniProt (https://www.uniprot.org/) was used to analyze the annotations and functions of a given gene. Additionally, the expression patterns of the predicated candidate genes of the novel QTL were obtained from the Triticeae Multi-omics Center (https://202.194.139.32/expression/index.html) and WheatExp (https://wheat.pw.usda.gov/WheatExp/#) using published data from IWGSC (2014) and IWGSC (2018).
Marker development and QTL validation
To validate the identified novel QTL, we converted a SNP marker AX-110413733 into a KASP maker as previously described (Ma et al. 2019a). Eighty lines randomly selected from three validation populations (SCN, M7C, MC), respectively, were used to do genotyping using this KASP marker. The amplification reactions were performed in a total volume of 10 μl containing 0.75 μl of template DNA, 5 μl of SsoFast EvaGreen mixture (Bio-Rad, Hercules, CA, USA), and 1.4 μl of mixture forward and reverse primers (KASP-AX-110413733F: GAAGGTGACCAAGTTCATGCTTTGTTTTGGATTCGGGTTTTC, KASP-AX-110413733H: GAAGGTCGGAGTCAACGGATTTTGTTTTGGATTCGGGTTTTG, KASP-AX-110413733R: GCAAGTTCAAAATCGAAAT), and DNase/RNase-free water up to the final value. CFX96™ Real-Time System made in Singapore was used to genotype. The PCR reaction procedure was as follows: 15 min at 94 °C, 40 cycles of 20 s at 94 °C, and 60 s at 61 °C–55°C (drop 0.6 °C, per cycle).
According to the genotype of the KASP marker in the three validation populations, the lines were divided into two groups: (1) lines with homozygous alleles from either of S849-8, MTL7, and MZ-5; (2) lines with homozygous alleles from CN16. Lines with heterozygous genotype were abandoned. Student’s t test (P < 0.01) was also used to evaluate the SEL between the above two groups.
Data analysis
The average values of SEL of more than three representative plants from each line were used in the succeeding analysis. The best linear unbiased prediction (BLUP) of target traits in different environments (Smith et al. 1998) and the broad-sense heritability (h2) were calculated using SAS V8.0 (SAS Institute, Cary, NC, USA; https://www.sas.com). IBM SPSS Statistic 26 (SPSS, Chicago, IL, USA; https://en.wikipedia.org/wiki/SPSS) was used to perform Student’s t test (P < 0.05) and correlation analysis of different phenotype values in different environments. Lines carrying different alleles at the major QTL from 2CN and 2SY populations were selected based on the genotype of its flanking markers and further compared for the SEL traits using Student’s t test (P < 0.05).
Results
Phenotypic variation of SEL traits
Significant differences between 20828 and CN16 for 2CN population (Fig. 1a) and between 20828 and SY95-71 for 2SY population (Fig. 1b) were observed. CN16 and SY95-71 had consistently and significantly higher values for SEL than 20828 (Table 1). The SEL ranged from 1.10 cm to 27.94 cm in the 2CN population and from 1.61 cm to 21.30 cm in the 2SY population (Table 1). The standard deviation (SD) of 2CN and 2SY populations ranged from 1.59 to 3.74 and from 2.21 to 4.21, respectively. The h2 of 2CN and 2SY populations were 0.88 and 0.94, respectively, indicating SEL was mainly controlled by genetic factors (Table 1).
Moreover, the frequency distribution of SEL in different environments was indicative of multigenic inheritance (Fig. S1). Significant and positive correlations for SEL were detected among six different environments, BLUP dataset and SEL|PH (P < 0.01). The correlation coefficients ranged from 0.493 to 0.950 for the 2CN population and from 0.583 to 0.940 for the 2SY population (Table 2).
Correlation analysis between SEL and yield-related traits
Phenotypic correlations between SEL and other yield-related traits are listed in Table 3. Significant and positive correlations (P < 0.05) between SEL and PH and TGW, and significant and negative correlation (P < 0.01) between SEL and SNS were observed in both 2CN and 2SY populations (Table 3, Fig. 2).
Effects of spike extension length (SEL) on other traits. Effects of SEL on plant height (PH), spikelet number per spike (SNS), and thousand-grain weight (TGW) in 2CN (a) and 2SY (b) populations, respectively. The relationships of SEL, PH, SNS, and TGW were evaluated by the BLUP dataset. The histogram of each trait was shown in the main diagonal. The scatter diagram of different traits was shown below and above the main diagonal
QTL for SEL
A total of 30 QTL for SEL were detected in 2CN (13 QTL) and 2SY (17 QTL) populations (Table 4). They were located on chromosomes: 1B, 1D, 2A, 2B, 2D, 3D, 4A, 4B, 4D, 5A, 5D, 6A, 6B, 6D, and 7A. Four of them were stably detected in at least three environments (Table 4).
In the 2CN population, two stable QTL QSEL.sicau-2CN-4D and QSEL.sicau-2CN-5A were detected. QSEL.sicau-2CN-4D, detected in four environments as well as the BLUP dataset, was located on chromosome arm 4DS. It explained between 5.17% and 8.74% of the phenotypic variance (Table 4, Fig. 3a). QSEL.sicau-2CN-5A with 5.31%–10.20% of the phenotypic variance, as a major locus, was detected in four environments as well as the BLUP dataset and SEL|PH on chromosome arm 5AS (Table 4, Fig. 3b). The positive alleles of these two stable QTL were both contributed by CN16. The remaining 11 QTL detected in less than three environments explained between 5.61% and 9.71% of the phenotypic variance (Table 4).
Two stable QTL QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 were also detected in the 2SY population. QSEL.sicau-2SY-4B.2 detected in three environments as well as the BLUP dataset was located on 4BS chromosome (Fig. 3c). This locus explained between 11.26% and 16.92% of the phenotypic variance (Table 4). QSEL.sicau-2SY-4D.1 was detected in three environments and BLUP dataset. It was located on chromosome arm 4DS and explained between 11.81% and 18.45% of the phenotypic variance (Table 4, Fig. 3d). The positive alleles of these two stable QTL were both contributed by SY95-71. The remaining 15 QTL were detected in a single environment and explained between 5.34% and 13.96% of the phenotypic variance (Table 4).
QTL × environment (QE) interaction analysis showed that a total of 18 QTL were detected in 2CN and 2SY populations (Table S2). Fourteen of them were the same as those detected in individual environment. For example, QSEL.sicau-2CN-4D, QSEL.sicau-2CN-5A, and QSEL.sicau-2SY-4D.1 were simultaneously detected by multi-environmental and individual environmental analyses, further indicating that they were major and stable QTL (Table S2).
Effects of QSEL.sicau-2CN-4D and QSEL.sicau-2CN-5A on SEL in the 2CN population
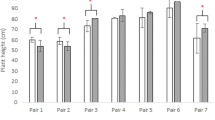
The positive alleles of both QSEL.sicau-2CN-4D and QSEL.sicau-2CN-5A were from CN16 (Table 4). Their effects on SEL were further analyzed in the 2CN population (Fig. 4a). Compared with those without any of the alleles increasing SEL, RILs possessing the positive allele from QSEL.sicau-2CN-4D significantly increased SEL by 26.69%; those possessing the positive allele from QSEL.sicau-2CN-5A significantly increased SEL by 22.48%; and those with the combination of QSEL.sicau-2CN-4D and QSEL.sicau-2CN-5A significantly increased SEL by up to 42.69%. In addition, RILs with the combination of positive alleles of QSEL.sicau-2CN-4D and QSEL.sicau-2CN-5A increased SEL by 12.63% and 16.50%, respectively, compared to those with the positive allele of either QSEL.sicau-2CN-4D or QSEL.sicau-2CN-5A in 2CN population.
Effects of QSEL.sau-2CN-4D and QSEL.sau-2CN-5A for SEL in the 2CN population (a) and those of QSEL.sau-2SY-4B.2 and QSEL.sau-2SY-4D.1 for SEL in the 2SY population (b). + and − represent lines with and without the positive alleles of the target quantitative trait loci (QTL) based on the flanking marker of the corresponding QTL, respectively. **Significant at P < 0.01, *Significant at P < 0.05. Differences between the two groups were labeled above the significant levels
Effects of QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 on SEL in the 2SY population
The positive alleles of both QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 were from SY95-71 in 2SY population (Table 4). Their effects on SEL were analyzed as well (Fig. 4b). Compared with those without any of the alleles increasing SEL, RILs possessing the positive allele from QSEL.sicau-2SY-4B.2 increased SEL by 22.13%; those with the combination of QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 significantly increased SEL by up to 52.55%. Furthermore, RILs with the combination of positive alleles of QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 significantly increased SEL by 24.91% and 50.45%, respectively, compared to those with the positive allele of either QSEL.sicau-2SY-4B.2 or QSEL.sicau-2SY-4D.1 in 2SY population. Besides, RILs with the positive allele of QSEL.sicau-2SY-4B.2 increased SEL by 20.45% compared to those of QSEL.sicau-2SY-4D.1.
Conditional QTL analysis-based determination of relationships between SEL and PH
SEL is an important part of PH. Thus, theoretically, SEL is closely correlated with PH. Conditional QTL analysis was carried out to determine the genetic relationships between SEL and PH. The LOD scores of the three major QTL QSEL.sicau-2CN-4D, QSEL.sicau-2SY-4B.2, and QSEL.sicau-2SY-4D.1 were significantly decreased below 2.5 when SEL was conditional on PH, implying they were closely related to PH (Fig. 5a, c, d). Interestingly, no significant difference was detected for the LOD score of QSEL.sicau-2CN-5A in 2CN population when SEL was conditional on PH (Fig. 5b). These results suggested that QSEL.sicau-2CN-5A was likely independent of PH.
Validation of the PH-independent QTL QSEL.sicau-2CN-5A
A newly developed KASP marker (KASP-AX-110413733) tightly linked to QSEL.sicau-2CN-5A was developed to validate the effects of QSEL.sicau-2CN-5A in three populations with different genetic backgrounds (SCN, M7C, and MC). The polymorphism between parent CN16 and the other three parents S849-8, MTL7 and MZ-5 was detected using the marker KASP-AX-110413733. According to the genotype (excluding heterozygotes), the lines were divided into two groups (Fig. 6a–c). As expected, Student’s t test detected significant differences (P < 0.01) between them (Fig. 6d–f). The lines with positive alleles from CN16 have significantly higher values than those without these alleles, and the differences between the two groups ranged from 13.04% to 36.95% with an average of 23.43% in three validation populations.
Validation of QSEL.sau-2CN-5A in three populations with different genetic backgrounds. Eighty lines randomly selected from SCN, M7C, and MC populations, respectively, were used to do genotyping using the KASP marker KASP-AX-110413733. Fluorescence PCR typing results of the Kompetitive Allele-Specific PCR (KASP) marker KASP-AX-110413733 in S849-8/ CN16 (SCN, a), MTL7/CN16 (M7C, b), and MZ-5/CN16 (MC, c) populations. Blue round represents lines with the allele of CN16 (FAM fluorescence); orange box frame represents lines with the allele of S849-8 (a), MTL7 (b), MZ-5 (c), respectively (HEX fluorescence); effects of QSEL.sau-2CN-5A in three validation populations of SCN (d), M7C (e), and MC (f), respectively. + and −represent lines with and without the positive alleles of the target quantitative trait loci (QTL) based on the flanking marker (KASP-AX-110413733) of the corresponding QTL, respectively. **Significant at P < 0.01; Differences between the two groups were labeled above the significant levels
Discussion
Correlation analysis of SEL and yield-related traits
SEL and SNS had a significant and negative correlation, indicating that there may be a competition between SEL and SNS during plant growth (Miralles et al. 1998; Bancal 2008). SEL was positively correlated with TGW, which is consistent with many previous reports (Yao et al. 2003; Ma et al. 2017). These results indicated that when plant height was determined, the longer SEL likely benefits the grain yield increasing. This is because SEL has a certain function of photosynthesis and temporary storage of energy and water, and thus, a longer SEL showing a longer distance between the panicle and flag leaf likely benefits the transportation of nutrients and water to seeds (Bridgemohan and Bridgemohan 2014; Ávila et al. 2017). Besides, a longer SEL was also helpful for ventilating and thus reducing the occurrence of FHB (Ma et al. 2017). Thus, a suitable SEL is likely beneficial for improving TGW.
Both of the LOD and PVE of SEL QTL on 5A indeed increased after the PH effect was removed in conditional analysis. However, according to conditional QTL analysis, no significant difference was detected for the LOD score of QSEL.sicau-2CN-5A in 2CN population when SEL was conditional on PH (Cui et al. 2011). Thus, QSEL.sicau-2CN-5A may be plant height-independent. Furthermore, there was no significant difference for PH between with and without the positive allele of QSEL.sicau-2CN-5A in 2CN population (Fig. S2). These results suggested that all loci controlling SEL might work together to increase PH, and thus, PH is positively correlated with SEL.
Stable QTL controlling SEL
To further identify relationships between QTL detected in this study and previous studies, we compared their physical intervals on the CS reference genome. QSEL.sicau-2SY-4B.2 was located in the interval between 37.87 and 45.42 Mbp close to QPel.HM.4B (Tao et al. 2019), suggesting that they are likely alleles (Table S3). QSEL.sicau-2CN-4D and QSEL.sicau-2SY-4D.1 were closely located on chromosome arm 4DS and may be allelic (Table S4). No QTL for SEL were detected on 4DS in previous studies to date. However, they were overlapped with QTL for PH (Table S4). QSEL.sicau-2CN-5A was located between 4.85 and 6.46 Mbp on chromosome arm 5AS (Table S5), which is different from previously reported SEL-related loci (Korzun et al. 1997; Wu et al. 2010; Jia et al. 2013; Linn et al. 2017; Zhang et al. 2017; Li et al. 2019b). These results showed that QSEL.sicau-2CN-5A may be a novel QTL controlling SEL identified in the present study.
Possible gene interaction between QSEL.sicau-2SY-4B and QSEL.sicau-2SY-4D.1
Two QTL controlling SEL were detected in 2SY population. When the positive allele from QSEL.sicau-2SY-4B.2 was present only, SEL increased significantly; when the positive allele from QSEL.sicau-2SY-4D.1 was present only, SEL increased insignificantly; however, the combination of positive alleles of QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 increased SEL significantly and extremely. Thus, we speculate that there is a gene interaction between the positive allele of QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1. Yu et al. (2002) clearly demonstrated the importance of epistatic interactions in genetic bases of QTL controlling plant height in rice. Zhang et al. (2008a) showed that epistatic effects were also important for genetic bases of PH in wheat. QSEL.sicau-2SY-4B.2 and QSEL.sicau-2SY-4D.1 were influenced by PH. Therefore, they might be also involved in epistatic interactions between each other in 2SY population, and the positive allele of QSEL.sicau-2SY-4D.1 can enhance the effect of QSEL.sicau-2SY-4B.2. However, fine mapping and map-based cloning are needed to further reveal their interaction mechanism.
Genetic relationships between SEL and PH
QSEL.sicau-2SY-4B.2 was located on chromosome 4BS (37.87–45.42 Mbp). Rht-B1b, an important PH-related gene, was also located on chromosome 4BS (Zhang et al. 2013). BLAST results suggested that Rht-B1b was located at 30.86 Mbp on CS RefSeqv.1.0 sequence (Jobson et al. 2019). Thus, QSEL.sicau-2SY-4B.2 is not likely allelic to Rht-B1b. However, it is co-located with QPh.sau.4B.1 for PH detected in the present study (Table S6) and overlapped with the PH-related QTL QPh.nau-4B.2 (Jia et al. 2013).
Both QSEL.sicau-2CN-4D and QSEL.sicau-2SY-4D.1 were located on 4DS. Rht-D1b as a PH gene was also located on chromosome 4DS and BLAST results suggested that Rht-D1b was located at 18.78 Mbp (Zhang et al. 2013). QSEL.sicau-2CN-4D and QSEL.sicau-2SY-4D.1 were located in the intervals 9.56–10.38 Mbp and 19.18–37.81Mbp, respectively. Although they were likely far from Rht-D1b, they were both co-located with the PH QTL detected in this study (Table S5) and overlapped with the PH-related QTL QPh-4D (Table S4) (Liu et al. 2014).
It is documented that the resolution of primary QTL mapping was rough due to the limited population size as well as the interference of environments (Dinka et al. 2007; Liu et al. 2018). Besides, based on different genetic backgrounds, we would be hard to rule out that QSEL.sicau-2SY-4B.2 is affected by Rht-B1 or QSEL.sicau-2SY-4D.1 and QSEL.sicau-2CN-4D are affected by Rht-D1. Additionally, this study clearly demonstrated that QSEL.sicau-2SY-4B.2, QSEL.sicau-2CN-4D, and QSEL.sicau-2SY-4D.1 were indeed affected by PH using conditional QTL analysis. Therefore, in order to further reveal the relationship between the three SEL-related QTL and Rht genes, we need to do fine mapping and even map-based cloning work.
QSEL.sicau-2CN-5A identified by unconditional and conditional QTL analysis methods was located at 4.85–6.46 Mbp on chromosome arm 5AS. The PH-related genes Rht-9 (Linn et al. 2017) and Rht-12 (Sun et al. 2019) were located at 558.34 Mbp and 678.29 Mbp, respectively, on chromosome arm 5AL, being clearly far from QSEL.sicau-2CN-5A (Table S5).
Thus, based on correlation, conditional QTL, genetic, and physical comparison analyses, SEL was significantly and positively correlated with PH. QSEL.sicau-2CN-4D, QSEL.sicau-2SY-4B.2, and QSEL.sicau-2SY-4D.1 may have a complex relationship with PH-related genes. While the stable QTL, QSEL.sicau-2CN-5A, successfully validated in three different genetic backgrounds, was not affected by PH and is worthy to be further fine mapped and used for breeding wheat variety with suitable SEL using the developed KASP marker.
The predicated genes in the interval of QSEL.sicau-2CN-5A
Given that the novel and stable QTL QSEL.sicau-2CN-5A was not dependent on PH, we thus attempt to predicate genes in the interval where this QTL was located on CS reference genome. Alignment of its flanking markers showed that it was located between 4.85 Mbp and 6.46 Mbp in the deletion bin 5AS7/10–0.98–1.00 on chromosome arm 5AS (Fig. S3). In this interval, 37 predicated genes were annotated in the CS (Table S7). Expression pattern analyses showed that 17 genes were expressed in stem and the other 20 genes were not expressed in stem (Fig. S4). Further, 3 genes (TraesCS5A01G008700, TraesCS5A01G008800 and TraesCS5A01G008900) had significantly higher expression levels in stem than other genes (Fig. S4) (IWGSC 2018). Interestingly, all of the three annotated genes encoded cytochrome P450 and they had the highest expression in stem at different growth stages (Fig. S5) (IWGSC 2018).
It was widely reported that cytochrome P450 was associated with the elongation of SEL. For example, Eui gene, a GA catabolism gene encoding cytochrome P450 monooxygenase was related to accumulation of active GAs in the uppermost internode thus affecting SEL in rice (Ma et al. 2006; Zhu et al. 2006; Zhang et al. 2008b). Another example is that the overexpression of cytochrome P450 enzyme, CYP94C2b, can modulate jasmonate metabolism and then increase the number of elongated cells in the uppermost internode to elongate SEL in rice (Kurotani et al. 2015). In addition, OsDWARF48, a gene encoding cytochrome P450, might control the SEL by regulating the synthesis and signal pathways of brassinosteroids in rice (Zhen et al. 2018).
Therefore, we speculated that the three genes (TraesCS5A01G008700, TraesCS5A01G008800 and TraesCS5A01G008900) encoding cytochrome P450 were likely closely related to SEL in wheat and they were useful in our following work of fine mapping and cloning of this locus.
References
Ávila L, E., Zerpa AJ, Santiago LS, (2017) Stem photosynthesis and hydraulics are coordinated in desert plant species. New Phytol 216:1119–1129
Bancal P (2008) Positive contribution of stem growth to grain number per spike in wheat. Field Crop Res 105:27–39
Berry PM, Sylvesterbradley R, Berry S (2007) Ideotype design for lodging-resistant wheat. Euphytica 154:165–179
Bridgemohan P, Bridgemohan SHR (2014) Evaluation of anti-lodging plant growth regulators on the growth and development of rice (Oryza sativa). J Cereals Oilseeds 5:12–16
Cui F, Li J, Ding A, Zhao C, Wang L, Wang X, Li S, Bao Y, Li X, Feng D (2011) Conditional QTL mapping for plant height with respect to the length of the spike and internode in two mapping populations of wheat. Theor Appl Genet 122:1517–1536
Dinka SJ, Campbell MA, Demers T, Raizada MN (2007) Predicting the size of the progeny mapping population required to positionally clone a gene. Genetics 176:2035–2054
Dixon L, Pasquariello M, Boden S (2020) TEOSINTE BRANCHED1 regulates height and stem internode length in bread wheat (Triticum aestivum). J Exp Bot:eraa252
Hoogendoorn J, Rickson J, Gale M (1990) Differences in leaf and stem anatomy related to plant height of tall and dwarf wheat (Triticum aestivum L.). J Plant Physiol 136:72–77
IWGSC (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788
IWGSC (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:eaar7191
Ji H, Han C, Lee G, Jung K, Kang D, Oh J, Oh H, Cheon K, Kim S, Choi I (2019) Mutations in the microRNA172 binding site of SUPERNUMERARY BRACT (SNB) suppress internode elongation in rice. Rice 12:1–14
Jia H, Wan H, Yang S, Zhang Z, Kong Z, Xue S, Zhang L, Ma Z (2013) Genetic dissection of yield-related traits in a recombinant inbred line population created using a key breeding parent in China’s wheat breeding. Theor Appl Genet 126:2123–2139
Jobson E, Johnston R, Oiestad A, Martin J, Giroux M (2019) The impact of the wheat Rht-B1b semi-dwarfing allele on photosynthesis and seed development under field conditions. Front Plant Sci 10:51
Korzun V, Röder M, Worland A, Börner AJPB (1997) Intrachromosomal mapping of genes for dwarfing (Rht12) and vernalization response (Vrn1) in wheat by using RFLP and microsatellite markers. Plant Breed 116:227–232
Ku L, Cao L, Wei X, Su H, Tian Z, Guo S, Zhang L, Ren Z, Wang X, Zhu Y (2015) Genetic dissection of internode length above the uppermost ear in four RIL populations of maize (Zea mays L.). G3: Genes. Genomes, Genetics 5:281–289
Kurotani KI, Hattori T, Takeda S (2015) Overexpression of a CYP94 family gene CYP94C2b increases internode length and plant height in rice. Plant Signal Behav 10:e1046667
Law C, Snape J, Worland A (1978) The genetical relationship between height and yield in wheat. Heredity 40:133–151
Li C, Ma J, Liu H, Ding P, Yang C, Zhang H, Qin N, Lan X (2019a) The wheat55K SNP based detection of QTL for spike length and plant height in wheat. J Triticeae Crop 131(11):1284–1292 (in Chinese)
Li F, Wen W, Liu J, Zhang Y, Cao S, He Z, Rasheed A, Jin H, Zhang C, Yan J (2019b) Genetic architecture of grain yield in bread wheat based on genome-wide association studies. BMC Plant Biol 19:168
Lin H, Qian H, Zhuang J, Lu J, Min S, Xiong Z, Huang N, Zheng K (1996) RFLP mapping of QTLs for yield and related characters in rice (Oryza sativa L.). Theor Appl Genet 92:920–927
Linn T, Bachir D, Chen L, Hu Y (2017) Effects of gibberellic acid responsive dwarfing gene Rht9 on plant height and agronomic traits in common wheat. Am J Agric Fores 5:102–111
Liu G, Jia L, Lu L, Qin D, Zhang J, Guan P, Ni Z, Yao Y, Sun Q, Peng H (2014) Mapping QTLs of yield-related traits using RIL population derived from common wheat and Tibetan semi-wild wheat. Theor Appl Genet 127:2415–2432
Liu J, Luo W, Qin N, Ding P, Zhang H, Yang C, Mu Y, Tang H, Liu Y, Li W (2018) A 55 K SNP array-based genetic map and its utilization in QTL mapping for productive tiller number in common wheat. Theor Appl Genet 131(11):2439–2450
Liu J, Tang H, Qu X, Liu H, Li C, Tu Y, Li S, Habib A, Mu Y, Dai S, Deng M, Jiang Q, Liu Y, Chen G, Wang J, Chen G, Li W, Jiang Y, Wei Y, Lan X, Zheng Y, Ma J (2020) A novel, major, and validated QTL for the effective tiller number located on chromosome arm 1BL in bread wheat. Plant Mol Biol. 104:173–185
Ma H, Zhang S, Ji L, Zhu H, Yang S, Fang X, Yang R (2006) Fine mapping and in silico isolation of the EUI1 gene controlling upper internode elongation in rice. Plant Mol Biol 60:87–94
Ma J, Sun M, Ding P, Luo W, Zhou X, Yang C, Zhang H, Qin N, Yang Y, Lan X (2017) Genetic identification of QTL for neck length of spike in wheat. J of Triticeae Crop 37(3):319–324 (in Chinese)
Ma J, Ding P, Liu J, Li T, Zou Y, Habib A, Mu Y, Tang H, Jiang Q, Liu Y, Chen G, Wang J, Deng M, Qi P, Li W, Pu Z, Zheng Y, Wei Y, Lan X (2019a) Identification and validation of a major and stably expressed QTL for spikelet number per spike in bread wheat. Theor Appl Genet 132:3155–3167
Ma J, Qin N, Cai B, Chen G, Ding P, Zhang H, Yang C, Huang L, Mu Y, Tang H, Liu Y, Wang J, Qi P, Jiang Q, Zheng Y, Liu C, Lan X, Wei Y (2019b) Identification and validation of a novel major QTL for all-stage stripe rust resistance on 1BL in the winter wheat line 20828. Theor Appl Genet 132:1363–1373
Ma J, Zhang H, Li S, Zou Y, Li T, Liu J, Ding P, Mu Y, Tang H, Deng M (2019c) Identification of quantitative trait loci for kernel traits in a wheat cultivar Chuannong16. BMC Genet 20:77
Ma J, Tu Y, Zhu J, Luo W, Liu H, Li C, Li S, Liu J, Ding P, Habib A (2020) Flag leaf size and posture of bread wheat: genetic dissection, QTL validation and their relationships with yield-related traits. Theo Appl Genet 133:297–315
Miralles D, Katz S, Colloca A, Slafer G (1998) Floret development in near isogenic wheat lines differing in plant height. Field Crop Res 59:21–30
Qiao B, Wang Y, Zhu X, Hong D (2007) QTL analysis of the uppermost internode length in rice under different growing environments. Hereditas 29:1001–1007
Reynolds M, Foulkes M, Slafer G, Berry P, Parry M, Snape J, Angus W (2009) Raising yield potential in wheat. J Exp Bot 60:1899–1918
Semenov AM, Stratonovitch P (2013) Designing high-yielding wheat ideotypes for a changing climate. Food Energy Secur 2:185–196
Smith SE, Kuehl RO, Ray IM, Hui R, Soleri D (1998) Evaluation of simple methods for estimating broad-sense heritability in stands of randomly planted genotypes. Crop Sci 38:1125–1129
Sun L, Yang W, Li Y, Shan Q, Ye X, Wang D, Yu K, Lu W, Xin P, Pei Z, Guo X, Liu D, Sun J, Zhan K, Chu J, Zhang A (2019) A wheat dominant dwarfing line with Rht12, which reduces stem cell length and affects gibberellic acid synthesis, is a 5AL terminal deletion line. Plant J 97:887–900
Tao Y, Yi X, Lin Y, Wang Z, Wu F, Jiang X, Liu S, Deng M, Ma J, Chen G (2019) Quantitative trait locus mapping for panicle exsertion length in common wheat using two related recombinant inbred line populations. Euphytica 215:104
Tian J, Deng Z, Zhang K, Yu H, Jiang X, Li C (2015) Genetic analysis of main physiological and morphological traits. Springer, pp 351–443
Wu X, Wang Z, Chang X, Jing R (2010) Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. J Exp Bot 61:2923–2937
Yao R, Zhu W (1998) The correlation analysis of the stem traits and lodging in wheat. J Guangxi Agric Univ 17(16–18):23 (in Chinese)
Yao J, Yao G, Wang S, Yang X (2003) Inheritance of neck length of spike and its breeding value in wheat. J Guangxi Agric Univ:16–18 (in Chinese)
Yu M, Mao S, Chen G, Pu Z, Wei Y, Zheng Y (2014) QTLs for uppermost internode and spike length in two wheat RIL populations and their affect upon plant height at an individual QTL level. Euphytica 200:95–108
Yu SB, Li JX, Xu CG, Tan YF, Li XH, Zhang Q (2002) Identification of quantitative trait loci and epistatic interactions for plant height and heading date in rice. Theor Appl Genet 104:619–625
Zhang K, Tian J, Zhao L, Wang S (2008a) Mapping QTLs with epistatic effects and QTL x environment interactions for plant height using a doubled haploid population in cultivated wheat. J Genet Genomics 35:119–127
Zhang Y, Zhu Y, Peng Y, Yan D, Li Q, Wang J, Wang L, He Z (2008b) Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice. Cell Res 18:412–421
Zhang J, Dell B, Biddulph B, Drake-Brockman F, Walker E, Khan N, Wong D, Hayden M, Appels R (2013) Wild-type alleles of Rht-B1 and Rht-D1 as independent determinants of thousand-grain weight and kernel number per spike in wheat. Mol breeding 32:771–783
Zhang N, Fan X, Cui F, Zhao C, Zhang W, Zhao X, Yang L, Pan R, Chen M, Han J (2017) Characterization of the temporal and spatial expression of wheat (Triticum aestivum L.) plant height at the QTL level and their influence on yield-related traits. Theor Appl Genet 130:1235–1252
Zhang W, Li Z, Fang H, Zhang M, Duan L (2018) Analysis of the genetic basis of plant height-related traits in response to ethylene by QTL mapping in maize (Zea mays L.). PloS one 13:e0193072
Zhen L, Qingguo W, Jiaowen P, Wei L, Center BR (2018) Functional analysis of height regulation of cytochrome P450 gene OsDWARF48 in Rice. Shandong Agricul Sci:1–9 (in Chinese)
Zhu J (1995) Analysis of conditional genetic effects and variance components in developmental genetics. Genetics 141:1633–1639
Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, Mao B, Hanada A, Zhou H, Wang R, Li P, Zhu X, Mander LN, Kamiya Y, Yamaguchi S, He Z (2006) ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18:442–456
Acknowledgments
This work is supported by the National Natural Science Foundation of China (31971937 and 31970243), the Applied Basic Research Programs of Science and Technology Department of Sichuan Province (2020YJ0140), and the Key Projects of Scientific and Technological Activities for Overseas Students of Sichuan Province. We thank the anonymous referees for critical reading and revising this manuscript.
Author information
Authors and Affiliations
Contributions
CL carried out the entire research and participated in drafting this manuscript. HPT and WL participated in phenotype measurement and data analysis. XMZ, YM, and MD participated in fieldwork and data analysis. YXL and QTJ assisted with data collection and analysis. GYC, JRW, PFQ, ZEP, and YFJ performed data processing, QTL analysis and manuscript revision. YMW and YLZ discussed results and revised the manuscript. XJL initiated the project, guided the study, and revised the manuscript. JM designed the experiments, guided the entire study, participated in data analysis, wrote and extensively revised this manuscript. All authors participated in the research and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical standards
All experiments and data analyses were conducted in Sichuan. All authors contributed to the study and approved the final version for submission. The manuscript has not been submitted to any other journal.
Additional information
Communicated by Takao Komatsuda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Tang, H., Luo, W. et al. A novel, validated, and plant height-independent QTL for spike extension length is associated with yield-related traits in wheat. Theor Appl Genet 133, 3381–3393 (2020). https://doi.org/10.1007/s00122-020-03675-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03675-0