Abstract
Key message
Fine mapping of Yr47 and Lr52 in chromosome arm 5BS of wheat identified close linkage of the marker sun180 to both genes and its robustness for marker-assisted selection was demonstrated.
Abstract
The widely effective and genetically linked rust resistance genes Yr47 and Lr52 have previously been mapped in the short arm of chromosome 5B in two F3 populations (Aus28183/Aus27229 and Aus28187/Aus27229). The Aus28183/Aus27229 F3 population was advanced to generate an F6 recombinant inbred line (RIL) population to identify markers closely linked with Yr47 and Lr52. Diverse genomic resources including flow-sorted chromosome survey sequence contigs representing the orthologous region in Brachypodium distachyon, the physical map of chromosome arm 5BS, expressed sequence tags (ESTs) located in the 5BS6-0.81-1.00 deletion bin and resistance gene analog contigs of chromosome arm 5BS were used to develop markers to saturate the target region. Selective genotyping was also performed using the iSelect 90 K Infinium wheat SNP assay. A set of SSR, STS, gene-based and SNP markers were developed and genotyped on the Aus28183/Aus27229 RIL population. Yr47 and Lr52 are genetically distinct genes that mapped 0.4 cM apart in the RIL population. The SSR marker sun180 co-segregated with Lr52 and mapped 0.4 cM distal to Yr47. In a high resolution mapping population of 600 F2 genotypes Yr47 and Lr52 mapped 0.2 cM apart and marker sun180 was placed 0.4 cM distal to Lr52. The amplification of a different sun180 amplicon (195 bp) than that linked with Yr47 and Lr52 (200 bp) in 204 diverse wheat genotypes demonstrated its robustness for marker-assisted selection of these genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common wheat (Triticum aestivum L.) is among the most important cereal crops grown around the world for human consumption (Gustafson et al. 2009). Demand for wheat is expected to increase by 60% by 2050 (Rosegrant et al. 2008). Stripe rust and leaf rust lead the list of diseases that result in 14 to 27% yield losses in wheat every year in many countries (Kosina et al. 2007). These diseases have been estimated to cause annual losses of A$139 million in Australia alone (Murray and Brennan 2009).
Deployment of rust resistance genes in new cultivars is one of the key objectives of wheat breeding programs worldwide. Pyramiding of two or more genes in new cultivars has been suggested to be an effective disease resistance breeding strategy (Bariana and McIntosh 1995; Singh et al. 2000). Combinations of all stage resistance (ASR) and adult plant resistance (APR) genes provide effective and long lasting resistance (Bariana et al. 2007; Kolmer 2013). Pyramiding of ASR and APR genes in a single genotype can be difficult to select phenotypically due to complete or near complete protection provided by single ASR genes. This limitation is slowly being overcome with advances in the identification of robust marker-trait associations (Bariana 2003; Bariana et al. 2007; Choudhary et al. 2008; http://maswheat.ucdavis.edu/; Yang et al. 2015), which allow direct selection for rust resistance genes without phenotyping. Molecular markers linked closely with several rust resistance genes have been identified in wheat (Bariana et al. 2007; http://maswheat.ucdavis.edu/) and are now being used to produce desired combinations of genes in future cultivars.
Donor sources that carry linked resistance to more than one disease have been exploited in wheat breeding programs. Triple rust resistance (Sr31/Lr26/Yr9) carried on the short arm of rye chromosome 1RS was commonly deployed in wheat cultivars released from the CIMMYT germplasm (Singh et al. 2006). Similarly, sources of linked rust resistance (Sr38/Lr37/Yr17 and Sr24/Lr24) have been used intensively in Europe and Australia (H.S. Bariana unpublished results). Matching virulences for these genes have now been detected (Bariana et al. 2007; Jin et al. 2008; Pretorius et al. 2000; Wellings 2007; http://sydney.edu.au/agriculture/documents/pbi/cereal_rust_report_2014_vol_12_3.pdf).
Several sources of linked ASR genes for resistance to stripe rust and leaf rust including Yr35/Lr53 (Marais et al. 2005), Yr40/L57 (Kuraparthy et al. 2007), Yr42/Lr62 (Marais et al. 2009), Yr47/Lr52 (Bansal et al. 2011) and Yr70/Lr76 (Bansal et al. 2015) have been identified in the last decade. All these genes, except Yr47/Lr52 (Aus28183), are located on translocated segments from related species and may result in linkage drag of deleterious traits. Aus28183 is not expected to suffer from this phenomenon. Yr47 and Lr52 confer resistance against predominant Australian, Indian and Kenyan pathotypes (H.S. Bariana unpublished work). Lr52 was shown to be effective against 29 Pt isolates and was located in the short arm of chromosome 5B by Hiebert et al. (2005). Bansal et al. (2011) mapped Yr47 in the short arm of chromosome arm 5B in two F3 populations (Aus28183/Aus27229 and Aus28187/Aus27229) and showed its genetic association with Lr52. These genes were flanked by markers gwm234 and cfb309 at about 10 cM distally and proximally, respectively. This study utilised available genomic resources to saturate the Yr47 and Lr52 carrying chromosome arm 5BS region to develop robust DNA markers that can be reliably used for marker-assisted selection of these genes in wheat breeding programs.
Materials and methods
Plant materials
A recombinant inbred line (RIL) F6 population of 120 lines was developed from the Aus28183/Aus27229 F3 population. A set of 84 Australian and 120 Nordic wheat genotypes was used to validate the linkage of DNA markers with Yr47 and Lr52. Aus28183 was crossed with an Australian cultivar Ventura and F1 plant was backcrossed (BC) with Ventura to generate BC3F1. BC3F1 plants were selfed to generate BC3F3 population which was phenotyped for rust resistance and six homozygous resistant families were selected. These six backcross derivatives carrying Yr47 and Lr52 were also tested with the linked markers.
Greenhouse studies
The Aus28183/Aus27229 RIL population and parents Aus28183 and Aus27229 were screened against Puccinia striiformis f. sp. tritici (Pst) and P. triticina (Pt) in the greenhouse. Nine centimeter diameter pots were filled with soil comprising a mixture of sand and pine bark in the ratio of 2:1. Water soluble fertilizer Aquasol (20 g/10L of tap water) was applied to all pots before sowing. Four lines per pot with eight seeds per line were sown and the pots were placed in a rust-free microclimate room at 20 °C. Seven days after sowing, urea was applied to the seedlings at the same rate as Aquasol. Seedlings were inoculated with the Pst pathotype 134 E16A+Yr17+Yr27+ (culture 617) and the Pt pathotype 104 1,(2),3,(6),(7),11,13 (culture 547) at the 2-leaf stage. Rust spores were suspended in the light mineral oil Isopar-L and atomized on seedlings using an aerosol pressure pack (McIntosh et al. 1995). Stripe rust inoculated seedlings were shifted to the incubation room on water filled steel trays and covered tightly with polythene hoods for 24 h at 9–12 °C, whereas leaf rust inoculated seedlings were shifted to a humidified chamber for 24 h. Stripe rust and leaf rust inoculated seedlings were then transferred to microclimate rooms set at 17 ± 2 and 25 ± 2 °C, respectively. Seedling rust response assessments were made 12–16 days after inoculation using the scales described in McIntosh et al. (1995).
DNA extraction and quantification
DNA was extracted from 10-day-old seedlings of the RIL population and parents Aus28183 and Aus27229 using the protocol described in Bansal et al. (2014a). DNA samples were quantified using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies) and diluted to 30 ng/µl concentration.
Marker development and saturation of the target 5BS region
Previously reported markers cfb306, cfb309, gwm234 and mag705 (Bansal et al. 2011) were genotyped on the entire RIL population. The sequences of these primers and their annealing temperatures are given in Table 1.
Various genomic resources were used to develop new markers in the target region based on the approach described in Bansal et al. (2014a, b). Flow-sorted chromosome survey sequence (CSS) 5BS contigs of variety Chinese Spring (CS) (International Wheat Genome Consortium, http://www.wheatgenome.org) were initially used as BlastN queries against the Brachypodium distachyon genome sequence to identify orthologous regions. Once identified, B. distachyon genes in the orthologous region were used to identify additional CSS contigs through reciprocal BlastN analysis. In all cases, the CSS query with the best hit was considered to be syntenic to B. distachyon. CSS contigs within the target region were then tested for nucleotide repeats using the SSR IT tool (http://www.gramene.org/db/markers/ssrtool) and SSR markers were developed. Primers were designed using Primer 3 software (http://frodo.wi.mit.edu/primer3/). In addition, SSR markers from the physical map (Nesterov et al. 2015) of chromosome arm 5BS were also used in mapping. An M13 sequence tail was added to the 5′ end of each forward primer (Table 1). The new primers were labeled as “sun” (Sydney University) and “icg” (Institute of Cytology and Genetics).
Selective genotyping was performed on DNA from eight homozygous resistant and eight homozygous susceptible RILs using the Illumina iSelect 90 K Infinium wheat array (Wang et al. 2014) to identify SNPs associated with Yr47 and Lr52. Closely linked SNPs were converted into Kompetitive allele specific PCR (KASP) having two allele specific forward primers and one common reverse primer. Twenty expressed sequence-tags (ESTs), gene-specific, SSR and STS based markers developed by Shi et al. (2016) were also used to saturate the chromosome 5BS region.
Markers flanking Yr47 and Lr52 were also used to screen 600 F2 plants from the Aus27729/Aus28183 cross. The recombinant genotypes were raised to F3 generation for phenotypic evaluation against stripe rust and leaf rust.
PCR amplification and visualization
For all SSR, STS, EST and gene-based markers, PCR was carried out in a 10 µl reaction mixture containing 60 ng DNA, 1× Immolase buffer, 1.5 mM MgCl2, 250 μM dNTPs, 100 nM M13 tailed forward primer, 200 nM reverse primer, 5 nM M13 dye with labeled fluorescence (IR-700 or IR-800), 0.04 U of Immolase DNA polymerase (Bioline). Thermocycling was performed with the touch down profile: initial denaturation at 95 °C for 10 min, followed by 92 °C for 30 s, 65 or 60 °C (with 1 °C drop down every cycle) for 30 s and 72 °C for 30 s for 5 cycles, and 35 cycles of 30 s at 92 °C, 30 s at 60 or 55 °C (depending upon the annealing temperature of primers) and 30 s at 72 °C and a final extension step of 72 °C for 10 min.
The amplified PCR products were visualized on 2% agarose gel stained with GelRedTM (Biotium) using a UV gel documentation unit (UVP-GelDoc-It). A 1 Kb DNA ladder (Fermentas) was run alongside the products to define allele sizes. To resolve small base pair differences, the amplified products were separated in 6.5% polyacrylamide gel on a LICOR 4300 DNA analyser using the protocol described in Randhawa et al. (2015).
KASP genotyping
KASP assays were performed in an 8 µl reaction mixture containing 3 µl of 30 ng/µl genomic DNA, 0.11 µl of primer mix having 12 µM each of the two allele specific forward primers and 30 µM of common reverse primer, 0.89 µl of double distilled water and 4 µl of KASP mix comprised of Taq polymerase, dNTPs, buffer with MgCl2, universal FAM and HEX fluorescence resonance energy transmitted cassettes and ROX™ reference dye (KD Biosciences).
PCR was performed using the Bio-Rad, USA CFX96 Touch™ real time PCR detection system. The PCR profile included an initial denaturation step of 94 °C for 15 min followed by 9 touchdown cycles at 94 °C for 20 s and 61 °C for 60 s dropping 0.6 °C per cycle and 38 cycles at 94 °C for 20 s and 55 °C for 60 s. Allelic discrimination was achieved using the Bio-Rad CFX Manager software (Bio-Rad, USA).
Statistical analysis and molecular mapping
Chi-squared analysis was employed to observe the goodness of fit of the observed phenotypic segregations to the expected genetic ratios. The genetic linkage map was constructed using MapDisto v.1.8 software (Lorieux 2012) using the Kosambi mapping function (Kosambi 1943) and the linkage map was drawn using Map Chart version 2.2 (Voorrips 2002).
Results
Phenotypic evaluation
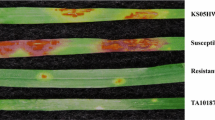
The resistant parent Aus28183 produced infection type (IT) ;C-1CN against Pst pathotype 134 E16A+Yr17+Yr27+ and IT0;-;1 against Pt pathotype 104-1,(2),3,(6),(7),11,13 (Fig. 1). The susceptible parent Aus27729 exhibited IT3+ for both rust diseases (Fig. 1). The RIL population was classified as homozygous resistant (HR) and homozygous susceptible (HS) on the basis of rust responses in the greenhouse. Chi-squared analysis of rust response variation data for both leaf rust and stripe rust conformed to monogenic segregation among the Aus28183/Aus27729 RIL population (Table 2). Two recombinants between Yr47 and Lr52 were identified. One recombinant showed susceptibility to leaf rust but was resistant to stripe rust and the other recombinant exhibited opposite responses (Table 2).
Molecular mapping
Sixty SSR markers designed through the identification of orthologous genes in Brachypodium distachyon from the wheat genome survey sequences and 21 markers from the physical map (Nesterov et al. 2015) were used to saturate the Yr47 and Lr52 region. Each marker was tested for linkage to Yr47 and Lr52 using the parental lines and a pair of DNA bulks comprised of RILs with resistance and susceptibility to the two rust diseases. Twenty-five markers that showed linkage between bulks were genotyped on the entire RIL population and seven markers (sun180, sun183, sun480, icg16c004_2, icg16c008, icg16c041 and icg16c041_2) were used for genetic map construction (Table 1).
Twenty SSR and STS markers developed from wheat ESTs located in the 5BS6-0.81-1.00 deletion bin (Shi et al. 2016) and resistance gene analog (RGA) contigs from chromosome arm 5BS were similarly tested for association with Yr47 and Lr52. Four markers (fcp652, fcp657, mag705 and TC252302) showing linkage were genotyped on the entire RIL population and used for linkage map construction (Table 1).
Selective genotyping performed using the Illumina iSelect 90 K Infinium wheat array identified 36 SNPs that showed linkage with Yr47 and Lr52. Single-marker KASP assays designed for each linked SNP were tested on the parental lines. Sixteen KASP assays giving good SNP allele discrimination were genotyped on the entire RIL population. Eight KASP markers (KASP_75279, KASP_12199, KASP_25183, KASP_1736, KASP_36048, KASP_8814, KASP_7565 and KASP_6180) were incorporated into the genetic map (Table 3).
A total of 22 unambiguous markers showing linkage with Yr47 and Lr52 were used to construct a genetic map for the RIL population (Fig. 2). The map included the previously reported SSR markers gwm234, cfb306 and cfb309 (Bansal et al. 2011), which were also genotyped on the entire RIL population. The total genetic distance of the map was 11.2 cM. Yr47 and Lr52 mapped 0.4 cM apart. SSR marker sun180 co-segregated with Lr52 and mapped 0.4 cM distal to Yr47. The SSR marker sun180 corresponded to the Brachypodium gene Bradi4g00550.
Genetic linkage map of chromosome 5BS: a Aus28183/Aus27229 F3 (Bansal et al. 2011) b Aus28183/Aus27229 F6 RIL c B. distachyon chromosome 4 marked with significant hits with wheat survey sequences within the Yr47/Lr52 interval and d High resolution map of Aus27229/Aus28183
Construction of a high-resolution linkage map
Marker sun180 that co-segregated with Lr52 and the flanking markers sun480, fcp652, icg16c008 and gwm234 spanning a 5.1 cM region were genotyped on 600 F2 (Aus27729/Aus28183) plants to identify recombinants for the construction of a high resolution genetic map. Forty-five recombination events were identified from the 1200 gametes screened. Recombinant F2 plants were selected and raised to generate F3 generation. The F3 generation was tested against Pst and Pt pathotypes. The recombinant F2 progenies were scored as homozygous resistant, homozygous susceptible and segregating.
Marker sun180, which co-segregated with Lr52 in the lower resolution map of the RIL population, mapped 0.4 and 0.6 cM to Lr52 and Yr47, respectively. Yr47 and Lr52 mapped 0.2 cM apart (Fig. 2). Markers sun480 and fcp652 mapped 0.4 cM and 0.6 cM distal to sun180, respectively. The interval between Yr47 and markers gwm234 and icg16c008 was reduced to 1.4 and 1.8 cM, respectively, in the high resolution map.
Evaluation of markers on Australian and Nordic wheats
Markers sun180, sun480, mag705 and fcp652, which showed close linkage with Yr47 and Lr52 among the RIL population, were evaluated on 84 Australian and 120 Nordic wheat genotypes. Marker sun180 amplified a 200 bp fragment in the resistant parent Aus28183 and a 195 bp product in the susceptible parent Aus27729. None of the cultivars contained the 200 bp allele associated Yr47 and Lr52 (Table 4; Fig. 3). Markers sun480, mag705 and fcp652 amplified 32 false positives in Australian and Nordic wheat cultivars indicating their loose genetic association with Yr47 and Lr52. Thus, marker sun180 proved to be diagnostic for the selection of Yr47 and Lr52. Six backcross derived genotypes carrying Yr47 and Lr52 in the wheat cultivar Ventura background produced a 200 bp amplicon with sun180, demonstrating its usefulness for marker-assisted selection.
Discussion
Breeding for durable rust resistance is challenging due to continuous evolution of virulence in pathogen populations. Pyramiding of two or more genes in new cultivars is essential to reduce the rate of breakdown of host resistance due to evolution of virulence in pathogen populations. This can be best achieved through the incorporation of modern molecular technologies, such as marker-assisted selection, in wheat improvement programs. Bansal et al. (2011) identified a genetic association between rust resistance genes Yr47 and Lr52, with a recombination distance of 3.3 cM based on F3 analysis. The advancement of the F3 population to F6 in this study reduced this distance to 0.4 cM. Likewise the genetic distance between the flanking markers gwm234 and cfb309 was also significantly reduced (Fig. 1b). Mapping of markers developed from various genomic resources changed the orientation of the genetic map originally reported in Bansal et al. (2011). Marker cfb309 mapped 1.3 cM distal to Lr52, whereas gwm234 mapped 4.3 cM proximal to Yr47. This marker order corresponds with that reported by Alfares et al. (2009), where cfb306 was completely linked with the Skr gene and gwm234 mapped 0.3 cM proximal to it. Further, high resolution mapping reduced the total genetic distance between markers fcp652 and icg16c008 to 3 cM, compared with 5.1 cM in the lower resolution map developed using the RIL population. The identification of recombinants in the high resolution mapping increased the genetic distance distal to Lr52 (sun180 which co-segregated in the low resolution map was mapped 0.4 cM distal to Lr52), but decreased the distance proximal to Yr47. Recombination breakpoints and the ratio of physical to genetic distance determines the position of target locus in the region (Dawson et al. 2016) and, therefore, the recombinants identified in this study can be used to provide a step forward for map-based cloning of Yr47 and Lr52. Two high resolution mapping studies involving Tsn1 (Lu et al. 2006) and Snn3-B1 (Shi et al. 2016) loci on the short arm of chromosome 5B of wheat also resulted in the identification of closely linked markers.
The availability of extensive genomic resources provided critical information for saturating the Yr47 and Lr52 region. The Chinese Spring genomic sequences proved to be the best resource in this study for saturating the region. Markers developed from the Chinese Spring flow-sorted chromosome survey sequence contigs (IWGSC 2014) showed a higher level of polymorphism compared to markers developed from other genomic resources. The chromosome survey sequence contigs successfully identified the orthologous region in chromosome 4 of Brachypodium distachyon and allowed the development of 60 new SSR markers within the chromosome arm 5BS region containing Yr47 and Lr52. High colinearity between the wheat and Brachypodium genomes in this region led to the development of closely linked marker (sun180) to Yr47 and Lr52 (supplementary Table S1 and Fig. 2c). The syntenic interval in Brachypodium, delineated by genes Bradi4g00490 and Bradi4g00550, carries four genes with putative kinase domains. There appears to be a gap between Bradi4g00550 and Bradi4g00620. While markers developed from the chromosome arm 5BS physical map and iSelect 90 K wheat SNP genotyping were useful as well, but they did not yield markers closely linked to Yr47 and Lr52. The high resolution genetic linkage map constructed in this study will be useful for map-based cloning of Yr47 and Lr52. The recent development of a high-quality wheat reference genome sequence combined with the availability of the physical map and BAC clones will facilitate faster determination of the physical region carrying Yr47 and Lr52. For example, the nine genes in the syntenic interval in Brachypodium were contained in nine scaffolds of the most recent publicly released assembly of the bread wheat genome (TGACv1, http://plants.ensembl.org/) developed by The Centre for Genome Analysis (TGAC) at the Earlham Institute, the United Kingdom. The nine TGAC scaffolds had a total length of 1.273 Mbp, compared to 115 Kbp for the nine CSS contigs in which the same Brachypodium genes were located (supplementary Table S2). This 11-fold increase in availability of assembled genome sequence illustrates the potential for faster map-based gene cloning in the near future. Validation of markers developed from bi-parental mapping populations on diverse genotypes carrying and/or lacking the target locus is essential to demonstrate their diagnostic value in marker-assisted selection (Sharp et al. 2001; Bariana et al. 2016). The absence of the Yr47- and Lr52-linked sun180 allele in 84 Australian and 120 Nordic wheat cultivars demonstrated its robustness for use in marker-assisted selection of Yr47 and Lr52 in these genetic backgrounds. Markers linked with several widely effective rust resistance genes have been reported in the last decade and protocols are available on MASWheat website (http://maswheat.ucdavis.edu/ ) for use in breeding programs. The marker sun180 is currently being used in the marker-assisted pyramiding of Yr47 and Lr52 with Lr34/Yr18/Sr57, Lr67/Yr46/Sr55 and Sr22 in the Australian Cereal Rust Control Program to produce triple rust resistant material based on ASR and APR genes to achieve durable rust control.
Author contribution statement
NQ drafted the manuscript; HB and UB developed segregating population; NQ, HB and UB did rust phenotyping; BK, TW, JF, ES provided primer information; NQ did marker work; UB, KF and MH designed primers; UB, HB, BK, TW, JF, ES, KF and MH edited the manuscript.
References
Alfares W, Bouguennec A, Balfourier F, Gay G, Bergès H, Vautrin S, Sourdille P, Bernard M, Feuillet C (2009) Fine mapping and marker development for the crossability gene SKr on chromosome 5BS of hexaploid wheat (Triticum aestivum L.). Genetics 183:469–481
Bansal UK, Forrest K, Hayden M, Miah H, Singh D, Bariana H (2011) Characterisation of a new stripe rust resistance gene Yr47 and its genetic association with the leaf rust resistance gene Lr52. Theor Appl Genet 122:1461–1466
Bansal UK, Kazi AG, Singh B, Hare RA, Bariana HS (2014a) Mapping of durable stripe rust resistance in a durum wheat cultivar Wollaroi. Mol Breed 33:51–59
Bansal U, Bariana H, Wong D, Randhawa M, Wicker T, Hayden M, Keller B (2014b) Molecular mapping of an adult plant stem rust resistance gene Sr56 in winter wheat cultivar Arina. Theor Appl Genet 127:1441–1448
Bansal M, Kaur S, Dhaliwal H, Bariana HS, Bansal UK, Chhuneja P (2015) Introgression of linked rust resistance genes Lr76 and Yr70 from Aegilops umbellulata to wheat chromosome 5DS. Plant Pathol. doi:10.1111/ppa.12549
Bariana HS (2003) Breeding for disease resistance. In: Thomas B, Murphy DJ, Murray BG (eds) Encyclopedia of applied plant sciences. Harcourt, Academic Press, UK, pp 244–253
Bariana H, McIntosh R (1995) Genetics of adult plant stripe rust resistance in four Australian wheats and the French cultivar ‘Hybride-de-Bersée’. Plant Breed 114:485–491
Bariana HS, Brown GN, Bansal UK, Miah H, Standen GE, Lu M (2007) Breeding for triple rust resistance wheat cultivars for Australia using conventional and marker assisted selection technologies. Aust J Agric Res 58:576–587
Bariana HS, Forrest K, Qureshi N, Miah H, Hayden M, Bansak UK (2016) Adult plant stripe rust resistance Yr71 maps close Lr24 in chromosome 3D of common wheat. Mol Breed. doi:10.1007/s11032-016-0528-1
Choudhary K, Choudhary O, Shekhawat N (2008) Marker assisted selection: a novel approach for crop improvement. Am-Eurasian J Agron 1:26–30
Dawson AM, Ferguson JN, Gardiner M, Green P, Hubbard A, Moscou MJ (2016) Isolation and fine mapping of Rps6: an intermediate host resistance gene in barley to wheat stripe rust. Theor Appl Genet 129:1–13
Gustafson P, Raskina O, Ma X, Nevo E (2009) Wheat evolution, domestication, and improvement. Sci Trade, Wheat, pp 5–30
Hiebert C, Thomas J, McCallum B (2005) Locating the broadspectrum wheat leaf rust resistance gene Lr52 (LrW) to chromosome 5B by a new cytogenetic method. Theor Appl Genet 110:1453–1457
International Wheat Genome Sequencing Consortium (2014) A chromosome-based draft sequence of the hexaploid bread wheat genome. Science 345:1251788
Jin Y, Szabo L, Pretorius Z, Singh R, Ward R, Fetch T Jr (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 92:923–926
Kolmer J (2013) Leaf rust of wheat: pathogen biology, variation and host resistance. Forests 4:70–84
Kosambi DD (1943) The estimation of map distances from recombination values. Annu Eugen 12:172–175
Kosina P, Reynolds M, Dixon J, Joshi A (2007) Stakeholder perception of wheat production constraints, capacity building needs, and research partnerships in developing countries. Euphytica 157:475–483
Kuraparthy V, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, Gill BS (2007) Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor Appl Genet 114:1379–1389
Lorieux M (2012) MapDisto: fast and efficient computation of genetic linkage maps. Mol Breed 30:1231–1235
Lu HJ, Fellers JP, Friesen TL, Meinhardt SW, Faris JD (2006) Genomic analysis and marker development for the Tsn1 locus in wheat using bin-mapped ESTs and flanking BAC contigs. Theor Appl Genet 112:1132–1142
Marais G, Pretorius Z, Wellings C, McCallum B, Marais A (2005) Leaf rust and stripe rust resistance genes transferred to common wheat from Triticum dicoccoides. Euphytica 143:115–123
Marais F, Marais A, McCallum B, Pretorius Z (2009) Transfer of leaf rust and stripe rust resistance genes and from req. ex Bertol. to common wheat. Crop Sci 49:871–879
McIntosh RA, Welling CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO, Melbourne, p 200
Murray GM, Brennan JP (2009) Estimating disease losses to the Australian wheat industry. Aust Plant Pathol 38:558–570
Nesterov MA, Afonnikov DA, Sergeeva EM, Miroshnichenko LA, Bragina MK, Bragin AO, Vasiliev GV, Salina EA (2015) Identification of microsatellite loci according to BAC sequencing data and their physical mapping to the bread wheat 5B chromosome. Vavilov J Genet Breed 19:707–714
Pretorius Z, Singh R, Wagoire W, Payne T (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84:203
Randhawa M, Bariana H, Mago R, Bansal U (2015) Mapping of a new stripe rust resistance locus Yr57 on chromosome 3BS of wheat. Mol Breed 35:1–8
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rosegrant MW, Msangi S, Ringler C, Sulser TB, Zhu T, Cline SA (2008) International model for policy analysis of agricultural commodities and trade (IMPACT): model description. International Food Policy Research Institute, Washington DC
Sharp PJ, Johnston S, Brown G, McIntosh RA, Palotta M, Carter M, Bariana HS, Khatkar S, Lagudah ES, Singh RP, Khairallah M, Potter R, Jones MGK (2001) Validation of molecular markers for wheat breeding. Aust J Agric Res 52:1357–1366
Shi G, Zhang Z, Friesen TL, Bansal U, Cloutier S, Wicker T, Rasmussen JB, Faris JD (2016) Marker development, saturation mapping, and high-resolution mapping of the Septoria nodorum blotch susceptibility gene Snn3-B1 in wheat. Mol Genet Genomics 291:107–119
Singh RP, Huerta-Espino J, Rajaram S (2000) Achieving near immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathol Entomol Hung 35:133–139
Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB reviews: perspectives in agriculture, veterinary science, nutrition and natural resources 1:1–13
Voorrips R (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, International Wheat Genome Sequencing C, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo MC, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E (2014) Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotech J 12:787–796
Wellings C (2007) Puccinia striiformis in Australia: a review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006. Aust J Agric Res 58:567–575
Xue S, Zhang Z, Lin F, Kong Z, Cao Y, Li C, Yi H, Mei M, Zhu H, Wu J, Xu H, Zhao D, Tian D, Zhang C, Ma Z (2008) A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags. Theor Appl Genet 117:181–189
Yang H, Li C, Lam HM, Clements J, Yan G, Zhao S (2015) Sequencing consolidates molecular markers with plant breeding practice. Theor Appl Genet 128:779–795
Acknowledgements
Naeela Qureshi acknowledges the University of Sydney for the USYDIS award. We thank the Grains Research and Development Corporation (GRDC) Australia for financial support through the Australian Cereal Rust Control Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have read the manuscript and declare that they have no conflict of interest.
Additional information
Communicated by A Zhang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qureshi, N., Bariana, H., Forrest, K. et al. Fine mapping of the chromosome 5B region carrying closely linked rust resistance genes Yr47 and Lr52 in wheat. Theor Appl Genet 130, 495–504 (2017). https://doi.org/10.1007/s00122-016-2829-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2829-5