Abstract
Colony size in social insects is one of the most important factors in shaping their self-organized system. It affects a wide variety of traits such as foraging and defense strategies, social immune responses, the degree of polymorphism, and reproductive output. However, colony size estimation of subterranean termites in the field has been challenging, due to their extremely cryptic biology and multiple site–nesting behavior. Since natural selection favors workers that maximize the number of their siblings, the amount of egg production may reflect the number of workers in the colony. Here, we report a method for inferring colony size in the field using total egg production in each colony from a subterranean termite, Reticulitermes speratus. Our investigation of field colonies revealed that the body weight of queens reaches a peak and had the largest variance in June and July and accurately predicts the number of eggs laid by the queen per 24 h. Using laboratory-reared colonies, we found that the total egg production in each colony is proportional to the number of workers. We also estimated the colony size of 198 field colonies and found that the median and maximum colony size was 24,500 and 451,800 workers per colony. The method for inferring colony size presented here may also be applicable to termite species with a clear seasonality in egg production. The colony size estimate will contribute to understanding the life history strategies and social systems of termites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Division of labor between reproductive and non-reproductive castes is fundamental to the ecological success of social insects (Wilson 1971; Oster and Wilson 1978; Beshers and Fewell 2001; Hölldobler and Wilson 2009). Societies of social insects can be considered a superorganism, with the number of non-reproductive individuals in a colony (i.e., colony size) in eusocial species corresponding to the body size in solitary organisms (Bourke 1999; Dornhaus et al. 2012; Kennedy et al. 2017; Boomsma and Gawne 2018). The colony size is an important social characteristic shaping defense and foraging strategies, task specialization, the initiation time of the production of fertile dispersers (i.e., alates), and the lifelong reproduction of alates (Wilson 1971; Thorne 1997; Su 2003; Hölldobler and Wilson 2009). To comprehensively understand the regulatory mechanisms and dynamics of life history traits in social insects, it is essential to conduct researches that take into account colony size under natural conditions.
In termites, especially subterranean species, investigation of the colony size in the field can be challenging due to multiple-site nesting behavior and a cryptic biology. Several methods for investigating the colony size have been proposed, including destructive sampling by collecting termite nests and habitats (King and Spink 1969; Howard et al. 1982; Darlington 1990) or trapping (Haverty et al. 1975; La Fage et al. 1983), and non-destructive methods such as using radioisotopes (Spragg and Paton 1980) and mark–release–recapture protocols (Su and Scheffrahn 1988; Su et al. 1993; Su 1994; Kenneth Grace et al. 1995; Tsunoda et al. 1999; Su and Lee 2008). The mark–recapture methods have been widely used for the estimation of population size in field studies; however, since subterranean termites have large colony sizes and extensive foraging systems, it is difficult to accurately estimate the whole population size by these methods (Thorne et al. 1996; Evans et al. 1999). Although some successful colony size estimation methods have been proposed for laboratory-kept colonies (Su 2013; Patel et al. 2020), to date, no method is suitable for estimating the whole population size in the field termites.
In this study, we developed a method for estimating colony size under field conditions in a subterranean termite Reticulitermes speratus. They live mostly in pine and Japanese cedar forests in Japan (Takematsu 1999; Park et al. 2006). A single colony uses multiple logs which are connected by underground tunnels (Abe 1987; Shellman-Reeve 1997). Mature colonies are typically headed by one primary king (PK) and multiple secondary queens (SQs), which are produced by parthenogenesis and eventually replace the primary queen (Matsuura et al. 2009, 2018). Egg production is a seasonal event and is limited from June to August (Matsuura et al. 2007; Nozaki and Matsuura 2021; Takata et al. 2023a). The resources required for egg production are produced by workers and predominantly consumed by queens (Konishi et al. 2023). We initially validated the mark–recapture method in R. speratus; however, the method was not applicable for estimating the whole colony size. We then developed a new method for estimating colony size based on the fecundity of the queens. A previous study reported that the body weight of a queen strongly correlates with the number of workers captured in and around the nests in Macrotermes subhyalinus (Darlington 1990). Although workers who were away from the nest were not taken into account in the study, there could be a correlation between the queen's fecundity and the total number of individuals in the colony. Thus, the objective of the present study was to determine whether the fecundity of queens could be used to estimate the number of workers in the field colonies (Fig. 1). First, we investigated the seasons with the greatest differences in fecundity of the queens among colonies. Second, we measured the relationship between the body weight of the queen and her fecundity during those seasons. Third, we evaluated the relationship between daily egg production and colony size under laboratory conditions also during those seasons. Finally, the number of workers was estimated in the field colonies.
Schematic representation of the nutritional contribution of workers on egg production in small (a) and large colonies (b). A single colony uses multiple logs which are connected by tunnels. Even if the workers forage from multiple logs, the nutrients necessary for egg production are concentrated in the queens, so the number of eggs produced is likely to reflect the number of workers in the colony. The size of the queens varies remarkably among colonies
Materials and methods
Termite sampling
Decayed logs containing a primary king and secondary queens of R. speratus were collected in pine or Japanese cedar forests in Kyoto, Osaka, Nara, Shiga, Hyogo, and Fukui, Japan, from 2017 to 2022 (see Dataset 1 for details). Six colonies were used for a mark–release–recapture program. Another 342 colonies were used to investigate the seasonal body weight of queens. To investigate the relationship between the body and ovaries weight of the queen, 25 out of the 342 colonies were used. To investigate the relationship between the body weight of the queen and her daily egg production, 18 out of the 25 colonies were used. Fifteen colonies, including 13 out of the 342 colonies used to investigate the queen weight and an additional 2 colonies, were used to investigate the presence or absence of satellite nests. An additional 15 colonies were used to evaluate the relationship between daily egg production and colony size. To estimate the number of workers in field colonies, 188 out of the 342 colonies were used. In every experiment, each colony was individually processed.
Evaluation of mark–recapture methods
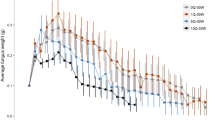
To investigate whether the assumption of the mark–recapture methods (i.e., the workers distribute equally in the colony) are met in R. speratus, the proportion of marked workers was compared between different colony areas under laboratory conditions. Parts of the log containing and not containing royals were collected for each of the six colonies. The colonies were individually transferred into a container (70 × 50 × 40 cm: W × D × H) lined with 1 cm of moistened mountain soil (Takagi Kenzai Co., Ltd., Japan), and maintained at 25°C under dark conditions. Six months later, 500 workers were extracted from the edge of each log, and marked by sprays (Tamiya Color TS-15 and TS-35). Different colors were applied to workers from each log (royal chamber/foraging area: green/blue or blue/green). Then, the workers were returned to their original logs. The logs were returned to the original position in the container and maintained at 25°C under dark conditions. Two weeks later, all termites were extracted from each log, and the number of marked and unmarked workers was recorded in three colonies. Four weeks later, the remaining three colonies were processed in the same manner.
Relationship between queen weight and daily egg production in field colonies
To determine the month with the largest variance in the body weight of queens, 342 colonies with kings and queens were collected during the breeding season (from April to September). Within 3 days of collection, all termites were extracted from the log, and the fresh body weight of queens was recorded to the nearest 0.1 mg.
Since the body weight of queens had the largest variance in June and July, 25 colonies were collected in June and July to evaluate the relationships between the queen’s body weight and her fecundity in the field colonies. Within 24 h of collection, all termites were extracted and the fresh body weight of queens was recorded to the nearest 0.1 mg. To evaluate the relationships between the body weight of queens and ovary size, one SQ from each of the colonies was individually dissected in PBS buffer (Wako Pure Chemical Industry Co., Ltd., Japan) within 24 h of collection. Their ovaries were removed and transferred into a centrifuge tube to avoid desiccation after the removal of water on its surface. The fresh weight of their ovaries was recorded to the nearest 0.01 mg. Twenty-five SQs were used in total.
To evaluate the relationships between the body weight of queens and egg production, another two SQs were randomly selected from each of 18 out of the 25 colonies, individually transferred into dishes (ca. 30 mm) lined with a moist unwoven cloth and 10 non-nestmate workers (including both sexes), and maintained at 25°C under dark conditions. Thirty-six SQs were used in total. After 24 h, the number of eggs laid by SQs was recorded.
Relationship between queen weight and colony size in laboratory conditions
To evaluate the relationship between daily egg production and colony size, decayed logs of Japanese cedar containing kings and secondary queens were collected in April (n = 15 colonies). The colonies were individually transferred into a container (626 × 426 × 365 mm: W × D × H) keeping them separate from one another. The individual containers were then lined with 4 cm of moistened mountain soil. If the logs were too large to fit in a single container, they were split into multiple containers. Then an opening was made on the lower side of the containers connected by 15-cm tubes (6 mm inner diameter), so the workers could move between the containers. A moistened Japanese cedar log (ca. diameter 10 cm, length 35 cm) was added to each container as an additional food source. Then, the logs were maintained at ambient temperature. In June and early July, all termites were extracted from the container. The number of individuals who died during the extraction process was recorded by counting the number of individuals (or more precisely, the number of heads) directly for each caste. To calculate the ratio of the surviving individuals in each caste, all surviving termites were separated from their nest material and the fresh body weight of king and queens was recorded to the nearest 0.1 mg. The total weight of the remaining colony members was recorded to the nearest 1 mg. Then, a 500-mg subset of termite colony members was randomly selected and the numbers of each individual caste was recorded (worker, soldier, nymph, or larva). The number of surviving caste members was determined by the ratio of individuals in each caste from the randomly selected subset. The total number of members in each caste was determined by adding the calculated number of the surviving individuals to the counted number of the dead individuals.
Colony size estimation in the field colonies
Colony size estimation using queen fecundity requires the collection of all queens in the colony. Therefore, before estimating colony size in the field colonies, we confirmed the absence of satellite nests, which are apart from the location where the king is present and consist only of the queen and non-reproductive members. First, we searched for a log with one or more queens in the field. Once the queens were found, we brought the entire log back to the laboratory for further examination (n = 15 colonies). Within eight days of collection, we examined whether there was a king in the logs.
To estimate the number of workers in field colonies, 188 colonies with kings and queens were collected in June and July. Within 3 days of collection, all secondary queens were extracted, and their fresh body weight was recorded to the nearest 0.1 mg. The daily egg production in each colony was calculated using a regression formula between the queen's body weight and her daily egg production. Then, the number of workers in the colony was estimated using a regression formula between daily egg production and the number of workers in the colony.
Statistical analysis
To investigate whether the workers distribute randomly in the colony as assumed in mark–recapture–methods, the proportion of marked workers was compared using a generalized linear mixed model (GLMM) with a binomial error distribution and logit link function. The response variable was the proportion of marked workers, and the explanatory variables were the log (whether the marked workers were introduced in the log or not), test period, and their interactions. Colony was included as a random factor. The proportion of marked workers was compared in each test period using the same model without the interaction term. Two-tailed paired F-tests were used to compare the variance in the mean body weight of the queens in each colony among different months. A two-sided Bonferroni-corrected significance level of P < 0.0033 was set. We analyzed the relationship between the body weight of queens and the size of their sexual organs using a generalized linear model (GLM) with a gamma error distribution and log link function. The response variable was the weight of the sexual organ, and the explanatory variable was the body weight of the queens. We also analyzed the relationship between the body weight of the queens and egg production using a GLM with a Poisson error distribution and log link function. The response variable was the number of eggs laid, and explanatory variable was the log-transformed the body weight of the queens. We analyzed the correlation between egg production and colony size using a GLM with a Poisson error distribution and log link function. The response variable was the number of workers, and explanatory variable was the log-transformed the daily egg production in each colony. We also analyzed the effect of the body weight of primary king, mean weight of secondary queen, number of the queens, or total weight of the queens on the colony size using a GLM with a Poisson error distribution and log link function. The response variable was the number of workers, and explanatory variable was the log-transformed the body weight of primary king, mean weight of secondary queen, number of the queens, or total weight of the queens in each colony.
All analyses were performed by the software R v4.2.3 (R Core Team 2022), with the lme4 and car packages. For the GLMMs and GLMs, likelihood ratio tests (LRTs) were used to determine the statistical significance of each explanatory variable. A significance value of p < 0.05 was considered to indicate statistical significance.
Results
Distribution of marked workers
The marked workers were not distributed equally in the colony but were found at a higher frequency from the logs in which they were introduced (GLMM, LRT: χ2 = 13.537, df = 1, p < 0.001, Fig. 2). The interaction between the log (whether the marked workers were introduced in the log or not) and the test period was not significant (GLMM, LRT: χ2 = 0.044, df = 1, p = 0.834). There were statistically significant differences in the proportion of marked workers between the logs in the 2-week (GLMM, LRT: χ2 = 9.571, df = 1, p = 0.002, Fig. 2a) and 4-week test period group (GLMM, LRT: χ2 = 4.010, df = 1, p = 0.045, Fig. 2b).
Relationship between queen weight and daily egg production in field colonies
In total, 15,637 SQs from 342 colonies were collected from the field from April to September. We found that the body weight of the SQs begins to increase in May, peaks in June, and then decreases through September (Fig. 3a). The variance in mean body weight of the queens among the colonies was the largest in June and July (F-test with Bonferroni correction). In June and July, the body weight of queens correlated with their ovaries weight (GLM, LRT: χ2 = 610.89, df = 1, p < 0.001, R2 = 0.951, Fig. 3b) and the number of eggs produced in 24 hours (GLM, LRT: χ2 = 805.36, df = 1, p < 0.001, R2 = 0.914, Fig. 3c). The regression formula between body weight of queens and their daily egg production is as follows:
Body weight and fecundity of secondary queens in Reticulitermes speratus. a Seasonal changes in the variance in mean body weight of queens among colonies. Plots indicate the mean body weight of queens in each colony (n = 342 colonies). Different letters indicate significant differences in variance (F-test with Bonferroni correction, p < 0.05). b Correlation between queen weight and ovaries weight in field colonies. The fresh weights of the ovaries are plotted as a function of the body weight of queens (n = 25 secondary queens from 25 colonies). The solid line represents the estimated regression line, yi = e ^ (−3.071019 + 1.754776 * log(xi)). The yi and xi are the ovaries and the body weight of the queen i, respectively. c Correlation between queen weight and daily egg production in field colonies. The number of eggs laid by queens is plotted as a function of her body weight (n = 36 secondary queens from 18 colonies). The solid line represents the estimated regression line, yi = e ^ (−1.052582 + 1.855425 * log(xi)). The yi is the number of eggs produced in 24 h by the queen i, and xi is the body weight of the queen i
where yi is the number of eggs produced in 24 h by the queen i and xi is the body weight of the queen i.
Relationship between queen weight and colony size in laboratory conditions
Using laboratory-reared colonies, we found that colony size is predictable from the body weight of SQs in the colony. The total egg production in each colony was calculated from the regression formula (1), and was best explained the variation in the number of workers in a colony (GLM, LRT: χ2 = 1,571,616, df = 1, p < 0.001, AIC = 36,630, R2 = 0.975; null model: AIC = 1,608,244, Fig. 4). The number of workers was weakly corelated with the weight of primary king (GLM, LRT: χ2 = 887,905, df = 1, p < 0.001, AIC = 720,341, R2 = 0.645), the mean weight of the secondary queen (GLM, LRT: χ2 = 909,198, df = 1, p < 0.001, AIC = 699,048, R2 = 0.310), the number of queens (GLM, LRT: χ2 = 847,66, df = 1, p < 0.001, AIC = 1,523,480, R2 = 0.034), and the total weight of the queens (GLM, LRT: χ2 = 1,265,010, df = 1, p < 0.001, AIC = 343,236, R2 = 0.820). The regression formula between the total daily egg production and the number of workers is as follows:
Correlation between the amount of daily egg production and the number of workers under laboratory conditions. The number of workers in each colony is plotted as a function of the total number of daily eggs laid in the colony (n = 15 colonies). The solid line represents the estimated regression line, w = e ^ (0.8946205 + 1.411185 * log(∑yi)). The w is the number of workers in the colony and ∑yi is the total number of eggs produced in the colony in 24 h
where w is the number of workers in the colony and ∑yi is the total number of eggs produced in the colony in 24 h.
Estimated colony size in the field colonies
We confirmed that satellite nests are either absent or extremely rare. In all 15 logs where queens were found, kings were also collected in each of them (see Dataset for details). The colony sizes were calculated using the regression formulas (1) and (2) in 188 field colonies collected in June and July. The first quartile, median, third quartile, and maximum of the estimated number of workers in a colony were 10,700, 24,500, 58,600, and 451,800, respectively (Fig. 5).
Histogram of colony size in Reticulitermes speratus in the field. The x-axis represents the estimated number of workers in each colony, and the y-axis represents the number of colonies. The colony sizes were estimated for 188 field colonies. The estimated number of workers in a colony ranged from 10,700 (first quartile) to 24,500 (median) to 58,600 (third quartile) to 451,800 (maximum)
Discussion
We present a reliable method for estimating colony size in field colonies of R. speratus. We initially evaluated a mark–recapture method commonly used in estimating colony size for subterranean termite species (Grace et al. 1989; Su et al. 1993; Tsunoda et al. 1999). However, we found that the assumption of the mark–recapture method is not met in R. speratus, similar to other species of subterranean termites (Thorne et al. 1996; Evans et al. 1999). Our results showed that marked workers are not equally distributed throughout the colony, and the proportion of marked workers varies even between neighboring logs (Fig. 2). The mark–recapture methods requires an assumption of equilibrium (Su 2013), which is not met in R. speratus as presented here (see also Dataset) and Coptotermes formosanus (Su et al. 2017), as the different instars are distributed unequally over the distance from the central part of the nest. The actual degree of the relative lack of equilibrium may provide the data necessary to estimate population size more accurately. Although we do not completely reject the potential use of mark–recapture methods in R. speratus, we decided to consider an alternative approach. As a result, we found that the number of workers in the colony can be estimated from the body weight of the queens. The weight of the queen reflects the number of eggs laid per 24 h (Fig. 3), and the total number of eggs produced per unit of time in the colony is strongly correlated with the total number of workers (Fig. 4). The queens of this species are distributed in a single log and does not exist across multiple sites. Therefore, by collecting all the queens from a single log we can estimate the total egg production and the number of workers in the whole colony.
Our colony size estimation is based on the valid assumption that termite colonies are adapted to maximize egg production based on colony size within the constraints of a limited growing season. While termites are typically found in tropical regions, some species, such as R. speratus, have successfully adapted to and colonized temperate zones (Emerson 1955; Eggleton et al. 1994; Takematsu 1999; Eggleton 2000; Park et al. 2006; Evans et al. 2013). R. speratus exhibits a clear seasonality in egg production, which is limited to the period from June to August (Nozaki and Matsuura 2021). The queens are specialized for egg production, and their fecundity is reflected in their body weight (Fig. 3). The queens rely on a food supply from the workers for the nutrients required for egg production (Tasaki et al. 2023; Konishi et al. 2023), and unlike the workers, they have no symbiotic microorganisms which are essential for wood digestion (Shimada et al. 2013; Inagaki and Matsuura 2016). Therefore, it is reasonable to infer that the body weight of queens can serve as an indicator of worker population size within a colony, as it reflects the number of workers available for food provisions. The present study demonstrated that the number of workers affects egg production. The annual production of new members influences age demography and is fundamental for the maintenance and health of termite societies (Chouvenc et al. 2022). Future comparative studies that examine the impact of seasonal egg production patterns on overall colony functionality will be valuable in revealing how termite colonies adapt to cyclical changes in environmental conditions that occur, for example, in temperate regions where temperature fluctuates more dramatically than tropical regions.
Our method, which requires the dissection of the royal chamber to collect all the queens, is not suitable for monitoring and follow-up studies, but it allows for snapshot studies. The organization of colonial organisms is analogous to that of multicellular organisms, and colony size in eusocial species corresponds to body size in solitary species (Bourke 1999; Dornhaus et al. 2012; Kennedy et al. 2017; Boomsma and Gawne 2018). Colony size is one of the most important parameters that shape the collective organization of colonial organisms, as it influences defense and foraging strategies, task specialization, and the production of dispersers (Wilson 1971; Thorne 1997; Su 2003; Hölldobler and Wilson 2009). Furthermore, colony size may also play a crucial role in breeding systems and caste development, as seen with the replacement of primary queens with secondary queens, and changes in caste development occurring during the colony life cycle in R. speratus (Matsuura et al. 2009, 2018; Takata et al. 2023b). Our method has broad applicability in studying the role and contribution of colony size in these life history events and traits in the field and opens new avenues for understanding the life history strategies and social systems of termites.
The data must be interpreted carefully because the relationship between queen weight and the number of workers may vary slightly in the field, and our data may underestimate the population size of field colonies. In this study, termite nesting logs were placed adjacent to each other, but in the field, R. speratus foraging territories can extend up to 56.6 m2 per colony, with foraging occurring from logs as far as 10 m linear distance away (Tsunoda et al. 1999). Hence, additional studies are needed to investigate the potential influence of energy expenditure associated with nest-to-nest movement on egg production, as this would allow a more accurate estimation of population size.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
The codes for graphing and statistical analyses used for this paper are available at GitHub (https://github.com/MamoruTakata/Colony_size_estimate).
References
Abe T (1987) Evolution of life types in termites. In: Kawano S, Connell JH, Hidaka T (eds) Evolution and coadaptation in biotic communities. University of Tokyo Press, Tokyo, Japan, pp 125–148
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440. https://doi.org/10.1146/annurev.ento.46.1.413
Boomsma JJ, Gawne R (2018) Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation. Biol Rev 93:28–54. https://doi.org/10.1111/brv.12330
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257. https://doi.org/10.1046/j.1420-9101.1999.00028.x
Chouvenc T, Ban PM, Su N-Y (2022) Life and death of termite colonies, a decades-long age demography perspective. Front Ecol Evol 10:1–21. https://doi.org/10.3389/fevo.2022.911042
Darlington JPEC (1990) Populations in nests of the termite Macrotermes subhyalinus in Kenya. Insectes Soc 37:158–168. https://doi.org/10.1007/BF02224028
Dornhaus A, Powell S, Bengston S (2012) Group size and its effects on collective organization. Annu Rev Entomol 57:123–141. https://doi.org/10.1146/annurev-ento-120710-100604
Eggleton P (2000) Global patterns of termite diversity. In: Abe T, Bignell DE, Higashi M (eds) Termites, evolution, sociality, symbioses, ecology. Springer, Netherlands, Dordrecht, pp 25–51
Eggleton P, Williams PH, Gaston KJ (1994) Explaining global termite diversity: productivity or history? Biodivers Conserv 3:318–330. https://doi.org/10.1007/BF00056505
Emerson AE (1955) Geographic origins and dispersion of termite genera. Fieldiana Zool 37:465–521
Evans TA, Forschler BT, Grace JK (2013) Biology of invasive termites: a worldwide review. Annu Rev Entomol 58:455–474. https://doi.org/10.1146/annurev-ento-120811-153554
Evans TA, Lenz M, Gleeson PV (1999) Estimating population size and forager movement in a tropical subterranean termite (Isoptera: Rhinotermitidae). Environ Entomol 28:823–830. https://doi.org/10.1093/ee/28.5.823
Grace JK, Abdallay A, Farr KR (1989) Eastern subterranean termite (Isoptera: Rhinotermitidae) foraging territories and populations in Toronto. Can Entomol 121:551–556. https://doi.org/10.4039/Ent121551-7
Haverty MI, Nutting WL, Lafage JP (1975) Density of colonies and spatial distribution of foraging territories of the desert subterranean termite, Heterotermes aureus (Snyder). Environ Entomol 4:105–109. https://doi.org/10.1093/ee/4.1.105
Hölldobler B, Wilson EO (2009) The superorganism: the beauty, elegance, and strangeness of insect societies. W. W, Norton, New York
Howard RW, Jones SC, Mauldin JK, Beal RH (1982) Abundance, distribution, and colony size estimates for Reticulitermes spp. (Isoptera: Rhinotermitidae) in southern Mississippi. Environ Entomol 11:1290–1293. https://doi.org/10.1093/ee/11.6.1290
Inagaki T, Matsuura K (2016) Colony-dependent sex differences in protozoan communities of the lower termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Ecol Res 31:749–755. https://doi.org/10.1007/s11284-016-1387-2
Kennedy P, Baron G, Qiu B et al (2017) Deconstructing superorganisms and societies to address big questions in biology. Trends Ecol Evol 32:861–872. https://doi.org/10.1016/j.tree.2017.08.004
Kenneth Grace J, Yamamoto RT, Tamashiro M (1995) Relationship of individual worker mass and population decline in a Formosan subterranean termite colony (Isoptera: Rhinotermitidae). Environ Entomol 24:1258–1262. https://doi.org/10.1093/ee/24.5.1258
King EG, Spink WT (1969) Foraging galleries of the Formosan subterranean termite, Coptotermes formosanus, in Louisiana. Ann Entomol Soc Am 62:536–542. https://doi.org/10.1093/aesa/62.3.536
Konishi T, Tasaki E, Takata M, Matsuura K (2023) King- and queen-specific degradation of uric acid contributes to reproduction in termites. Proc R Soc B Biol Sci 290:20221942. https://doi.org/10.1098/rspb.2022.1942
La Fage JP, Su N-Y, Jones MJ, Esenther GR (1983) A rapid method for collecting large numbers of subterranean termites from wood. Sociobiology 7:305–309
Matsuura K, Kobayashi N, Yashiro T (2007) Seasonal patterns of egg production in field colonies of the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Popul Ecol 49:179–183. https://doi.org/10.1007/s10144-006-0030-4
Matsuura K, Mizumoto N, Kobayashi K et al (2018) A genomic imprinting model of termite caste determination: not genetic but epigenetic inheritance influences offspring caste fate. Am Nat 191:677–690. https://doi.org/10.1086/697238
Matsuura K, Vargo EL, Kawatsu K et al (2009) Queen succession through asexual reproduction in termites. Science 323:1687–1687. https://doi.org/10.1126/science.1169702
Nozaki T, Matsuura K (2021) Oocyte resorption in termite queens: seasonal dynamics and controlling factors. J Insect Physiol 131:104242. https://doi.org/10.1016/j.jinsphys.2021.104242
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton, NJ
Park YC, Kitade O, Schwarz M et al (2006) Intraspecific molecular phylogeny, genetic variation and phylogeography of Reticulitermes speratus (Isoptera: Rhinotermitidae). Mol Cells 21:89–103
Patel JS, Lee S-B, Chouvenc T, Su N-Y (2020) Inferring termite colony size using wood consumption in subterranean termites (Blattodea: Rhinotermitidae) in laboratory-rearing conditions. J Econ Entomol 113:905–910. https://doi.org/10.1093/jee/toz353
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Shellman-Reeve JS (1997) The spectrum of eusociality in termites. In: Choe JC, Crespi BJ (eds) Evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, UK, pp 52–93
Shimada K, Lo N, Kitade O et al (2013) Cellulolytic protist numbers rise and fall dramatically in termite queens and kings during colony foundatio. Eukaryot Cell 12:545–550. https://doi.org/10.1128/EC.00286-12
Spragg WT, Paton R (1980) Tracing, trophallaxis and population measurement of colonies of subterranean termites (Isoptera) using a radioactive tracer. Ann Entomol Soc Am 73:708–714. https://doi.org/10.1093/aesa/73.6.708
Su N-Y (2003) Behavioral ecology of foraging in subterranean termites. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, Dordrecht, Netherlands, pp 165–191
Su N-Y (1994) Field evaluation of a hexaflumuron bait for population suppression of subterranean termites (Isoptera: Rhinotermitidae). J Econ Entomol 87:389–397. https://doi.org/10.1093/jee/87.2.389
Su N-Y (2013) Estimating population size of large laboratory colonies of the Formosan subterranean termite using the capture probability equilibrium. J Econ Entomol 106:2442–2447. https://doi.org/10.1603/EC13258
Su N-Y, Ban PM, Scheffrahn RH (1993) Foraging populations and territories of the eastern subterranean termite (Isoptera: Rhinotermitidae) in southeastern Florida. Environ Entomol 22:1113–1117. https://doi.org/10.1093/ee/22.5.1113
Su N-Y, Lee S-H (2008) Estimating the population size and colony boundary of subterranean termites by using the density functions of directionally averaged capture probability. J Econ Entomol 101:592–604. https://doi.org/10.1093/jee/101.2.592
Su N-Y, Osbrink W, Kakkar G et al (2017) Foraging distance and population size of juvenile colonies of the Formosan subterranean termite (Isoptera: Rhinotermitidae) in laboratory extended arenas. J Econ Entomol 110:1728–1735. https://doi.org/10.1093/jee/tox153
Su N-Y, Scheffrahn RH (1988) Foraging population and territory of the Formosan subterranean termite (Isoptera: Rhinotermitidae) in an urban environment. Sociobiology 14:353–359
Takata M, Konishi T, Nagai S et al (2023a) Discovery of an underground chamber to protect kings and queens during winter in temperate termites. Sci Rep 13:8809. https://doi.org/10.1038/s41598-023-36035-1
Takata M, Nagai S, Inagaki T et al (2023b) Heritable effects on caste determination and colony-level sex allocation in termites under field conditions. iScience 26:106207. https://doi.org/10.1016/j.isci.2023.106207
Takematsu Y (1999) The genus Reticulitermes (Isoptera: Rhinotermitidae) in Japan, with description of a new species. Entomol Sci 2:231–243
Tasaki E, Mitaka Y, Takahashi Y et al (2023) The royal food of termites shows king and queen specificity. PNAS Nexus. https://doi.org/10.1093/pnasnexus/pgad222
Thorne BL (1997) Evolution of eusociality in termites. Annu Rev Ecol Syst 28:27–54. https://doi.org/10.1146/annurev.ecolsys.28.1.27
Thorne BL, Russek-Cohen E, Forschler BT et al (1996) Evaluation of mark–release–recapture methods for estimating forager population size of subterranean termite (Isoptera: Rhinotermitidae) colonies. Environ Entomol 25:938–951. https://doi.org/10.1093/ee/25.5.938
Tsunoda K, Matsuoka H, Yoshimura T, Tokoro M (1999) Foraging populations and territories of Reticulitermes speratus (Isoptera: Rhinotermitidae). J Econ Entomol 92:604–609. https://doi.org/10.1093/jee/92.3.604
Wilson EO (1971) The insect societies. Belknap Press, Cambridge, Massachusetts
Acknowledgements
We thank Matthew Tatsuo Kamiyama for the fruitful discussion, and Wu Yao, Chihiro Tamaki, Takehiro Morimoto, Hiroki Noda, Shuya Nagai for assistance in collecting termites and helpful discussion.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP18H05268, JP20K20380, and JP23H00332 to K.M., JP21K14863 to M.T., and JP20J20278 to T.K.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.T.; methodology, M.T. and K.Y.; validation, M.T.; formal analysis, M.T.; investigation, M.T., K.Y., T.N. S.M., T.K., and E.T.; resources, M.T., K.Y., T.N. S.M., T.K., and E.T.; writing—original draft: M.T.; writing—review and editing: M.T., K.Y., T.N. S.M., T.K., E.T., and K.M.; visualization, M.T. and T.K.; supervision, M.T. and K.M.; project administration, M.T.; funding acquisition, M.T., T.K., and K.M.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Sean O'Donnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 348 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takata, M., Yabe, K., Noro, T. et al. A method for estimating colony size using queen fecundity in termites under field conditions. Sci Nat 110, 35 (2023). https://doi.org/10.1007/s00114-023-01865-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-023-01865-6