Abstract
Metallic nanoparticles of different compositions have already found numerous applications in various branches of industry, agriculture, and medicine. Given the well-known antibacterial activity of Ag, silver nanoparticles (AgNPs) are constantly being investigated for their promising ability to fight antibiotic-resistant pathogens. A promising candidate for AgNPs biosynthesis is chili pepper Capsicum annuum, cultivated worldwide and known for accumulating significant amounts of active substances. Phytochemical screening of aqueous extract of C. annuum pericarps demonstrated accumulation of 4.38 mg/g DW of total capsaicinoids, 14.56 mg GAE/g DW of total phenolic compounds, 1.67 mg QE/g DW of total flavonoids, and 1.03 mg CAE/g DW of total phenolic acids. All determined aromatic compounds carry various active functional groups, which effectively participate in the biosynthesis of AgNPs and are characterized by high antioxidant potential. Therefore, the present research focused on the facile, quick, and effective procedure for the biosynthesis of AgNPs, which were analyzed for their morphology such as shape and size through UV–visible, Fourier-transform infrared spectroscopy (FTIR) assays, and scanning electron microscopy. We found that the AgNPs biosynthesis resulted in changes in FTIR spectra, depicting the rearrangement of numerous functional groups, while the nanoparticles themselves were shown to be stable, spherical, 10–17 nm in size. Also we investigated the antibacterial properties of biosynthesized AgNPs, obtained with C. annuum fruit extracts, against a common phytopathogen Clavibacter michiganensis subsp. michiganensis. As was shown by zone inhibition assay, AgNPs showed dose-dependent 5.13–6.44 cm antibacterial activity, greatly exceeding the 4.98 cm inhibition area, produced by the precursor salt, AgNO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biosynthesis of silver nanoparticles (AgNPs) with plant extracts is an important subtype of green chemistry approaches. Plant tissues containing specific bioactive compounds are able to reduce and stabilize metallic salts used as precursors in AgNPs synthesis. Such reductive properties against silver ions are known for various molecules that can be identified in the chemical composition of a plant, including ketones, amino acids, aldehydes and vitamins. The low production costs and the profound effectiveness are advantages that can overcome the traditional chemical route of nanoparticle synthesis (Nicolae-Maranciuc et al. 2022). The bioreducers found in plants also include enzymes, proteins, polysaccharides, organic acids and a vast diversity of secondary metabolites such as phenolic compounds and capsaicinoids that are potentially able to reduce metal ions (Smirnov et al. 2021).
Throughout the last decade, AgNPs, biosynthesized from different parts of Capsicum species, have received much consideration for various applications due to their substantial ability to disperse in aqueous systems combined with biological compatibility, antibacterial activities, high adsorption and catalytic activity, as well as the ease of their application in chemo- and biosensing fields (Shankar et al. 2017; Kumar et al. 2021; Dzhagan et al. 2022). Extracts enriched with bioreductants, obtained from different species of the genus Capsicum (C. annuum, C. frutescens, C. baccatum, and C. chinense) were shown to be a good choice for the biosynthesis of metallic nanoparticles. However, few studies have used extracts from plant tissues other than the fruits, such as leaves (Velgosova and Veselovský 2019; Lomelí-Rosales et al. 2022).
Genus Capsicum is a member of Solanaceae family and is believed to originate from Central and South America. It is the most commonly cultivated dicotyledonous plant – an indispensable spice used as a basic ingredient in a great variety of cuisines all over the world (Samrot et al. 2018; Rajam et al. 2021). Chilli pepper is a crop of great economic importance, as this vegetable is widely used in traditional cuisine providing flavour, aroma, and colour to various national dishes, and in addition, it is used in food cosmetics and pharmacy (Caicedo-Lopez et al. 2022). Also it has potential applications in herbal and traditional medicine, because it contains compounds such as flavonoids, ascorbic acid, tocopherol, lycopene, minerals and especially capsaicinoids with their antimicrobial, antiseptic, anticancer, counterirritant, appetite stimulator, antioxidant and immunomodulatory activities (Batiha et al. 2020).
The average percentage of food losses may reach 40% worldwide between postharvest and distribution in the fruit and vegetable supply chain including Solanaceae food crops such as tomatoes, potatoes, chilli peppers and eggplants. This food is substantially perishable and often has a fragile physical constitution, leading to a relatively short shelf life (Costa et al. 2021). One of the main infections affecting Solanaceae food crops is the bacterial canker caused by Clavibacter michiganensis subsp. michiganensis (Cmm) (Solano-Alvarez et al. 2022). The European and Mediterranean Plant Protection Organization classify four subspecies of Cmm as hazardous quarantine microorganisms because of the serious economic danger that they instigate. Solanaceae food crops infected with Cmm strains show diverse symptoms that depend on the host plant cultivar receptivity and virulence of microorganism pathovar, along with specific environmental conditions. Fruits and seeds that are externally contaminated with Cmm strains, contaminated soil, and infected plant debris can serve as an initial source of inoculum for systemic infections (Valencia-Hernandez et al. 2022).
Chemically and physically synthesized AgNPs release silver ions leading to the formation of reactive oxygen species. These radicals in interaction with plants have shown adverse effects, not only by penetrating into the root or shoot, but also by causing physical damage to the surface of plant tissues. Very little attention has been paid concerning the prevalence of bacterial canker and no research has been done on measures for controlling it. The biosynthesis of AgNPs by C. annuum pericarps aqueous extract with high bactericidal effects and its ability to induce potential resistance in plants highlights its importance (Noshad et al. 2020; Méndez-Andrade et al. 2022).
In this study, AgNPs were biosynthesized using aqueous extracts of Capsicum annuum L. pericarps, which contained aromatic bioactive secondary metabolites with antioxidant activity, and the bactericidal activity of obtained AgNPs was evaluated against phytopathogenic Clavibacter michiganensis subsp. michiganensis as hazardous and quarantine bacteria.
Materials and methods
Collection and preparation of plant extract
The plant material – pericarps of Capsicum annuum L. (cv. ‘Teja’) were purchased from the popular market near ESC “Institute of Biology and Medicine” in Kyiv, Ukraine. Fresh pericarps of C. annuum were washed several times in deionized water for removal of soil and any organic impurities and then air dried at 60 ºC to eliminate the residual moisture. Cleaned and dried pericarps of C. annuum were cut into small pieces and powdered into finely dispersed flour. Two grams of plant samples were put in a flask with a flat bottom with 100 ml deionized water and boiled for 20 min at 100 ºC. The obtained extracts of pericarps were cooled at room temperature and filtered with Whatman No.1 filter paper.
Phytochemical screening of potential bioreductants
All spectrophotometric assays were performed using UV–vis spectrophotometer UV-1800 “Shimadzu” (Japan). Total capsaicinoids content (TCC) analysis was estimated with Gibbs reagent (2,6-dichloroquinone-4-chloroimide) by the technique described by Ryu et al. 2017 with slight modifications. Total capsaicinojids contents were calculated with the molar extinction coefficient of 2,6-dichlorophenol indophenol according to Armstrong 1964 and expressed in mg per g of dry weight (DW). The total phenolic content (TPC) was determined according to the method with Folin-Ciocalteu reagent, which is based on the quantification of the total concentration of hydroxyl groups that are present in the extract (Boudghane et al. 2022). The absorbance at 765 nm was measured and results were expressed in mg of gallic acid equivalents (GAE) per g of DW. The total flavonoid content (TFC) assay was performed according to the protocol described by Neupane and Lamichlane (Neupane and Lamichhane 2020) with minor modifications. The absorbance at 506 nm was measured and results were expressed as mg of quercetin equivalents (QE) per g of DW. The total phenolic acid content (TPAC) was determined according to the method with Arnova reagent (Vergun et al. 2021). The absorbance at 490 nm was measured and caffeic acid was used as a standard, the results were expressed in mg of caffeic acid equivalents (CAE) per g of DW.
Evaluation of antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) is a relatively stable radical. Radical scavenging activity determination was used to quantify the ability of bioreductants in plant extracts to put out the DPPH radical within 15 min. Changes in the reaction mixture colouration were measured at 517 nm wavelength (UV-1800 “Shimadzu” (Japan) UV–vis spectrophotometer), which corresponds to the reduction of DPPH• to a non-radical form. The decrease in absorption correlated with the percent inhibition in samples. The percentage of inhibition was calculated against blank (Nakagawa et al. 2021) as follows:
AgNPs biosynthesis
Ag nanoparticles were synthesized using cooled and filtered extracts of C. annuum pericarp tissues by the addition of 0.001 M silver nitrate (AgNO3). For the reduction of silver ions and formation of AgNPs, 10 mL of extract was mixed with 40 mL of AgNO3 solution. The resulting solution was incubated under a light-emitting diode lamp (Secret Jardin, 42 W, 6500 K) for 2 h at room temperature for the reduction of silver salt.
Characterization of biosynthesized AgNPs
Ultraviolet spectrophotometric analysis of biosynthesized AgNPs were recorded with UV-1800 “Shimadzu” (Japan) UV–vis spectrophotometer at range of 200–700 nm with 0.5 nm resolution after 2 h of synthesis procedure. Fourier transform infrared spectroscopy (FT-IR, Bruker model VERTEX 80v) was performed to analyse the functional groups of the plant extract and biosynthesized AgNPs in the range of 500 cm−1 to 4000 cm−1. The size distribution and morphology was estimated after desiccation of the purified AgNP solutions at 60 ºC by scanning electron microscopy (SEM, Tescan Mira 3 MLU).
Antibacterial activity evaluation
The antibacterial activity of test samples against phytopathogenic Clavibacter michiganensis subsp. michiganensis (Cmm) was evaluated with agar diffusion test method. The culture of the test bacteria was grown in nutrient broth (Himedia) and adjusted to 2.0 McFarland turbidity standards. 1 mL of 72-h Cmm culture suspension was inoculated on the surface of solidified Mueller-Hilton agar in Petri dishes. Then, one well per one dish was made in agar with cylindrical metal tube and 100 μL aliquots of test samples were added to the wells. Commercially available single antibiotic disc impregnated with gentamicin GEN30 (Himedia) were aseptically placed on the Mueller–Hinton agar dishes as a positive control. As a negative control, we used the extract of C. annuum pericarps. Petri dishes were incubated at 20° C for 6 days and the diameters of zones of inhibition for the six separate determinations were recorded.
Statistical analysis
Each experiment was performed at least in triplicate. The results were expressed as mean ± standard deviation (SD). The analysis of variance (ANOVA) followed by Tukey’s multiple range test were used for significance tests. Means and standard deviations were calculated using Microsoft Office Excel (Microsoft Office 2010). A value of P < 0.05 was considered significant.
Results and discussion
Capsicum annuum is recognized worldwide as irreplaceable crop, valued for its nutritional potential, antioxidant compounds, flavour, pungency, brilliant colours, and texture (Caicedo-Lopez et al. 2022). Almost all parts of the C. annuum are considered to be rich in aromatic compounds including polyphenols, flavonoids and capsaicinoids. One of the significant biological properties of bioactive compounds is their ability to act as antioxidants to reduce reactive oxygen species (Akhtar et al. 2021). Determination of total capsaicinoids (TCC), total phenolic compounds (TPC), total flavonoids (TFC) and total phenolic acids (TPAC) contents in extracts from pericarps of C. annuum showed the presence all of these compounds in experimental extract (Table 1). As shown in Table 1, experimental aqueous extract demonstrated 4.38 mg/g DW of total capsaicinoids, 14.56 mg GAE/g DW of total phenolic compounds, 1.67 mg QE/g DW of total flavonoids, and 1.03 mg CAE/g DW of total phenolic acids. All determined aromatic compounds carry many active functional groups which effectively participate in biosynthesis of AgNPs and act as probable bioreducing, capping as well as a stabilizing agents and are characterized by high antioxidant potential (Khatoon et al. 2022).
The total antioxidant activity of aqueous extracts of C. annuum pericarps was investigated with stable DPPH radical. DPPH• is used in a basic in vitro screening method for evaluating the radical scavenging activity of natural or artificial compounds, different plant extracts because this technique is simple and relatively fast (La et al. 2021). The extract from C. annuum pericarps showed the high level of DPPH inhibition at 76% (Table 1).
All identified phytochemicals are potent bioreductive agents. The prominent total antioxidant activity of C. annuum pericarps aqueous extracts could be attributed to the substantial amount of TPC. These bioactive molecules present in extracts bear the responsibility for the high DPPH radical scavenging activity and act as reducing agents that in turn play a central role in the process of green synthesis of AgNPs. In addition, there are several studies on the action of capsaicin on AgNPs by the direct reduction of silver nitrate in the aqueous phase, without the use of any other reducing agents (Amruthraj et al. 2015; Dong et al. 2021). Antioxidant activity of extract can affect the experimental optimization of operating parameters needed for the biosynthesis of AgNPs from pericarps of C. annuum.
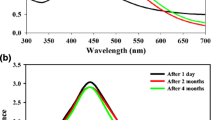
The formation of AgNPs as a result of green synthesis with C. annuum pericarp extracts was detected with UV–vis spectrometry. It has been previously shown that solution of metallic nanoparticles shows an increased absorbance at a specific wavelength, which depends on shape, size and composition of nanostructures (Buda et al. 2017; Fahmy et al. 2019). Such optical activity is a result of surface plasmon resonance (SPR) – a characteristic feature of nanoparticles that arises from the interaction of external energy of light with the densely-distributed energy fields on the surface of nanounits (Lee and Jun 2019). In our study, the formation of AgNPs in experimental solutions was accompanied by the increased absorbance in visible spectrum, with a peak at 435 nm (Fig. 1). After the completion of green synthesis, the width and height of this peak remained constant, proving the stability of obtained AgNP solution. The reduction of silver ions and accumulation of AgNPs could also be easily detected with a naked eye due to increased turbidity of reaction medium, combined with the change of its colour to brown (Fig. 1, inset). These effects are also the result of SPR propagation combined with increased light scattering, which is typical for any nanocolloidal solution (Balachandar et al. 2022; Nayak et al. 2022).
From the FTIR data presented in Fig. 2, we can assume which functional groups or molecular species may be involved in the NP stabilization. The vibration bands that are similar in position and intensity in the pure extract and in AgNP sample are those at 1043, 1076, 1134, 1217, 1234, 1740 cm−1 (Fig. 2a), as well as all the peaks in the range 2800–3000 cm−1 (Fig. 2b).
Infrared transmission spectra of phytosynthesized AgNPs in the range of 700–1900 cm−1 (a) and of 2400–3600 cm−1 (b). The spectra shifted vertically for convenience. To avoid overloading the figure, the wavenumbers of absorption peaks are marked only in those spectra where they occur at the largest intensity or are most distinct
The intensity ratio of the bands at 1355 and 1400 cm−1 was reversed in the AgNP sample. The same observation is for the pair of bands at 1610 and 1670 cm−1, which in addition change their positions to 1628 and 1658 cm−1 in the AgNP sample. Most likely, the functional groups related with these vibrations are involved in the stabilization of the AgNPs. According to the literature, the 1355 cm−1 band can be due to N = O of the aliphatic nitro group, reported in Ulaeto et al. 2020 at 1363 cm−1 and/or C–H bending in cellulose and hemicellulose, reported around 1365–1375 cm−1 (Türker-Kaya and Huck 2017). The 1400 cm−1 band can be attributed to C − H bend of alkanes (CH3), observed at 1395 cm−1 in Ulaeto et al. 2020 and/or O–H bending in polysaccaride cell wall, alcohol, and carboxylic acid, observed at 1420–1430 cm−1 in Türker-Kaya and Huck 2017.
The candidates for the 1610 (1628) cm−1 are C = C of aromatic group, N − H bending in amides according to Ulaeto et al. 2020, C − C skeletal vibrations/N = H deformations, C = O aromatic stretch: lignin, alkaloid (Huang et al. 2008). The 1670 (1658) cm−1 band is most likely related with amide I (C = O stretch) in protein, pectin, water associated cellulose or lignin, alkaloids (Türker-Kaya and Huck 2017).
The band at 1547 cm−1 is not registered for bare extract, only for the NP sample. The vibrations around this frequency were attributed in the related literature (on phytoextracts) to N − H bending in amides & the interaction between N − H bending and C − N stretching of C − N–H group and amide II (C = N and N–H stretch) in protein (Türker-Kaya and Huck 2017).
Morphological features of obtained AgNPs were described with scanning electron microscopy. Analysis of obtained SEM images (Fig. 3) showed that nanoparticles were uniform and spherical in shape, with an average size of 10–17 nm. Notably, we detected low number of AgNP aggregates, while the main part of the solution was represented with finely-dispersed nanostructures.
It is known that stability of nanoparticles greatly depends on the exact composition of the solution as well as concentration of particular molecules. Specifically, the presence of capping agents in the reaction medium during nanoparticle formation enables effective creation of the protective layer on AgNPs, promoting their repulsion and, thus, decreasing the aggregation tendency (Ali et al. 2021; Bélteky et al. 2019). We therefore might postulate that bioactive compounds in C. annuum extract may serve as effective stabilizing agents for AgNPs. Silver nanoparticles of similar size, shape and stability were also obtained in other plant-based green synthesis approaches. For instance, Salayová et al. 2021 received 15–75 nm AgNPs with extracts from five different plant species, while Ahn et al. 2019 reported synthesis of 8–35 nm particles with 30 different extracts. All authors underline that properties of obtained AgNPs are specific for the plant species and therefore the selection of suitable source of bioreducing compounds may affect the possible range of applications of green-synthesized AgNPs.
The antimicrobial activity of the biosynthesized AgNPs in different concentrations (20 mg/L, 40 mg/L, and 80 mg/L) was assessed against phytopathogenic bacteria Clavibacter michiganensis subsp. michiganensis (Cmm). The results indicated that test samples exhibited varying antimicrobial activities against the tested phytopathogen. Antibacterial activity was estimated by the size of the zone of bacterial culture growth inhibition in comparison with the gentamicin, 0.001 M silver nitrate solution, and the primary extract of C. annuum pericarps (Fig. 4).
Antimicrobial susceptibility agar diffusion method against Clavibacter michiganensis subsp. michiganensis: zone of inhibition of gentamicin (a), extract of dry pericarps Capsicum annuum (b), 0.001 M silver nitrate (c), AgNPs in 20 mg/L (d), 40 mg/L (e), 80 mg/L (f) obtained by biosynthesis using aqueous extracts of Capsicum annuum pericarps
Gentamicin, which was used as positive control, is an aminoglycoside antibiotic used in the treatment of several bacterial infections. The highest antimicrobial activity was recorded for the AgNPs at a concentration of 80 mg/L (6.44 cm) followed by those for the AgNPs at a concentration of 40 mg/L (5.86 cm). The effect of experimental samples of AgNPs at a concentration of 20 mg/L and 0.001 M silver nitrate solution as precursor salt were not statistically significantly different (5.13 cm and 4.98 cm respectively). In contrast, the lowest antimicrobial action was recorded for gentamicin influence (1.92 cm), at the same time primary extract of C. annuum pericarps did not affect Cmm bacterial culture at all (Table 2).
The activity of biosynthesized AgNPs against Cmm bacterial culture was also evaluated by Noshad et al. 2019, where filamentous fungi A. fumigatus and T. harzianum were used as bioreducers. However, our results indicate the prevalent anti-Cmm activity of C. annuum-derived AgNPs, proposing its potential wide application for food processing and agriculture.
Conclusions
The present study confirms the possibility of AgNPs biosynthesis by aqueous extracts of Capsicum annum pericarps. The phytoscreening of plant-derived extract showed accumulation of bioreducing compounds, whose activity was confirmed by the DPPH quenching assay. The obtained AgNP solutions showed remarkable stability combined with structural uniformity of nanostructures, as verified by UV–vis, SEM and FT-IR analyses. Plant-derived AgNPs showed excellent antibacterial activity against phytopathogen Clavibacter michiganensis subsp. michiganensis, compared to the traditional antibiotic gentamycin. Therefore, C. annuum may be used as an effective and eco-friendly source of bioactive AgNPs for their application in agriculture and food technology.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- ANOVA:
-
Analysis of variance
- Cmm:
-
Clavibacter michiganensis Subsp. michiganensis
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DW:
-
Dry weight
- FT-IR:
-
Fourier transform infrared spectroscopy
- GAE:
-
Gallic acid equivalents
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscopy
- SPR:
-
Surface plasmon resonance
- TCC:
-
Total capsaicinoids content
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoids content
- TPAC:
-
Total phenolic acids content
- UV-vis:
-
Ultraviolet-visible spectroscopy
References
Ahn EY, Jin H, Park Y (2019) Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater Sci Eng, C 101:204–216. https://doi.org/10.1016/j.msec.2019.03.095
Akhtar A, Asghar W, Khalid N (2021) Phytochemical constituents and biological properties of domesticated capsicum species: a review. Bioact Compd Health Dis 4(9):201–225. https://doi.org/10.31989/bchd.v4i9.837
Ali A, Sattar M, Hussain F, Tareen MHK, Militky J, Noman MT (2021) Single-step green synthesis of highly concentrated and stable colloidal dispersion of core-shell silver nanoparticles and their antimicrobial and ultra-high catalytic properties. Nanomaterials 11(4):1007. https://doi.org/10.3390/nano11041007
Amruthraj NJ, Preetam Raj JP, Lebel A (2015) Capsaicin-capped silver nanoparticles: its kinetics, characterization and biocompatibility assay. Appl Nanosci 5(4):403–409. https://doi.org/10.1007/s13204-014-0330-5
Armstrong JM (1964) The molar extinction coefficient of 2, 6-dichlorophenol indophenol. Biochimica et Biophysica Acta (BBA)-General Subjects 86(1):194–197. https://doi.org/10.1016/0304-4165(64)90180-1
Balachandar R, Navaneethan R, Biruntha M, Kumar KKA, Govarthanan M, Karmegam N (2022) Antibacterial activity of silver nanoparticles phytosynthesized from Glochidion candolleanum leaves. Mater Lett 311:131572. https://doi.org/10.1016/j.matlet.2021.131572
Batiha GES, Alqahtani A, Ojo OA, Shaheen HM, Wasef L, Elzeiny M, Hetta HF (2020) Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int J Mol Sci 21(15):5179. https://doi.org/10.3390/ijms21155179
Bélteky P, Rónavári A, Igaz N, Szerencsés B, Tóth IY, Pfeiffer I, Kónya Z (2019) Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int J Nanomed 14:667. https://doi.org/10.2147/IJN.S185965
Boudghane LC, Bouabdellah N, Bouanane S, Ahmed FZB, Laroussi MA, Bendiaf Y, Merzouk H (2022) Phytochemical, antioxidant, and antimicrobial attributes of different extracts of seeds: the Algerian variety of dates ‘Deglet Nour’(Phoenix dactylifera L.). Vegetos, 1–7. https://doi.org/10.1007/s42535-022-00413-3
Buda S, Shafie S, Rashid SA, Jaafar H, Sharif NFM (2017) Enhanced visible light absorption and reduced charge recombination in AgNP plasmonic photoelectrochemical cell. Results Phys 7:2311–2316. https://doi.org/10.1016/j.rinp.2017.07.009
Caicedo-Lopez LH, Guevara-Gonzalez RG, Ramirez-Jimenez AK, Feregrino-Perez AA, Contreras-Medina LM (2022) Eustress application trough-controlled elicitation strategies as an effective agrobiotechnology tool for capsaicinoids increase: a review. Phytochem Rev 1–28. https://doi.org/10.1007/s11101-022-09818-z
Dong C, Tao J, Fu Z (2021) Green Synthesis and Characterization of Silver Nanoparticles Using Capsicum annuum L. Extract. Nanosci Nanotechnol-Asia 11(4):72–78. https://doi.org/10.2174/2210681210999200905130447
dos S Costa D, Alviano Moreno DS, Alviano CS, da Silva AJR (2021) Extension of Solanaceae Food Crops Shelf Life by the Use of Elicitors and Sustainable Practices During Postharvest Phase. Food Bioprocess Technol 1–26. https://doi.org/10.1007/s11947-021-02713-z
Dzhagan V, Smirnov O, Kovalenko M, Mazur N, Hreshchuk O, Taran N, Zahn DR (2022) Spectroscopic Study of Phytosynthesized Ag Nanoparticles and Their Activity as SERS Substrate. Chemosensors 10(4):129. https://doi.org/10.3390/chemosensors10040129
Fahmy HM, Mosleh AM, AbdElghany A, Shams-Eldin E, Serea ESA, Ali SA, Shalan AE (2019) Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv 9(35):20118–20136. https://doi.org/10.1039/C9RA02907A
Huang J, Lin L, Li Q, Sun D, Wang Y, Lu Y, He N, Yang K, Yang X, Wang H (2008) Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried Cinnamomum camphora leaf in tubular microreactors. Ind Eng Chem Res 47:6081–6090. https://doi.org/10.1021/ie701698e
Khatoon A, Syed JA, Buledi JA, Shakeel S, Mallah A, Solangi AR, Shah MR (2022) Bio-green fabrication of bell pepper mediated silver nanoparticles: an efficient material for electrochemical sensing of arbutin in cosmetics. J Iran Chem Soc 1–14. https://doi.org/10.1007/s13738-022-02558-z
Kumar B, Smita K, Awasthi SK, Debut A, Cumbal L (2021) Capsicum baccatum (Andean Chilli)-assisted phytosynthesis of silver nanoparticles and their H2O2 sensing ability. Part Sci Technol 1–9. https://doi.org/10.1080/02726351.2021.2006381
La J, Kim MJ, Lee J (2021) Evaluation of solvent effects on the DPPH reactivity for determining the antioxidant activity in oil matrix. Food Sci Biotechnol 30(3):367–375. https://doi.org/10.1007/s10068-020-00874-9
Lee SH, Jun BH (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20(4):865. https://doi.org/10.3390/ijms20040865
Lomelí-Rosales DA, Zamudio-Ojeda A, Reyes-Maldonado OK, López-Reyes ME, Basulto-Padilla GC, Lopez-Naranjo EJ, Velázquez-Juárez G (2022) Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum chinense Plant. Molecules 27(5):1692. https://doi.org/10.3390/molecules27051692
Méndez-Andrade R, Vallejo-Perez MR, Loera-Alvarado E, de los Santos-Villarreal G, García-Cerda LA, Vera-Reyes I (2022) Efficacy of biosynthesized silver nanoparticles from Larrea tridentata against Clavibacter michiganensis. J Phytopathol 170(2):91–99. https://doi.org/10.1111/jph.13058
Nakagawa S, Ohmura R, Toshima S, Park H, Narasako Y, Hirano T, Kunitake H (2021) Changes in polyphenols, anthocyanins, and DPPH radical-scavenging activities in sweetpotato (Ipomoea batatas L.) during tuber growth. Sci Hortic 284:110100. https://doi.org/10.1016/j.scienta.2021.110100
Nayak S, Goveas LC, Kumar PS, Selvaraj R, Vinayagam R (2022) Plant-mediated gold and silver nanoparticles as detectors of heavy metal contamination. Food Chem Toxicol 113271. https://doi.org/10.1016/j.fct.2022.113271
Neupane P, Lamichhane J (2020) Estimation of total phenolic content, total flavonoid content and antioxidant capacities of five medicinal plants from Nepal. Vegetos 33(2):360–366. https://doi.org/10.1007/s42535-020-00116-7
Nicolae-Maranciuc A, Chicea D, Chicea LM (2022) Ag Nanoparticles for Biomedical Applications-Synthesis and Characterization – A Review. Int J Mol Sci 23(10):5778. https://doi.org/10.3390/ijms23105778
Noshad A, Iqbal M, Hetherington C, Wahab H (2020) Biogenic AgNPs – a nano weapon against bacterial canker of tomato (BCT). Adv Agric 2020:9630785. https://doi.org/10.1155/2020/9630785
Noshad A, Hetherington C, Iqbal M (2019) Impact of AgNPs on seed germination and seedling growth: A focus study on its antibacterial potential against Clavibacter michiganensis subsp. michiganensis infection in Solanum lycopersicum. J Nanomater 2019. https://doi.org/10.1155/2019/6316094
Rajam MV, Nandy S, Pandey R (2021) Biotechnology of Red Pepper. In Genetically Modified Crops (pp. 53–83). Springer, Singapore. https://doi.org/10.1007/978-981-15-5932-7_3
Ryu WK, Kim HW, Kim GD, Rhee HI (2017) Rapid determination of capsaicinoids by colorimetric method. J Food Drug Anal 25(4):798–803. https://doi.org/10.1016/j.jfda.2016.11.007
Salayová A, Bedlovičová Z, Daneu N, Baláž M, LukáčováBujňáková Z, Balážová Ľ, Tkáčiková Ľ (2021) Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 11(4):1005. https://doi.org/10.3390/nano11041005
Samrot AV, Shobana N, Jenna R (2018) Antibacterial and antioxidant activity of different staged ripened fruit of Capsicum annuum and its green synthesized silver nanoparticles. BioNanoScience 8(2):632–646
Shankar T, Karthiga P, Swarnalatha K, Rajkumar K (2017) Green synthesis of silver nanoparticles using Capsicum frutescence and its intensified activity against E. coli. Resour-Efficient Technol 3(3):303–308. https://doi.org/10.1016/j.reffit.2017.01.004
Smirnov OE, Kalynovskyi VY, Yumyna YM, Zelena PP, Skoryk MA, Dzhagan VM, Taran NY (2021) Green synthesis of silver nanoparticles using aqueous extract of hot chili pepper fruits and its antimicrobial activity against Pseudomonas aeruginosa. Ukr Biochem J 93(5):102–110. https://doi.org/10.15407/ubj93.05.102
Solano-Alvarez N, Valencia-Hernández JA, Vergara-Pineda S, Millán-Almaraz JR, Torres-Pacheco I, Guevara-González RG (2022) Comparative Analysis of the NDVI and NGBVI as Indicators of the Protective Effect of Beneficial Bacteria in Conditions of Biotic Stress. Plants 11(7):932. https://doi.org/10.3390/plants11070932
Türker-Kaya S, Huck CW (2017) A review of mid-infrared and near-infrared imaging: Principles, concepts and applications in plant tissue analysis. Molecules 22:168. https://doi.org/10.3390/molecules22010168
Ulaeto SB, Mathew GM, Pancrecious JK, Nair JB, Rajan TPD, Maiti KK, Pai BC (2020) Biogenic Ag Nanoparticles from Neem Extract: Their Structural Evaluation and Antimicrobial Effects against Pseudomonas nitroreducens and Aspergillus unguis (NII 08123). ACS Biomater Sci Eng 6:235–245. https://doi.org/10.1021/acsbiomaterials.9b01257
Valencia-Hernandez JA, Solano-Alvarez N, Rico-Rodriguez MA, Rodriguez-Ontiveros A, Torres-Pacheco I, Rico-Garcia E, Guevara-Gonzalez RG (2022) Eustressic Dose of Cadmium in Soil Induces Defense Mechanisms and Protection Against Clavibacter Michiganensis in Tomato (Solanum lycopersicum L.). J Plant Growth Regul 1–8. https://doi.org/10.1007/s00344-021-10559-0
Velgosova O, Veselovský L (2019) Synthesis of Ag nanoparticle using R. officinalis, U. dioica and V. vitisidaea extracts. Mater Lett 248:150–152. https://doi.org/10.1016/j.matlet.2019.04.027
Vergun O, Svydenko L, Grygorieva O, Sedláčková VH, Šramková KF, Ivanišová E, Brindza J (2022) Polyphenol component and antioxidant activity of Thymus spp. Potravinarstvo 15(1):1–14. https://doi.org/10.5219/1715
Acknowledgements
We would like to thank Dr. M.A. Skoryk from G.V. Kurdyumov Institute for Metal Physics, National Academy of Sciences of Ukraine for the SEM investigation and Dr. M. Vuichyk from ISP NASU for IR measurements of biosynthesized solutions of AgNPs.
Funding
This work was not supported by any funding agency.
Author information
Authors and Affiliations
Contributions
Oleksandr Smirnov and Vitalii Kalynovskyi project conceptualization and edited the manuscript, Pavlina Zelena and Yuliia Yumyna designed the experiments with microorganisms, Volodymyr Dzhagan performed the FTIR analysis, Mariia Kovalenko wrote the first draft of the manuscript, Yevheniia Konotop and Nataliya Taran analyzed the manuscript contents and made the manuscript corrections. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Lukasz Stepien
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smirnov, O., Kalynovskyi, V., Zelena, P. et al. Bactericidal activity of Ag nanoparticles biosynthesized from Capsicum annuum pericarps against phytopathogenic Clavibacter michiganensis. Sci Nat 110, 15 (2023). https://doi.org/10.1007/s00114-023-01844-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-023-01844-x