Abstract
Key message
Resistance to Puccinia striiformis in 18 barleys was conferred by one or more genes. In two genotypes, resistance mapped to chromosomes 5HL and 7HL (seedling), and 5HS (adult plant).
Abstract
Twenty barley genotypes were assessed for resistance to a variant of P. striiformis [barley grass yellow rust (BGYR)] that is adapted to wild Hordeum sp. (barley grass) and is known to be virulent on several Australian barley cultivars. With the exception of Biosaline-19, all of the genotypes tested were resistant to BGYR. Genetic analyses of 16 Australian and three exotic barley lines indicated that each carried at least a single gene for resistance. Seedling resistance genes identified in the doubled haploid population developed from a cross between Franklin and Yerong were mapped to the long arm of chromosomes 5H and 7H, respectively. These genes were given the temporary designations of Rpsp-hFranklin and Rpsp-hYerong. Three QTL were detected in the same population when tested at the adult plant stage, two of them being in a similar position to Rpsp-hFranklin and Rpsp-hYerong and the third one was mapped to 5HS. Allelism tests between genotypes that exhibited seedling infection type responses to BGYR that were similar to Franklin and Yerong revealed that resistance in most were genetically independent of Rpsp-hFranklin and Rpsp-hYerong.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stripe rust, caused by Puccinia striiformis, is an important disease affecting cereals worldwide. The pathogens that cause stripe rust of wheat (Puccinia striiformis f. sp. tritici: Pst) and barley (P. striiformis f. sp. hordei: Psh) are considered separate formae speciales (special forms) even though the host ranges of these pathogens overlap (Stubbs 1985). In Australia, Pst was first detected in 1979 (O’Brien et al. 1980) and while it continues to be a serious disease of wheat, it is not an economically important disease of barley because all Australian barley cultivars are highly resistant to locally occurring Pst isolates (Wellings 2007). Psh has not been recorded in Australia; however, tests of Australian barley cultivars in CIMMYT (International Maize and Wheat Improvement Centre) Mexico revealed that a majority of cultivars are susceptible to Psh Race 24 (Wellings et al. 2000b).
In 1998, Wellings et al. (2000b) detected an unusual variant of P. striiformis that was isolated from wild Hordeum species in Australia. This variant was avirulent on most seedlings of the standard wheat stripe rust differential genotypes, including cultivars Morocco, Lemhi and Michigan Amber, which are recognised as standard international susceptible genotypes of Pst, but partially virulent on the wheat differential Chinese 166, which is used to assay for pathogenicity with respect to resistance gene Yr1 (Wellings et al. 2000a, b). Given that some pathotypes of Psh are partially virulent on Yr1 (Stubbs 1985), it was initially thought that the isolate from Hordeum spp. may have been Psh. However, subsequent detailed seedling tests of Australian barley cultivars revealed that most were resistant despite offshore testing showing they were broadly susceptible to Psh (Wellings et al. 2000b).
Molecular studies differentiated isolates of Pst, Psh and other P. striiformis taxa from this new pathogen, suggesting that it represented an exotic stripe rust incursion into Australia (Keiper et al. 2003; Spackman et al. 2010; Wellings et al. 2000a). Based on pathogenic and molecular characteristics, it was determined that the pathogen represented a new formae speciales of P. striiformis. Although referred to as barley grass yellow rust (BGYR), the designation P. striiformis f. sp. pseudo-hordei was proposed by Wellings (2010) to reflect the distinct pathogenic and molecular biology of this variant. BGYR currently represents the most immediate stripe rust threat to Australian barley production (Wellings et al. 2000b).
Previous studies revealed that the resistance of selected Australian barley cultivars to BGYR appeared to be based on a small number of genes (Golegaonkar 2007). This implied that simple mutational events in the BGYR pathogen could result in Psp-h gaining virulence on Australian barley cultivars and becoming a significant problem for the industry. In order to explore the current and potential impact of BGYR on the barley industry in Australia more fully, an analysis of genetic diversity and the inheritance of resistance with respect to Psp-h was undertaken among Australian and diverse barley germplasm.
Materials and methods
Genetic material
The pedigrees of 20 barley genotypes used in the present study are provided in Table 1 and Fig. 1. Three F2 and F3 populations based on crosses between the BGYR susceptible genotype “Biosaline-19” and the resistant cultivars Franklin, Molloy and Yerong were used to investigate the genetic basis of resistance to BGYR.
F2 populations derived from 15 crosses (Franklin/Molloy, Gilbert/Franklin, Franklin/Harrington, Franklin/Kaputar, Milby/Franklin, Franklin/Parwan, Franklin/Torrens, Windich/Franklin, Franklin/Yagan, ZUG159/Franklin, Dash/Yerong, Yerong/Fitzgerald, Naso nijo/Yerong, Yerong/Tilga and ZUG405/Yerong) and three doubled haploid (DH) populations (Franklin/Arapiles, Harrington/Gilbert and Yerong/Franklin) were used to examine linkage relationships between resistance genes in each parent.
Seedling inoculation procedures and disease assessment
Seedlings of all populations and parental lines were grown in 90 mm pots containing a mixture of pine bark fines and coarse sand, in a ratio of 4:1. Pots were fertilized with a soluble nitrogenous fertilizer (Aquasol®, Hortico Pty. Ltd., Revesby, NSW, Australia) at the rate of 35 g per 3 L for 100 pots, prior to sowing. Wheat stripe rust differential lines used to identify Pst pathotypes in Australia were sown five lines per pot, with ~8–10 seeds per line (Wellings and McIntosh 1990). Lines of the DH population were sown as two lines per pot. Approximately 150 F2 seedlings were phenotyped in the greenhouse for response to Psp-h and individuals were then transplanted into the field at 10 cm spacing. These plants were harvested independently at maturity to develop F3 populations. Twenty-five to 30 seeds of each F3 line were sown in a single pot for seedling disease assessments. All lines were maintained in the greenhouse before inoculation at 16–20 °C.
Seedlings were inoculated when the first leaf was fully expanded (8–10 days after sowing) with BGYR isolate 981549, isolated from infected barley grass in Victoria in 1998. Approximately 10 mg of urediniospores was suspended in 10 ml of light mineral oil (IsoparL®, Univar, NSW, Australia) and sprayed with a mist atomizer over the top of seedlings. Inoculated seedlings were lightly misted with double distilled water (to maintain leaf wetness for spore germination) and incubated in an enclosed chamber in the dark, overnight at 9–12 °C. After incubation the seedlings were transferred to naturally lit microclimate rooms, where temperatures were maintained at 16–20 °C.
Disease response was assessed 16–18 days post inoculation, using a 0–4 infection type (IT) scale as described for Pst by McIntosh et al. (1995). Where ‘0’ = no visible symptoms, ‘;’ = necrotic flecks, ‘;n’ = necrotic areas without sporulation, ‘1’ = necrotic and chlorotic areas with restricted sporulation; ‘2’ = moderate sporulation with necrosis and chlorosis, ‘3’ sporulation with chlorosis, ‘4’ abundant sporulation without chlorosis. Variations of the ITs were indicated by use of ‘−’ (less than average for the class), ‘+’ (more than average for the class), ‘c’ (chlorosis) and/or ‘n’ (necrosis). Where two predominant ITs are observed both ITs are recorded and separated with a comma (e.g. IT = ‘1,1+’ and IT = ‘3,3+’). Infection types of ‘3’ or higher were considered to indicate a compatible response (susceptibility in the host) (McIntosh et al. 1995).
Adult plant tests
The Yerong/Franklin doubled haploid population was evaluated in the 2006–2007 growing season in the birdcage at the Mt Pleasant Laboratories in Launceston, Tasmania. Each line was grown in a 2 m row with 0.4 m between rows. Fertilizer was applied at 150 kg DAP per ha. All experiments were arranged in a randomised complete block design with two replications. Disease severity was recorded for naturally occurring symptoms of BYGR at the ear emergence stage. A 0–3 scale was used to assess disease severity, where 0 indicated resistance and 3 indicated susceptibility in the host.
QTL analysis
A genetic linkage map produced for the Yerong/Franklin DH population using 496 DArT and 28 microsatellite (SSR) markers (Li et al. 2008) was used for QTL analysis. The software package MapQTL6.0 (Van Ooijen and Kyazma 2009) was used to detect QTL, which were first analysed by interval mapping. The closest marker at each putative QTL identified using interval mapping was selected as a cofactor and the selected markers were used as genetic background controls in the approximate multiple QTL model (MQM). Logarithm of the odds (LOD) threshold values, applied to declare the presence of a QTL, were estimated by performing the genome wide permutation tests implemented in MapQTL version 6.0, using at least 1,000 permutations of the original data set for each trait, resulting in a 95 % LOD threshold around 3.0. The percentage of variance explained by each QTL (R 2) was obtained using restricted MQM mapping implemented with MapQTL6.0.
Statistical analysis
The F2, F3 and DH populations were subjected to Chi squared (χ 2) analyses, in order to determine the goodness-of-fit of observed segregation frequencies to expected genetic ratios. Results are presented as P values, where χ 2 = 3.84 at P 1 df = 0.05. The minimum family size required to test a genetic hypothesis was calculated according to the formula: n = (logP)/logq (Hanson 1959), where P is the probability threshold being tested and q is the probability of failing to observe the phenotype.
Results
Seedling responses of the parental barley genotypes to BGYR are presented in Table 2. With the exception of susceptible genotype Biosaline-19 IT ‘3c’, all cultivars were resistant and ITs ranged from ‘0;’ to ‘11+c’. Cultivars were classified into three response groups (RGs) based on infection types. Very low ITs were observed in genotypes of RG1 (IT ‘0;’) and RG2 (IT ‘;+cn’ to ‘11+c’). RG3 consisted of the single susceptible genotype Biosaline-19. The similarity in IT between cultivars within RGs suggested that lines within these groups may carry a gene or genes in common. The observed seedling ITs were therefore used to design and select genetic populations, to determine the inheritance of resistance and to study the genetic diversity of genes conferring resistance to BGYR among these barley cultivars. Due to the presence of a DH population derived from Yerong (RG1) and Franklin (RG2), they were chosen as representative genotypes for their respective groups.
Genetic analysis
Three F2 populations derived from crossing resistant cultivars Franklin, Yerong and Molloy to the susceptible genotype Biosaline-19 were assessed for response to BGYR at the seedling growth stage. They were developed to investigate the genetic basis of resistance to BGYR in these cultivars. Individual F2 lines from these populations were scored as either resistant or susceptible, and results are presented in Table 3. The IT responses of parental genotypes are illustrated in Fig. 2. The frequency of resistant to susceptible lines observed in the Franklin/Biosaline-19 (\(\chi^{2}_{3:1}\) = 0.15, P = 0.70) and Yerong/Biosaline-19 (\(\chi^{2}_{3:1}\) = 2.46, P = 0.12) populations conformed to a 3:1 ratio consistent with the segregation of a single dominant resistance gene. The resistance in Yerong conferred an IT of ‘0;’, while that in Franklin conferred an IT of ‘11=c’ (Table 2). The Molloy/Biosaline-19 population segregated in the ratio of 13 resistant: 3 susceptible plants, which conformed to the segregation of a dominant and a recessive gene acting independently (\(\chi^{2}_{13:3}\) = 2.65, P = 0.10) (Table 3).
In order to determine genotypes of the F2 lines phenotyped as resistant and susceptible, F3 progeny obtained from individually harvested F2 plants were screened with BGYR isolate 981549 and scored as non-segregating resistant (NSR), segregating (Seg) and non-segregating susceptible (NSS). Results are presented in Table 4. The ratio of F3 lines derived from Franklin/Biosaline-19 conformed to a 1 NSR: 2 Seg: 1 NSS ratio, confirming the F2 prediction of a single gene segregating within this population (\(\chi^{2}_{1:2:1}\) = 0.43, P = 0.81) (Online Resource 1). F3 lines of the Molloy/Biosaline-19 population segregated 27 NSR: 33 Seg: 4 NSS (\(\chi^{2}_{7:8:1}\) = 0.29, P = 0.87), consistent with results obtained for the F2 generation (Table 3) and supporting the presence of two seedling resistance genes. Segregating lines within the latter F3 population conformed to ratios of 3:1, 13:3 and 1:3 resistant to susceptible (Online Resource 2), confirming the presence of two independent resistance genes, one dominant and one recessive (Table 4). However, the F3 lines from the Yerong/Biosaline-19 F2 population segregated in a two-gene segregation ratio (\(\chi^{2}_{7:8:1}\) = 2.30, P = 0.32), which differed from the expected one-gene segregation ratio (3:1) obtained in F2 (Table 4). The discrepancy observed between the F2 and F3 populations may have been due to the loss of field grown F2 plants as a result of severe rust infection compounded by unusually wet harvest conditions. This may explain the apparent deficiency in Seg and NSS F3 lines. Examination of the 32 Seg F3 lines revealed evidence for a single resistance gene (\(\chi^{2}_{\text{heterogeneity}}\) = 13.83, P 31df > 0.99) (Online Resource 3), which confirmed the prediction based on F2 tests.
The Yerong/Franklin DH population segregated in a 3 resistant: 1 susceptible ratio at the seedling stage (\(\chi^{2}_{3:1}\) = 0.01, P = 0.93) providing further evidence that single independent genes for resistance to BGYR isolate 981549 exists in both Yerong and Franklin (Table 5, Online Resource 4). The Yerong/Franklin DH population also segregated in a 3 resistant: 1 susceptible ratio at the adult plant stage (\(\chi^{2}_{3:1}\) = 1.94, P = 0.16).
Tests of allelism
Resistant barley genotypes in RG1 were crossed to Yerong and resistant barley genotypes in RG2 were crossed to Franklin because both these cultivars were shown to possess single independent dominant resistance genes to BGYR (Tables 3, 4 and Online Resource 3). Individual lines within these F2 populations were scored as either resistant or susceptible. Results from each F2 cross are presented in Table 6.
No susceptible segregates were observed in the crosses Franklin/Harrington, Windich/Franklin, Franklin/Parwan, Naso Nijo/Yerong, Dash/Yerong, and Yerong/Fitzgerald, indicating the presence of at least one common seedling resistance gene in the parental genotypes of each cross (Table 6). The distribution of resistant to susceptible lines within the F2 populations of Gilbert/Franklin (\(\chi^{2}_{15:1}\) = 1.61, P = 0.20), Milby/Franklin (\(\chi^{2}_{15:1}\) = 1.38, P = 0.24), ZUG159/Franklin (\(\chi^{2}_{15:1}\) = 4.92, P = 0.03), ZUG405/Yerong (\(\chi^{2}_{15:1}\) = 0.02, P = 0.89) and Yerong/Tilga (\(\chi^{2}_{15:1}\) = 0.54, P = 0.46) conformed to a segregation ratio of 15 resistant: 1 susceptible, indicating that it is likely that each parental genotype possessed a single independent dominant gene for resistance to BGYR (Table 6). A 63 resistant: 1 susceptible ratio was observed in the F2 populations of Franklin/Torrens (\(\chi^{2}_{63:1}\) = 0.63, P = 0.43), Franklin/Cowabbie (\(\chi^{2}_{63:1}\) = 0.14, P = 0.63), Molloy/Franklin (\(\chi^{2}_{63:1}\) = 0.09, P = 0.77) and Franklin/Yagan (\(\chi^{2}_{63:1}\) = 0.18, P = 0.68), indicating the segregation of three independent genes (Table 6). The frequency of resistant and susceptible lines in the Franklin/Kaputar F2 population fitted a 13 resistant: 3 susceptible ratio, indicating segregation for one dominant and one recessive gene (\(\chi^{2}_{13:3}\) = 0.69, P = 0.41) (Table 6).
Results from two DH populations, in addition to the Yerong/Franklin population, were included in this study. The Franklin/Arapiles DH population segregated 30 resistant: 2 susceptible lines (P = 1) indicating the presence of four seedling resistance genes in this population. The Harrington/Gilbert DH population segregated in a 15 resistant: 1 susceptible ratio (\(\chi^{2}_{15:1}\) = 0.04, P = 0.83), indicating the segregation of four independent genes (Table 5). The Yerong/Franklin population exhibited a 3 resistant: 1 susceptible ratio in both inoculated greenhouse tests and in naturally infected field trials, indicating that there were two genes segregating within this population. The availability of a molecular map for the Yerong/Franklin DH population allowed further investigations into the chromosomal location of resistance to BGYR in these cultivars.
Mapping BGYR resistance in the Yerong/Franklin DH population
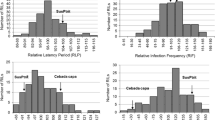
The phenotypic response data obtained from greenhouse tests were used to map loci conferring resistance to BGYR at the seedling growth stage in the Yerong/Franklin DH population (Online Resource 4). The analysis identified major QTLs on the long arms of chromosomes 5H and 7H (Fig. 3). The QTL located on chromosome 7HL corresponded to the seedling resistance gene identified in Yerong (LOD value of 12.01). The 7HL QTL explained 27 % of the total phenotypic variance (Table 7) and was associated with DArT marker bPb-6167. This QTL was tentatively designated Rpsp-hYerong. The second QTL contributed by Franklin was located on the long arm of chromosome 5H and explained 9 % of the total phenotypic variance (Table 7). It was associated with DArT marker bPb-8462 with a LOD value of 4.52 (Table 7), and was tentatively designated Rpsp-hFranklin. Three QTLs were detected for resistance at the adult plant stage from the field trial, two of them being in the similar positions of Rpsp-hYerong and Rpsp-hFranklin. The third QTL contributed by Franklin was identified on chromosome 5HS and explained 7.8 % of total genetic variation. Because this gene was not detected in seedling tests, it is likely that it confers adult plant resistance (APR). It was associated with DArT marker bPb-7651 and had a LOD value of 3.78 (Table 7; Fig. 4).
Discussion
Stripe rust of barley grass (BGYR) caused by P. striiformis f. sp. pseudo-hordei is a relatively new disease in Australia (Wellings 2010). It was initially recognised as a new forma specialis based on its virulence pattern on the Australian wheat stripe rust differential set (Wellings et al. 2000a). Although BGYR is adapted to wild Hordeum sp., it can cause significant disease on certain Australian commercial barley cultivars (Skiff and Skiff derivatives) under greenhouse and field conditions, with yield losses of up to 10 % recorded in Victoria (Wellings et al. 2000a, b). Golegaonkar (2007) revealed that in addition to Skiff (and the derivative cultivar Tantangara), six Australian barley cultivars (Clipper, Cutter, Keel, Ketch, Maritime, and Prior) were also potentially vulnerable to the BGYR pathogen.
In the present study, 20 barley genotypes were screened at the seedling growth stage against a standard isolate of BGYR, and with the exception of the susceptible genotype Biosaline-19, all exhibited resistant reactions. The genotypes were classified into three phenotype groups based on IT response. Results obtained from screening the Yerong (RG1)/Franklin (RG2) DH population revealed that each parent carried a single independent resistance gene, effective against BGYR isolate 981549. Segregation ratios observed in F2 and F3 populations developed from a cross between Yerong and Franklin with Biosaline-19 (susceptible), indicated that resistance in these cultivars were governed by dominant genes. The genes for seedling resistance in Franklin and Yerong were mapped to the long arms of chromosomes 5H and 7H, respectively, and temporarily designated Rpsp-hFranklin and Rpsp-hYerong. An additional gene for adult plant resistance was mapped to chromosome 5HS.
Only two genes for resistance to BGYR have been mapped in barley. Golegaonkar (2007) mapped a recessive seedling gene (Bgyr1) in barley cultivar Sahara 3771 to the long arm of chromosome 7H, and an APR gene (Bgyr2) in Clipper to chromosome 3HL. In this study, the seedling resistance observed in Yerong (Rpsp-hYerong) was also mapped to chromosome 7HL, linked to DArT marker bPb-6167. In addition to BGYR resistance, Sui et al. (2010) identified A dominant gene (YrpstY1) in the Chinese barley line Y12, conferring resistance to Pst pathotype CYR32. This gene was flanked by SSR markers EBmac755 and AWBMS022, and positioned at roughly 162 cM on chromosome 7H (Sui et al. 2010; Wenzl et al. 2006). Based on the barley linkage maps of Alsop et al. (2011) and Wenzl et al. (2006) Rpsp-hYerong and YrpstY1 are <2 cM apart. Field data obtained in CIMMYT (Toluca, Mexico) for the Yerong/Franklin DH population revealed that Yerong and Franklin carried genes effective against Psh Race 24 (Online Resource 5). The resistance to Psh in Yerong was also linked to DArT marker bPb-6167 and is likely to be conferred by Rpsp-hYerong (Online Resource 6). This indicates that this gene may be effective against both BGYR and Psh and may play an important role in protecting barley from both formae speciales of P. striiformis.
The relationship between Bgyr1, YrpstY1 and Rpsp-hYerong is currently unknown. Phenotypic differences observed between Yerong (IT ‘0;’) and Sahara 3771 (IT ‘1++2cn’) in response to BGYR isolate 981549 suggests that these genes are most likely different. Rpsp-hYerong was also shown to be dominant, contrasting to the recessive nature of Bgyr1. Further tests are needed to study the allelic relationship between the three genes.
Similar IT reactions observed in each response group suggested that lines in these groups may carry a common gene or genes. Populations developed to study the relationship between the single gene observed in Yerong and genotypes classified in RG1 and the single gene observed in Franklin and genotypes classified in RG2 resulted in the identification of a number of additional genes. Tests of allelism conducted between crosses developed from Naso Nijo/Yerong, ZUG405/Yerong, Yerong/Tilga, Dash/Yerong, Yerong/Fitzgerald indicated that Dash, Naso Nijo and Fitzgerald also carry Rpsp-hYerong. This is because no segregation was observed in these populations. Tilga and ZUG405 were each shown to carry a gene independent of Rpsp-hYerong. The genetic relationship between these genes, however, is currently unknown.
No segregation in BGYR response was observed among progeny derived from crosses Franklin/Harrington, Windich/Franklin and Franklin/Parwan. This suggests that cultivars Harrington, Windich and Parwan most likely carry Rpsp-hFranklin. It is possible that the resistance observed in these cultivars is provided by barley genotype Kenia, as all four are derivatives of Kenia (Fig. 1). Single genes independent of Rpsp-hFranklin were observed in Gilbert, Milby, ZUG159 and Kaputar. Segregation ratios observed in the Franklin/Kaputar population suggest that resistance in Kaputar was governed by a recessive gene. A three gene segregation ratio was observed in the Franklin/Torrens, Franklin/Cowabbie, Franklin/Yagan and Molloy/Franklin populations, suggesting that Torrens, Cowabbie, Yagan and Molloy each carry two genes effective against BGYR isolate 981549, independent of Rpsp-hFranklin. Resistance in Molloy was shown to be governed by a dominant and a recessive gene. Nover and Scholz (1969) and Chen and Line (1999, 2003) reported that recessive genes conferring resistance to P. striiformis were common in barley. The high incidence of recessive genes suggests that although resistance is most often reported to be dominant in genetic studies of plant disease resistance (De Wit 1992), recessive resistance may be just as frequent (Chen and Line 1999, 2003).
Diagrammatic representation of the genetic relationships between barley genotypes (Franklin, Harrington, Windich and Parwan) based on pedigree information (Table 1)
Major QTL on chromosome 5H and chromosome 7H associated with resistance to the BGYR isolate 981549 at the seedling stage in the Yerong/Franklin DH population, including thresholds for the logarithm of odd ratio (LOD = 3) where Rpsp-hFranklin represents resistance in Franklin and Rpsp-hYerong represents resistance in Yerong
Screening the Franklin/Arapiles and Harrington/Gilbert DH populations revealed that each likely segregated for four independent genes. Based on this, resistance to BGYR in Arapiles and Harrington appear to be governed by three genes. Since no segregation was observed in the Franklin/Harrington F2 population, resistance in Harrington is therefore governed by Rpsp-hFranklin and two additional genes. In order to determine whether these DH populations are carrying linked genes, further tests using larger populations (to recover rare recombinants) and/or multiple BGYR pathotypes (to test for multiple alleles at a single locus) would be needed.
Of the 20 barley genotypes investigated, only two (Franklin and Tilga) have been studied previously. Golegaonkar (2007) showed that resistance to BGYR in Franklin was governed by two genes, contrasting to the results obtained in this study. Field tests of the Yerong/Franklin DH population to BGYR in Launceston Tasmania revealed that in addition to the major seedling gene in Franklin, on chromosome 5HL, a second resistance gene was present on chromosome 5HS (Table 7; Fig. 4). This gene is believed to confer adult plant resistance. While APR is typically expressed at adult growth stages, it can be detected in seedling tests under certain conditions (e.g. Park and McIntosh 1994; Pretorius et al. 1984). It is therefore possible that Golegaonkar (2007) may have observed the segregation of the second APR gene in Franklin in seedling greenhouse rust screening. Tilga was reported to carry a single dominant gene (Golegaonkar 2007), which is in agreement with the current findings. Resistance in Tilga was shown to be genetically distinct from the minor gene for resistance detected in Skiff and Tantangara (Golegaonkar 2007) and shown in this study to be independent of Rpsp-hYerong.
Unlike the nature and genetics of resistance to stripe rust of wheat and barley, resistance to BGYR has been studied in relatively few barley genotypes and few genes have been identified. Information on the number of genes for resistance and their mode of inheritance will be useful for monitoring pathogenicity in BGYR (i.e., identifying and differentiating new pathotypes of the pathogen) and in avoiding the narrowing of genetic diversity of resistance to this pathogen in commercially grown barley cultivars. Results from the present study revealed that most barley cultivars possessed at least a single gene for resistance against the BGYR pathogen, in agreement with findings by Golegaonkar (2007). Genetic studies conducted on 20 barley genotypes using F2 and F3 populations revealed the presence of 15 dominant and two recessive genes effective against the BGYR pathogen. Studies of allelism in two DH populations (Franklin/Arapiles, Harrington/Gilbert) revealed an additional five genes. Cultivars that did not segregate with resistance in Franklin (Windich and Parwan) and Yerong (Naso Nijo, Dash and Fitzgerald) may carry additional genes for resistance. Further studies should be conducted to determine the number of genes present within these genotypes. Intercrossing these resistant lines will help determine whether they share any common genes. The relationship between the genes observed in Arapiles, Cowabbie, Harrington, Kaputar, Milby, Molloy, Parwan, Tilga, Torrens, Yagan, ZUG159 and ZUG405 is currently unknown and allelism tests are needed to characterise the potential for diversity of resistances observed in these genotypes.
A relatively low number of seedling genes were identified in each cultivar (one to two genes) and the presence of common genes between some of these cultivars indicate that the BGYR pathogen could become a major concern if it acquires virulence for these genes progressively, such as that experienced in Australia with wheat against Pst (Wellings 2007) and in North America with barley against Psh (Brown et al. 2003). It is therefore important to continue to monitor the BGYR pathogen population closely for changes in virulence with respect to single gene stocks identified in this work. The single independent seedling resistance genes Rpsp-hYerong and Rpsp-hFranklin identified and the single genes identified in previous studies (Skiff and Sahara 3771) (Golegaonkar 2007) could become the basis of a barley differential set for monitoring the BGYR pathogen. Current approaches to BGYR control include avoiding the release of susceptible genotypes, as this could provide opportunities for further evolution within the pathogen population, potentially leading to new pathogenic variants with increased virulence on wheat and/or barley (Wellings 2007).
Author contribution statement
LD, RFP and DS Conceived and designed the experiments, LD Performed the experiments, LD and DS Analysed the data, LD Wrote the paper and LD, DS and RFP Commented on the manuscript before submission.
References
Alsop BP, Farre A, Wenzl P, Wang JM, Zhou MX, Romagosa I, Kilian A, Steffenson BJ (2011) Development of wild barley-derived DArT markers and their integration into a barley consensus map. Mol Breed 27:77–92
Brown WM, Hill JP, Velasco VR (2003) Barley yellow rust in North America. Phytopathology 39:367–384
Chen X, Line RF (1999) Recessive genes for resistance to Puccinia striiformis f. sp. hordei in barley. Phytopathology 89:226–232
Chen X, Line RF (2003) Identification of genes for resistance to Puccinia striiformis f. sp. hordei in 18 barley genotypes. Euphytica 129:127–146
De Wit P (1992) Molecular characterization of gene-for-gene systems in plant-fungus interactions and the application of avirulence genes in control of plant pathogens. Annu Rev Phytopathol 30:391–418
Golegaonkar PG (2007) Genetic and molecular analysis of resistance to rust diseases in barley. Dissertation, University of Sydney, Australia
Hanson WD (1959) Minimum family sizes for planning of genetic experiments. Agron J 51:711–715
Keiper FJ, Hayden MJ, Park RF, Wellings CR (2003) Molecular genetic variability of Australian isolates of five cereal rust pathogens. Mycol Res 107:545–556
Li HB, Vaillancourt R, Mendham NJ, Zhou MX (2008) Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genom 9:401–413
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. Kluwer Academic Publishers, London
Nover I, Scholz F (1969) Genetische Untersuchungen zur Resistenz der Gerste gegen Gelbrost (Puccinia striiformis West.). Theor Appl Genet 39:150–155
O’Brien L, Brown JS, Young RM, Pascoe I (1980) Occurrence and distribution of wheat stripe rust in Victoria and susceptibility of commercial wheat cultivars. Australas Plant Pathol 9:14
Park RF, McIntosh RA (1994) Adult plant resistances to Puccinia recondita f.sp. tritici in wheat. N Z J Crop Hortic Sci 22:151–158
Pretorius ZA, Wilcoxson RD, Long DL, Schafer JF (1984) Detecting wheat leaf rust resistance gene Lr 13 in seedlings. Plant Dis 68:585–586
Spackman ME, Ogbonnaya FC, Brown JS (2010) Hypervariable RAPD, ISSR and SSR markers generate robust taxonomic groups among Puccinia striiformis formae speciales of importance to Australian agriculture. Australas Plant Pathol 39:226–233
Stubbs RW (1985) Stripe rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts. Academic Press, Sydney, pp 61–102
Sui X, He Z, Lu Y, Wang Z, Xia X (2010) Molecular mapping of a non-host resistance gene YrpstY1 in barley (Hordeum vulgare L.) for resistance to wheat stripe rust. Hereditas 147:176–182
Van Ooijen JW, Kyazma BV (2009) MapQTL® 6: Software for the mapping of quantitative trait loci in experimental populations. Wageningen, The Netherlands
Wellings CR (2007) Puccinia striiformis in Australia: a review of the incursion, evolution, and adaptation of stripe rust in the period 1979-2006. Aust J Agric Res 58:567–575
Wellings CR (2010) Global status of stripe rust, BGRI 2010 Technical Workshop. St Petersburg, Russia
Wellings CR, McIntosh RA (1990) Puccinia striiformis f. sp. tritici in Australasia: pathogenic changes during the first 10 years. Plant Pathol 39:316–325
Wellings CR, Burdon JJ, McIntosh RA, Wallwork H, Raman H, Murray GM (2000a) A new variant of Puccinia striiformis causing stripe rust on barley and wild Hordeum species in Australia. Plant Pathol (Oxford) 49:803
Wellings CR, Read BJ, Moody DB (2000b) Stripe rust affecting barley in Australia—current and potential threats. In: Logue S (ed) Proceedings of the 8th International Barley Genetics Symposium. Adelaide Convention Centre, Adelaide, pp 197–199
Wenzl P, Li H, Carling J, Zhou MX, Raman H, Edie P, Hearnden C, Maier C, Xia L, Craig V, Ovensna J, Cakir M, Poulsen D, Wang J, Raman R, Smith KP, Muehlbauer GJ, Chalmers KJ, Kleinhofs A, Huttner E, Kilian A (2006) A high density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and phenotypic traits. BMC Genom 7:206–228
Acknowledgments
The research was supported by the Australian Grains and Research Development Corporation through a Postgraduate Research Scholarship (GRS139).
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patrick M. Hayes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Derevnina, L., Zhou, M., Singh, D. et al. The genetic basis of resistance to barley grass yellow rust (Puccinia striiformis f. sp. pseudo-hordei) in Australian barley cultivars. Theor Appl Genet 128, 187–197 (2015). https://doi.org/10.1007/s00122-014-2323-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2323-x