Abstract
Calcium oxalate (CaOx) crystals have challenged human curiosity since the advent of microscopy. These crystals are linked to the control of calcium levels in plant cells, but they have also been attributed several other functions, including protection against herbivory. However, the protection offered by CaOx crystals against herbivory may be overstated, as claims have been mainly based on their shapes and hard and indigestible nature rather than on experimental evidence. I contend that it is improbable that a constitutive defense, present since very early in the evolution of plants, has not been superseded by herbivores, especially insects. Here, I present arguments and evidence that suggest that these crystals have low efficiency in protecting plants against herbivores. First, I argue that insects with chewing mouthparts possess a semipermeable structure that protects their midgut, minimizing damage from crystals. Second, the action of CaOx crystals is purely mechanical and similar to other inert materials such as sand. Therefore, CaOx crystals only provide effective protection from herbivory in very particular cases and should not be considered an effective defense without supporting experimental evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 90% of the calcium in the body of plants of many species can be in the form of calcium oxalate (CaOx) crystals (Nakata 2003, and references therein). Furthermore, such crystals are widespread throughout the plant kingdom (Franceschi and Nakata 2005; Bauer et al. 2011). These crystals were originally described in the earliest microscopic observations of plant cells by Leeuwenhoek (1675), and many functions have since been attributed to them (Franceschi and Nakata 2005; Nakata 2003). Calcium regulation, calcium reserve, and defense against herbivores stand out among the many functions attributed to CaOx crystals (Paiva 2019).
Numerous studies refer to the supposed protective role of CaOx crystals against herbivores, mainly chewing insects (Franceschi and Nakata 2005; Park et al. 2009; Karabourniotis et al. 2020). However, these claims are mostly based on the morphological traits of the crystals (Franceschi and Nakata 2005) as some of them possess pointed or sharp edges. Besides their morphological attributes, the hard and indigestible nature of CaOx crystals in plant cells can provide a mechanical barrier to chewing insects (Hudgins et al. 2003).
The shapes of these crystals seem to have been relevant in provoking speculation about their effects against herbivory. Antonie van Leeuwenhoek (1632–1723), one of the first to observe CaOx crystals, made the first descriptions of their crystalline forms and associated them with some plant attributes. In one of his observations under a microscope, Leeuwenhoek (1675) mentioned: “… I perceived also the little pipes in this expressed sap. Now it is likely, that these pipes in this herb are the cause of the smart that is felt in chewing the Arum, by the motion of the tongue in tasting: for that there may be a long-lasting motion, remaining in liquors or saps upon a little stirring (which in our case occasions the pain) (…) But such little pipes, as I have mentioned to be in the herb Arum, I have not discovered in any other plants, hitherto viewed by me.” It should be noted that, in this case, in addition to describing the crystals present in cell sap extracted from Arum sp. (Araceae), which today we know to be raphides — a needle-shaped crystals of calcium oxalate, Leeuwenhoek linked them to the itching and painful irritation caused by this plant. Thus, albeit indirectly, Leeuwenhoek provided the first report of a supposed CaOx-mediated anti-herbivory action, based mainly on crystal shape. Since then, and especially concerning herbivory, the shape and hardness of CaOx crystals have led several authors to attribute them alleged anti-herbivory properties. At first thought, it seems obvious to think that the ingestion of raphides, which are essentially needles, by a young caterpillar, for example, would cause severe damage to its body (note that the raphides can reach up to one-hundredth the size of a caterpillar). But the obvious, in nature, is not always the truth, as I argue here.
One must bear in mind that plants and insects have long coevolved while coexisting for 350 million years. Therefore, as their most common interaction involves the consumption of plant tissues by insects, chemical and physical defenses against herbivorous insects evolved in plants (Gatehouse 2002). At the same time, and as a result of this co-evolution, insects have also developed constitutive adaptations to overcome the defensive traits of plants. A classic example is the changes that occur in insect enzyme assortment that allows them to feed on potentially toxic plants (Mello and Silva-Filho 2002).
Soluble oxalate, CaOx crystals, and vertebrate herbivory
The coexistence of plants and herbivores resulted in structural and chemical defenses by plants (War et al. 2018). Among these, oxalate is interesting because it can behave as both a chemical (soluble oxalates) and a structural defense (oxalate crystals). Regarding vertebrates, reports of toxic effects of both soluble and crystalline forms of oxalate are mainly linked to mammals, especially humans and domestic animals (Gardner 1994, and references therein). Soluble forms of oxalate have greater potential for toxicity and cause severe poisoning, mainly by causing systemic hypocalcemia and kidney failure (Cortinovis and Caloni 2013; Rahman et al. 2013). However, according to Rahman et al. (2013), insoluble oxalate crystals, such as CaOx, are not harmful, at least to ruminants, because rumen bacteria help to degrade oxalate.
The different morphologies of CaOx crystals (e.g., raphides, druses, crystal sand, or prismatic crystals) are not the main cause of most of the injuries caused to vertebrates by plant materials; but in some cases, raphides seem to prevail, due to their piercing action. A classic example is dumb cane (Dieffenbachia sp., Araceae), a common ornamental plant. The toxic effect caused by this species seems to result from a combination of the mechanical action of raphides with a chemical toxin (Arditti and Rodriguez 1982; Gardner 1994). Therefore, at least some CaOx crystals, like raphides, seem to act merely as piercing agents (Herbert 1924; Arditti and Rodriguez 1982; Gardner 1994). The damage caused to vertebrates by the ingestion of CaOx crystals can be only mechanical or potentiated by plant sap (Le Coz and Ducombs 2006). Such mechanical damage mainly consists of mucosal wounds due to puncture or abrasion, which occurs regardless of crystal shape. However, injuries or irritation caused by such crystals are well-tolerated by most vertebrate herbivores. Raphides are frequent in the food of humans and other vertebrates, such as raw fruits (e.g., pineapple) and vegetables. The presence of these crystals in food rarely causes damage; in some cases, mucosal irritation occurs due to the simple mechanical action of the crystals (Perera et al. 1990; Le Coz and Ducombs 2006).
Especially with regard to raphides, the role of crystals in defense against herbivory by vertebrates seems to be indirect (see Gardner 1994). For example, some poisonous plants, such as fruits of Caryota spp. (Arecaceae) and leaf and tubers of several species of Araceae, are known to cause a painful burning sensation of the skin and oral cavity in humans (Le Coz and Ducombs 2006). In these plants, CaOx crystals, such as raphides, act together with unverified proteinaceous toxins (Nelson et al. 2007) or even organic acids. Recently, Konno et al. (2014) provided strong experimental evidence on the synergism between raphides and other plant-defensive mechanisms. These authors suggested a ‘‘needle effect’’ of raphides, which intensifies the bioactivities of other bioactive plant components by making holes in barriers (e.g., cell membrane, cuticle, epithelium) within the herbivore. These holes facilitate the passage of bioactive factors such that they reach their targets within the buccal and digestive apparatus. This combined action of crystals and chemical agents seems to be essential to the defensive properties of raphides.
CaOx crystals are not harmless to vertebrates, but this does not imply protection against herbivory. Some negative effects of such crystals are chronic and not noticeable by the individual. In mammals, including humans, hyperoxaluria and kidney stone formation are problems related to the intake of oxalates, mainly if there is a genetic predisposition for oxalate kidney stone disease (Franceschi and Nakata 2005). However, this effect is cumulative and chronic and does not prevent the consumption of the plant by herbivores, i.e., it does not have a protective effect against herbivory. According to Sanz and Reig (1992), poisoning by ingestion of oxalate-containing plants, such as raw rhubarb, is infrequent, and deaths are rare.
Even when the action of CaOx crystals is immediate, in the case of wounds in the mucosa or itching, their presence does not always discourage herbivores. Ward et al. (1997) found that dorcas gazelles (Gazella dorcas) eat only the portions of a particular desert lily (Pancratium sickenbergeri) in which CaOx crystals do not occur. The presence of CaOx in these lily plants is not an inducible defense, and the herbivores are still able to inflict damage to the plant. CaOx crystals are constitutive defenses that act as an effective herbivore-deterrent (Ward et al. 1997; Ruiz et al. 2002).
In most cases of protection against vertebrate herbivory, the action of CaOx crystals is purely mechanical and similar to other inert materials such as sand. In fact, entrapped sand makes plants less preferred by herbivores, such as mammals, but also invertebrates such as gastropods and insects (Lopresti and Karban 2016) and seems to provoke deterrence similar to that caused by CaOx crystals. Furthermore, crystal shape is of fundamental significance in these interactions involving vertebrates, with raphides being responsible for almost all reports of anti-herbivory action.
Soluble oxalate, CaOx crystals, and insect herbivory
Despite not being the rule, CaOx crystals seem to have some detrimental effects on insect development (see Doege 2003; Korth et al. 2006). However, experimental tests that demonstrate such effects are rare, compared to the numerous speculative statements. But even when such crystals are demonstrated to have some effect on plant–insect interactions, the effect is limited to physical factors causing abrasion to insect mandibles (Park et al. 2009). This physical effect, however, has also been observed for entrapped sand (Lopresti et al. 2018) and silica (Massey and Hartley 2009) on the surface of plant organs. For example, the caterpillar Hyles lineata (Sphingidae) was found to experience wear to the mandible and reduced larvae fitness when feeding on Abronia latifolia (Nyctaginaceae) (Lopresti et al. 2018).
Crystalline clusters with pointed projections (druses) or needle-like crystals (raphides) can cause abrasion or punctures, especially when contacting delicate body parts or membranes. Therefore, since some insects, especially in the larval phase, have soft and delicate bodies, they are likely not resistant to these crystalline forms. But herein lies one of the biggest problems with regard to understanding the action of crystals against herbivory: an anthropocentric and distorted view of reality that does not consider insect morphophysiology, which has led to undue generalizations.
The formation of CaOx crystals is essential to the control of cytosolic calcium levels in plants (see Paiva 2019), and so these crystals probably appeared very early in plant evolution (Karabourniotis et al. 2020). Thus, it is hard to conceive that these crystals, present in most plant species, could constitute an anti-herbivory defense that has never been supplanted by insects — a group of highly adaptable and diverse organisms. According to Janzen (1980), herbivore-deterrent traits usually exert selection pressure on herbivores to overcome plant resistance. Therefore, the question to be answered is not whether CaOx crystals act in defense against herbivorous insects but how insects have broken through the barrier imposed by them. The answer to this question is relatively simple: insects, especially those with chewing mouthparts, have a peritrophic matrix (PM).
The PM is a semipermeable structure that involves the midgut of some insects and consists of a network of chitin, proteins, glycoproteins, and proteoglycans (see Mohan et al. 2006, and references therein). The PM avoids contact between the food bolus and the midgut epithelium and protects the delicate epithelial microvilli from abrasion by food particles; therefore, it seems to be essential for nutrient absorption (Wang and Granados 2001; Terra 2001). Thus, inducing damage to the PM may be a host–plant resistance mechanism, as observed by Pechan et al. (2002) in maize plants that respond to herbivorous insect attack by synthesizing a cysteine protease that causes damage to the PM of the caterpillar Spodoptera frugiperda (Lepidoptera: Noctuidae). This protease permeabilizes the PM and impairs the growth of lepidopteran larvae (Mohan et al. 2006). Because the PM contains chitin, the role of chitinases in insect defense could be assumed (Lawrence and Novak 2006).
Since the PM protects against mechanical damage, CaOx crystals are not harmful to the insect midgut. The PM of caterpillars (Spodoptera exigua) feeding on Medicago truncatula plants that have CaOx crystals was not damaged (Park et al. 2009). Indeed, these authors attributed the negative impacts of these crystals in the diet of larvae to physical factors, highlighting their lack of toxicity. Lepidopteran larvae and other chewing insects can feed on plant tissues with CaOx crystals (Figs. 1 and 2), including raphides, without apparent damage and still complete their life cycles.
Insects and their feces or undigested material within the midgut that present CaOx crystals. A Larvae of unidentified lepidopteran feeding on leaves of Hippeastrum sp. (Amaryllidaceae). B, C Samples of feces from larvae shown in A, observed via light microscopy. Note the presence of raphides (arrows) under polarized light. D Adults of Acalymma sp. (Coleoptera, Chrysomelidae) feeding on Amaranthus retroflexus (Amaranthaceae) leaves. Intact druses (inside circles) within the midgut of adults of Acalymma sp. after feeding on Amaranthus, as shown in D
Insects and their faces or undigested material within the midgut that present CaOx crystals. A, B, C Larvae of unidentified lepidopteran that fed on leaves of Cissus gongylodes (Vitaceae). Note, in B, detail of the mouthparts and material regurgitated by the caterpillar showed in A, highlighting entire raphides. C Detail of the terminal portion of the caterpillar, with emphasis on a portion of recently released feces. Note the presence of bundles of raphides (long arrows), as well as entire druses (short arrows). D Larvae of unidentified lepidopteran that fed on leaves of Amaranthus spinosus (Amaranthaceae). Intact druses can be observed within the midgut of adults (inside circles) as well as in their feces (inside circle in E)
Studying silkworm, Bombyx mori (Lepidoptera: Bombycidae), larvae, Nagaoka et al. (2010) observed that the addition of CaOx crystals to the diet did not increase mortality in larval and pupal stages. Additionally, these authors reported the presence of crystals in larval feces, demonstrating the absence of interference with these crystals by the physiological processes of these insects. These results demonstrate the protection exerted by the PM in eliminating any potential mechanical damage that could be caused by crystals.
Interestingly, when purified raphides are applied to the leaf surface, they cause more damage to some delicate insect bodies than if they had been ingested. This is due to the fact that during the larval stages of some insects, there is no resistant external barrier that protects vulnerable tissues, as does the PM in the midgut (see Konno et al. 2014). Likewise, the action of inert dust seems to be a result of the abrasion of the insect cuticle, which can lead to water loss and desiccation, as pointed out by Showler (2002).
Therefore, CaOx crystals must be considered just a physical deterrent of insect herbivory, rather than a substance that provokes toxicity or midgut injuries. According to Bernays and Chapman (1987), the evolutionary adaptability of insects is such that “harmless resources are unlikely to remain un-utilized, and that the possession of a harmless compound simply causing behavioral deterrence is ultimately no protection against insect herbivory.” As defined by Dethier et al. (1960), a deterrent is “A chemical which inhibits feeding or oviposition when present in a place where insects would, in its absence, feed or oviposit.” As stated by Stout (2013), resistance results from plant traits that limit injury to the plant. In this way, CaOx crystals can play a role in the resistance of plants against herbivores. The question that remains to be answered is the extent and effectiveness of this protection, especially if we consider that this is not an induced defense and, above all, one with which herbivorous insects have probably lived together since the beginning of their evolutionary history.
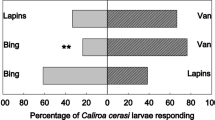
Regarding herbivore deterrence, we must consider that CaOx crystals can exclusively exert some effect on insect feeding preference if alternative food sources are available and, obviously, insects can move to reach them. For insects with reduced mobility, such as lepidopteran larvae, the choice between alternative food sources is not simple and does not always depend on the decision of the individual. In most cases, these larvae feed at the site or on the plant where the adults laid the eggs. As far as we know, lepidopteran adults cannot sense the presence of CaOx crystals inside plant cells. Therefore, if CaOx crystals act to protect plants against insects, this appears to be limited to a deterrent effect and depends on the stage of the life cycle and habit of the insects. Studying herbivory in Prunus avium cultivars, Peschiutta et al. (2020) showed that those with greater numbers of CaOx crystals in their leaves were the most preferred by Caliroa cerasi (Hymenoptera: Tenthredinidae). These authors considered these findings as evidence that CaOx crystals constitute an inducible defense in this plant species. On the other hand, we can consider these results as evidence of the absence of effects of these CaOx crystals on these herbivores, since a plant response of increasing the number of crystals in the face of the herbivory is very unlikely. It is important, in this case, to point out that the authors did not consider factors such as transpiration rate and the leaf microenvironment, as well as soil composition, which decidedly influence the quantity of crystals.
Soluble oxalate seems to constitute a more effective resistance trait against insect herbivory than CaOx crystals. As in vertebrates, when free oxalic acid is consumed by invertebrate herbivores, it can form insoluble calcium oxalate and lead to nephrolithiasis (kidney stones) as pointed by Hirata et al. (2012). These authors developed a nephrolithiasis model using Drosophila melanogaster, which develops CaOx stones upon dietary oxalate supplementation. Furthermore, oxalic acid was found to be a growth inhibitor of Helicoverpa armigera (Lepidoptera, Noctuidae). According to Yoshida et al. (1995), the inhibition of larval growth was not caused by antifeedant effects of oxalic acid but more likely by an antibiotic effect. Also, soluble oxalate seems to protect plants from herbivory by sucking insects, as demonstrated by Yoshihara et al. (1980) using the brown planthopper, Nilaparvata lugens, as a model.
Final remarks
In conclusion, CaOx crystals only provide effective protection against herbivory in very particular cases, such as inducing feeding preference in herbivores (mainly in mammals) due to itching and painful irritation. Obviously, such protection is dependent on the existence of an alternative food source. These crystals should not be considered effective against herbivores without the proper support of experimental evidence. Obviously, because of the absence of energetic or nutritional value, the presence of these crystals in plants is not advantageous for herbivores. We must also consider that CaOx crystals may be sufficient to have some detrimental effects on insect fitness by damaging their mouthparts. Indeed, a new plant resistance character can be effective in reducing herbivore attack, but this resistance is usually overcome by herbivores in a cycle of evolutionary interactions that, as stated by Rausher (2001), can explain much of Earth’s biological diversity. Therefore, it can hardly be expected that a physical and constitutive defense, such as CaOx crystals, would not have not been superseded in millions of years of evolution of highly adaptable organisms with short life cycles, such as insects.
Data availability
Not applicable.
Code availability
Not applicable.
References
Arditti J, Rodriguez E (1982) Dieffenbachia: uses, abuses and toxic constituents: a review. J Ethnopharmacol 5:293–302. https://doi.org/10.1016/0378-8741(82)90015-0
Bauer P, Elbaum R, Weiss IM (2011) Calcium and silicon mineralization in land plants: transport, structure and function. Plant Sci 180:746–756. https://doi.org/10.1016/j.plantsci.2011.01.019
Bernays EA, Chapman R (1987) The evolution of deterrent responses in plant-feeding insects. In: Chapman RF, Bernays EA, Stoffolano JG (eds) Perspectives in chemoreception and behavior. Springer-Verlag, New York, pp 159–173. https://doi.org/10.1007/978-1-4612-4644-2_10
Cortinovis C, Caloni F (2013) Epidemiology of intoxication of domestic animals by plants in Europe. Vet J 197:163–168. https://doi.org/10.1016/j.tvjl.2013.03.007
Dethier VG, Browne LB, Smith CN (1960) The designation of chemical in terms of the responses they ilicit from insects. J Econ Entomol 53:134–136. https://doi.org/10.1093/jee/53.1.134
Doege SJ (2003) The role of natural calcium oxalate crystals in plant defense against chewing insects. Inquiry: The University of Arkansas Undergraduate Research Journal, 4: Article 15. Available at: http://scholarworks.uark.edu/inquiry/vol4/iss1/15
Franceschi VR, Horner HT (1980) Calcium oxalate crystals in plants. Bot Rev 46:361–427. https://doi.org/10.1007/BF02860532
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Ann Rev Plant Biol 56:41–71. https://doi.org/10.1146/annurev.arplant.56.032604.144106
Gardner DG (1994) Injury to the oral mucous membranes caused by the common houseplant, Dieffenbachia: a review. Or Surg or Med or Pathol 78:631–633. https://doi.org/10.1016/0030-4220(94)90177-5
Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156:145–169. https://doi.org/10.1046/j.1469-8137.2002.00519.x
Herbert DA (1924) Stinging crystals in plants. Science 60:204–205. https://doi.org/10.1126/science.60.1548.204-a
Hirata T, Cabrero P, Berkholz DS, Bondeson DP, Ritman EL, Thompson JR, Dow JAT, Romero MF (2012) In vivo Drosophila genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiology 303:F1555–F1562. https://doi.org/10.1152/ajprenal.00074.2012
Hudgins JW, Krekling T, Franceschi VR (2003) Distribution of calcium oxalate crystals in the secondary phloem of conifers: a constitutive defense mechanism? New Phytol 159:677–690. https://doi.org/10.1046/j.1469-8137.2003.00839.x
Janzen DH (1980) When is it coevolution? Evolution 34:611–612
Karabourniotis G, Horner HT, Bresta P, Nikolopoulos D, Liakopoulos G (2020) New insights on the functions of carbon-calcium-inclusions in plants. New Phytol 228:845–854. https://doi.org/10.1111/nph.16763
Konno K, Inoue TA, Nakamura M (2014) Synergistic defensive function of raphides and protease through the needle effect. PLoS ONE 9(3):e91341. https://doi.org/10.1371/journal.pone.0091341
Korth KL, Doege SJ, Park S-H, Goggin FL, Wang Q, Gomez SK, Liu G, Jia L, Nakata PA (2006) Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol 141:188–195. https://doi.org/10.1104/pp.106.076737
Lawrence SD, Novak NG (2006) Expression of poplar chitinase in tomato leads to inhibition of development in Colorado potato beetle. Biotechnol Lett 28:593–599. https://doi.org/10.1007/s10529-006-0022-7
Le Coz CJ, Ducombs G (2006) Plants and plant products. In: Frosch PJ, Menne T, Leppoittevin JP (eds) Contact dermatitis, 4th edn. Springer, Berlin, pp 751–800
Leeuwenhoek A (1675) Microscopical observations. Philos Trans R Soc Lond 10:380–385
Lopresti EF, Grof-Tisza P, Robinson M, Godfrey J (2018) Entrapped sand as a plant defence: effects on herbivore performance and preference. Ecol Entomol 43:154–161. https://doi.org/10.1111/een.12483
Lopresti EF, Karban R (2016) Chewing sandpaper: grit, plant apparency, and plant defense in sand-entrapping plants. Ecology 97:826–833. https://doi.org/10.1890/15-1696.1
Massey FP, Hartley SE (2009) Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. J Anim Ecol 78:281–291. https://doi.org/10.1111/j.1365-2656.2008.01472.x
Mello MO, Silva-Filho MC (2002) Plant-insect interactions: an evolutionary arms race between two distinct defense mechanisms. Braz J Plant Physiol 14:71–81. https://doi.org/10.1590/S1677-04202002000200001
Mohan S, Ma PWK, Pechan T, Bassford ER, Williams WP, Luthe DS (2006) Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J Insect Physiol 52:21–28. https://doi.org/10.1016/j.jinsphys.2005.08.011
Nagaoka S, Katayama H, Fujibayashi Y, Sugimura Y (2010) Calcium oxalate crystals in mulberry leaves: no negative effect on feeding the silkworm, Bombyx mori. J Insect Biotech Sericol 79:71–74. https://doi.org/10.11416/jibs.79.2_071
Nakata PA (2003) Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci 164:901–909. https://doi.org/10.1016/S0168-9452(03)00120-1
Nelson LS, Shih RD, Balick MJ (2007) Handbook of poisonous and injurious plants. Springer-Verlag, Berlin
Paiva EAS (2019) Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol 223:1707–1711. https://doi.org/10.1111/nph.15912
Park S, Doege SJ, Nakata PA, Korth KL (2009) Medicago truncatula-derived calcium oxalate crystals have a negative impact on chewing insect performance via their physical properties. Entomol Exp Appl 131:208–215. https://doi.org/10.1111/j.1570-7458.2009.00846.x
Pechan T, Cohen A, Williams WP, Luthe DS (2002) Insect feeding mobilizes a unique plant defence protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Acad Sci USA 99:13319–13323. https://doi.org/10.1073/pnas.202224899
Perera CO, Hallett IC, Nguyen TT, Charles JC (1990) Calcium oxalate crystals: the irritant factor in kiwifruit. J Food Sci 55:1066–1069. https://doi.org/10.1111/j.1365-2621.1990.tb01599.x
Peschiutta ML, Bucci SJ, Goldstein G, Scholz FG (2020) Leaf herbivory and calcium oxalate crystal production in Prunus avium. Arthropod-Plant Inte 14:727–732. https://doi.org/10.1007/s11829-020-09781-6
Rahman MM, Abdullah RB, Wan Khadijah WE (2013) A review of oxalate poisoning in domestic animals: tolerance and performance aspects. J Anim Physiol an Nutr 97:605–614. https://doi.org/10.1111/j.1439-0396.2012.01309.x
Rausher MD (2001) Co-evolution and plant resistance to natural enemies. Nature 411:857–864. https://doi.org/10.1038/35081193
Ruiz N, Ward D, Saltz D (2002) Calcium oxalate crystals in leaves of Pancratium sickenbergeri: constitutive or induced defence? Funct Ecol 16:99–105. https://doi.org/10.1046/j.0269-8463.2001.00594.x
Sanz PMD, Reig RMD (1992) Clinical and pathological findings in fatal plant oxalosis - a review. Am J Foren Med Path 13:342–345
Showler AT (2002) Effects of kaolin-based particle film application on boll weevil (Coleoptera: Curculionidae) injury to cotton. J Econ Entomol 95:754–762. https://doi.org/10.1603/0022-0493-95.4.754
Stout MJ (2013) Re-evaluating the conceptual framework for applied research on host-plant resistance. Insect Sci 20:263–272. https://doi.org/10.1111/1744-7917.12011
Terra WR (2001) The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem 47:47–51. https://doi.org/10.1002/arch.1036
Wang P, Granados RR (2001) Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch Insect Biochem 47:110–118. https://doi.org/10.1002/arch.1041
War AR, Taggar GK, Hussain B, Taggar MS, Nair RM, Sharma HC (2018) Plant defence against herbivory and insect adaptations. AoB PLANTS 10:ply037; https://doi.org/10.1093/aobpla/ply037
Ward D, Spiegel M, Saltz D (1997) Gazelle herbivory and interpopulation differences in calcium oxalate content of leaves of a desert lily. J Chem Ecol 2:333–346. https://doi.org/10.1023/B:JOEC.0000006363.34360.9d
Yoshida M, Cowgill SE, Wightman JA (1995) Mechanism of resistance to Helicoverpa armigera (Lepidoptera, Noctuidae) in chickpea: role of oxalic acid in leaf exudate as an antibiotic factor. J Econ Entomol 88:1783–1786. https://doi.org/10.1093/jee/88.6.1783
Yoshihara T, Sogawa K, Pathak MD, Juliano BO, Sakamura S (1980) Oxalic acid as a sucking inhibitor of the brown planthopper in rice (Delphacidae, Homoptera). Entomol Exp Appl 27:149–155. https://doi.org/10.1111/j.1570-7458.1980.tb02959.x
Acknowledgements
I thank Dr. Clemens Schlindwein, Dr. Denise M. T. Oliveira, and Dr. Alberto Teixido for their helpful comments and suggestions. I am also indebted to Paula Roig, Dr. Harry Horner, and an anonymous reviewer for improving the article with valuable suggestions and comments. This work is dedicated to the memory of my greatest master, Olimpio Vieira de Paiva, whom I have always taken as a model.
Funding
This study was funded in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, process 305638/2018‒1).
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interest.
Additional information
Communicated by: Lukasz Stepien and Paula Roig-Boixeda
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paiva, É.A.S. Do calcium oxalate crystals protect against herbivory?. Sci Nat 108, 24 (2021). https://doi.org/10.1007/s00114-021-01735-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-021-01735-z