Abstract
Precopulatory mate-guarding behavior is a common strategy that maximizes male reproductive success when female receptivity to copulation is low. This behavior has been demonstrated in vertebrates, aquatic crustaceans, terrestrial isopods, and some species of insects, but there is very little available information about hymenopteran insects. A few studies have clarified the factor that determines the outcome of a contest between a guarding male and an invader male. We investigated the male–male contest and mating behavior of a saproxylic parasitoid wasp, Ibalia japonica (Hymenoptera: Cynipoidea: Ibaliidae) using field observations in Japan. These observations indicated that I. japonica males show precopulatory mate-guarding behavior and that four types of male–male contests occur on the Magnolia liliiflora (Magnoliales: Magnoliaceae) tree that virgin females emerge from. We show that the arrival order of I. japonica males that found the future emergence point of a female was key factor that allowed males to secure virgin females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mate-securing is critical for sexual reproduction in animals. Precopulatory mate-guarding behavior is a common strategy used by males to maximize reproductive success when females show low receptivity to copulation (Jormalainen 1998), and the strategy has been demonstrated in both vertebrates (Low 2006; Schubert et al. 2009; Brattli et al. 2018) and invertebrates (Jormalainen 1998; Wada et al. 1999; Iltis et al. 2017). In some species, male–male contests for a female occur between a guarding male and a newly arrived male. Male–male contests and precopulatory mate-guarding behavior by invertebrates are well documented in aquatic crustaceans (Wada et al. 1997, 1999; Jormalainen 1998; Murai and Backwell 2005), and some studies on insects, such as Odonata (Miller et al. 1984), Coleoptera (Arakaki et al. 2004; Chaudhary et al. 2017), and Lepidoptera (Bennett et al. 2012; Jarrige et al. 2016), are available. However, only a few studies have focused on mate-guarding behavior by hymenopteran insects (Longair 2004; Beani et al. 2014), and there is no available information about hymenopteran parasitoids, although many parasitoid species are known to have male–male contests to access virgin females (Godfray 1994). Furthermore, a few studies have demonstrated that there are several factors that can determine the outcome of contests between a guarding male and an invader male in mate-guarding contests, such as body size or the size of specific body parts (Brockerhoff and McLay 2005; Walker and Holwell 2018).
We investigated the precopulatory male–male contests and mating behavior of newly emerged Ibalia japonica (Hymenoptera: Cynipoidea: Ibaliidae), which is a primary solitary, koinobiont, endoparasitoid of the woodwasp Tremex apicalis (Hymenoptera: Siricidae) (Nordlander and Liu 1994; Choi et al. 2013; Kuramitsu et al. 2016; Kuramitsu et al. 2019). Ibalia spp. larvae parasitize eggs and larvae of wood-boring woodwasps, which are forest pests. Adult Ibalia spp. emerge from the host-infested wood after chewing an exit hole through the wood and bark (Nordlander and Liu 1994). Ibalia drewseni adults are known to mate after emergence from host-infested wood (Spradbery 1974), but the mating process for ibaliid species is largely unknown. Females of most parasitoid (Godfray 1994) and Cynipoidea species (Abe 1991) are known to mate only once in their life.
To clarify the determinants of the male–male contest, we investigated the arrival order of males to the female emergence point and body size effects on male–male contest outcomes. The principal aim of this study was (1) to determine whether the hymenopteran parasitoid, I. japonica, shows precopulatory mate-guarding behavior and (2) to clarify the determinant that is responsible for the male–male contest outcomes.
Materials and methods
Study site and study organisms
Field observations of I. japonica were carried out in the Tsukuba Experimental Forest (36° 07′ 10″ N; 140° 05′ 50″ E [DMS], ca. 25 m a.s.l.), University of Tsukuba, Ibaraki Prefecture, Japan. We identified one tree of Magnolia liliiflora (Magnoliales: Magnoliaceae) that had been infested by T. apicalis in 2017. This tree was approximately 12 m in height and 44 cm in diameter at breast height. To observe emergence and mating behavior of I. japonica, the infested parts of this tree were removed with a chainsaw on October 4, 2017 and the wood kept outside until May 2018. Behavior of males and females of I. japonica that emerged from the bolts of the infested wood was observed. In this study, we did not individually identify male wasps in the field. Therefore, we cannot deny the possibility that our data included some pseudoreplication.
Observation 1: descriptions of the I. japonica mating process and mate–mate contests
Field observation 1 lasted for 5 days from May 8 to May 15, 2018. We observed the woodwasp-infested M. liliiflora between 10:00 and 16:00 on each day. The behavior of both I. japonica males and females was recorded when we found newly emerging females on the wood. When a male mounts a female, we observed whether the female resists to copulation or not. Seven of the 34 female emergences were recorded by a video camera (Tough TG-5, Olympus Corporation, Tokyo, Japan or iPhone SE, Apple Inc., Cupertino, USA).
Observation 2: effects of arrival sequence and/or male body size on male–male contests
Observation 1 indicated that I. japonica males showed male–male contest behavior around the female emergence site (see below). To clarify the contributing factors that influence the outcome of their contests, we evaluated the effects of (1) the arrival sequence of the males at the potential female emergence point and (2) male body size.
The relationship between the order of arrival at the potential female emergence point and the outcome of the male–male contests was determined from videos that were taken during observation 1. Contest types and the outcome of the contests, such as the guarding male winning against an invader, were recorded. The competition was recorded as a draw when both the guarding male and the invader male left the female emergence point after a contest. Body size effects on the outcome of a male–male contests were determined by field observations that were carried out between May 15 and 20, 2018. When a male–male contest around a female emerging point was identified, we caught the losing male after their contest and measured its body length and the tibia length of a right foreleg with digital venire calipers (DN-150, Niigata Seiki Co., Ltd., Sanjo, Japan). The winning male was also caught after mating, and its body was measured in a similar way to the losing male. Losing males were released after mating of the female and the winning male. The winning male was also released after the measurement of its body size.
Finally, the successful mating rate of the first male (the male that found a newly emerging female first) was calculated based on information from observation 1.
Statistical analysis
All statistical analyses were performed using R v. 3.4.2 (R Core Team, 2015). Significance of winning percentage of wasps in each contest was analyzed using a binominal test.
Results
Observation 1: description of the mating process and the I. japonica male–male contests
We observed 34 emerging I. japonica females. A diagram of the normal mating sequence is shown in Fig. 1. A total of 94.1% mated immediately after emergence from the wood (Fig. 2; Online Resource 1). The male that stood by the female emergence point immediately mounted the female after her emergence. All of the guarding males (n = 32) touched females’ body using their antenna during female emergence. Before and during mating, the males stroked the female antenna using their own antenna (Online Resource 1). Copulation duration was 58.8 ± 18.4 s (mean ± SD, n = 7). During copulation, other males tried to interrupt the mating pairs 1.57 ± 1.51 times (mean ± SD, n = 7) per pair but no invading males obtain females. Female resistance to first copulation was never observed, but mated females were not receptive to further courtship after first mating. Two females emerged when no male was waiting near their emergence hole, and these females flew away from the wood.
Female emergence and mating behavior of Ibalia japonica. (mov 209433 kb)
Based on the video analysis, total 203 male–male contests performed by a cumulative total of 176 male–male pairs were observed on the wood in advance of mating. When another male approached the emergence spot, a contest between the guarding male and invader male occurred. The male–male contests can be classified to four types, i.e., repelling (running at the invader with wings flapping and sounds; Online Resource 2), mounting (Fig. 3a; Online Resource 3), biting (biting invader’s antenna; Fig. 3b; Online Resource 4), and foreleg spreading (Fig. 3c, d; Online Resource 5). In many cases, the guarding male raised his head and looked around the emergence spot, while the invader male approached the emergence spot with antennal searching on the wood. Therefore, it appears that the guarding male began to attack an invader before the invader noticed the guarding male. The guarding males guarded a hole for 9.14 ± 5.33 min (mean ± SD, n = 7) before a female came out. No females emerged from hole during male–male contests.
Male-male contests by Ibalia japonica males. Contest type: repelling. (mov 47919 kb)
Male-male contests by Ibalia japonica males. Contest type: mounting. (mov 85661 kb)
Male-male contests by Ibalia japonica males. Contest type: biting. (mov 34444 kb)
Male-male contests by Ibalia japonica males. Contest type: foreleg spreading. (mov 44632 kb)
Observation 2: effects of the order in which males found the female and body size on male–male contests
A transition diagram for 176 male–male pairs, based on 203 male–male contests, was analyzed. Repelling behavior was a dominant contest type (65.3%) followed by mounting (17.0%), foreleg spreading (15.9%), and biting (1.7%), which was rarely observed. A total of 84.6% of the couple interactions were settled by a single contest type, and the others (15.4%) were settled by two contest types. No pairs performed three or more contest types in the same male–male contest (Fig. 4).
The percentage of wins by a guarding male was 95.0% in total and 82.1–100% for each contest type (Fig. 5). Guarding males showed a significantly higher winning percentage (P < 0.01, binomial test, n = 202) in a contest against an invader (Fig. 5).
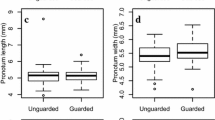
Ibalia japonica males showed small variations in body size, i.e., body length was 18.7 ± 1.3 mm (mean ± SD, n = 36) and foreleg length was 2.7 ± 0.2 mm (mean ± SD, n = 36). Guarding males won significantly more contests (binomial test, P < 0.01) against both smaller invaders (81.8%, n = 22) and larger invaders (88.5%, n = 26). The percentage of females that mated with a first arrival male (87.5%) was significantly higher (P < 0.001, binomial test, n = 32) than with second or later males (12.5%).
Discussion
Ibalia japonica males awaited female emergence and mated immediately with virgin females (Fig. 2; Online Resource 1). Along with many other parasitoid species (Ruther 2013), Ibalia japonica may also release a female-derived courtship pheromone, which is detected by the males and allows them to find and mate with newly emerging females. Rhyssini parasitoid males (Hymenoptera: Ichneumonidae), which are another woodwasp parasitoids, also aggregate and wait around the female emergence points (Quicke 2014). This suggests that waiting for female emergence on the wood surface is a common strategy for saproxylic parasitoid males. I. japonica males stroked the female antenna during mating (Online Resource 1). The behavior seems to be a signal for the female to accept the male, and this occurs amongst other Cynipoidea (Isidoro et al. 1999) and Vespidae (Romani et al. 2005). No females emerged from a hole during male–male contests. Female wasps may detect male movement or sound in order to emerge safety.
There were contests between a guarding male and invaders (176 male–male pairs showed 203 contests). Winners of the contest stayed at the female emergence point and the losers left. We conclude that the purpose of the contests is to secure a virgin female. Contest styles were classified into four types. A few individuals used two contest styles although there was no characteristic combination (Fig. 4). Some parasitoid Chalcidoidea (Hymenoptera) species are also known to perform male–male contests to secure newly emerged females and they also use multiple contest styles (Wilson 1961; van den Assem et al. 1980). For example, Wilson (1961) showed that once an Asolcus basali (Hymenoptera: Scelionidae) male takes possession of a host egg mass from which female wasps will emerge, the male drives away all other males by attacking or biting. Other chalcidoid parasitoids also show mounting, repellent, or biting behavior during male–male contests (van den Assem et al. 1980; Godfray 1994). Taken together, the data suggest that these three behaviors are positively selected in several parasitoid taxa. In contrast, foreleg spreading has never been observed in other species. Therefore, that behavior might be unique to I. japonica.
Most guarding males won the contest regardless of contest type (Fig. 5) and showed a high winning percentage even when an invader was bigger than a guarding male. In many cases, guarding males launched the attack on invaders without being noticed. These data clearly indicate that guarding males make a preemptive attack against invaders, and it is often sufficient to settle the contests. Body size and/or the size of specific body parts are major factors that affect the outcome of male–male fights in many animal taxa (Christy 1983; Longair 2004; Brockerhoff and McLay 2005; Walker and Holwell 2018). However, body size variation in I. japonica males was very small, which was similar to other koinobiont parasitoids because koinobiont parasitoids are less susceptible to host body size than idiobiont parasitoids (Godfray 1994; Harvey et al. 2014). Therefore, differences in body size are not an important factor in I. japonica male–male contests.
First arrival males can successfully fight off other males and mate with the female in high percentages. The results suggest that I. japonica shows precopulatory mate-guarding behavior. This is to the best of our knowledge the first report of precopulatory mate-guarding behavior performed by a hymenopteran parasitoid. Our observations indicate that the ability of male I. japonica to find newly emerging females is an important factor that enhances their reproductive success. Previous studies have demonstrated that the sensitivity of individual males to sex pheromones varied, even within the same populations, and this led to sexual selection by females in lepidopteran insects (Groot et al. 2014). Further studies are needed in order to clarify the main factor that determines the ability of I. japonica males to find newly emerging females in the field.
References
Abe Y (1991) Notices on courtship behaviour and copulation of Synergus japonicus Walker (Hym., Cynipidae). J Appl Entomol 111:478–483. https://doi.org/10.1111/j.1439-0418.1991.tb00350.x
Arakaki N, Kishita M, Nagayama A, Fukaya M, Yasui H, Akino T, Wakamura S (2004) Precopulatory mate guarding by the male green chafer, Anomala albopilosa sakishimana Nomura (Coleoptera: Scarabaeidae). Appl Entomol Zool 39:455–462. https://doi.org/10.1303/aez.2004.455
Beani L, Dessì-Fulgheri F, Cappa F, Toth A (2014) The trap of sex in social insects: from the female to the male perspective. Neurosci Biobehav Rev 46:519–533. https://doi.org/10.1016/j.neubiorev.2014.09.014
Bennett VJ, Smith WP, Betts MG (2012) Evidence for mate guarding behavior in the Taylor’s checkerspot butterfly. J Insect Behav 25:183–196. https://doi.org/10.1007/s10905-011-9289-1
Brattli MB, Egeland TB, Nordeide JT, Folstad I (2018) Spawning behavior of Arctic charr ( Salvelinus alpinus ): spawning synchrony, vibrational communication, and mate guarding. Ecol Evol 8:8076–8087. https://doi.org/10.1002/ece3.4277
Brockerhoff AM, McLay CL (2005) Comparative analysis of the mating strategies in grapsid crabs with special references to the intertidal crabs Cyclograpsus lavauxi and Helice crassa (Decapoda: Grapsidae) from New Zealand. J Crustac Biol 25:507–520. https://doi.org/10.1651/C-2548
Chaudhary DD, Mishra G, Omkar G (2017) Strategic mate-guarding behaviour in ladybirds. Ethology 123:376–385. https://doi.org/10.1111/eth.12606
Choi WY, Lee JW, Suh KI (2013) Taxonomic review of the family Ibaliidae (Cynipoidea: Hymenoptera) from Korea. Entomol Res 43:135–141. https://doi.org/10.1111/1748-5967.12015
Christy JH (1983) Female choice in the resource-defense mating system of the sand fiddler crab, Uca pugilator. Behav Ecol Sociobiol 12:169–180. https://doi.org/10.1007/BF00343209
Godfray HCJ (1994) Parasitoids behavioral and evolutionary ecology. Princeton University Press, Princeton
Groot AT, Schöfl G, Inglis O, Donnerhacke S, Classen A, Schmalz A, Santangelo RG, Emerson J, Gould F, Schal C, Heckel DG (2014) Within-population variability in a moth sex pheromone blend: genetic basis and behavioural consequences. Proc R Soc B Biol Sci 281:20133054. https://doi.org/10.1098/rspb.2013.3054
Harvey JA, Harvey IF, Thompson DJ (2014) Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology 75:1420–1428. https://doi.org/10.2307/1937465
Iltis C, Dechaume-Moncharmont FX, Galipaud M, Moreau J, Bollache L, Louâpre P (2017) The curse of being single: both male and female Gammarus pulex benefit energetically from precopulatory mate guarding. Anim Behav 130:67–72. https://doi.org/10.1016/j.anbehav.2017.06.013
Isidoro N, Bin F, Romani R, Pujade-Villar J, Ros-Farré P (1999) Diversity and function of male antennal glands in cynipoidea (Hymenoptera). Zool Scr 28:165–174. https://doi.org/10.1046/j.1463-6409.1999.00013.x
Jarrige A, Kassis A, Schmoll T, Goubault M (2016) Recently mated males of a lek-mating insect intensify precopulatory mate guarding under male competition. Anim Behav 117:21–34. https://doi.org/10.1016/j.anbehav.2016.04.012
Jormalainen V (1998) Precopulatory mate guarding in crustaceans: male competitive strategy and intersexual conflict. Q Rev Biol 73:275–304
Kuramitsu K, Kosaki A, Ishihara T, Yamada H, Watanabe K (2016) Infestation of the woodwasp Tremex apicalis Matsumura (Hymenoptera, Siricidae) on the large-leaf dogwood Swida macrophylla (Wall.) with biological notes on its parasitoid wasps. J Hymenopt Res 52:71–79. https://doi.org/10.3897/jhr.52.10060
Kuramitsu K, Ishihara T, Sugita A, Yooboon T, Lustig B, Matsumori Y, Yamada H, Kinoshita N (2019) The attraction of Tremex apicalis (Hymenoptera, Siricidae, Tremecinae) and its parasitoid Ibalia japonica (Hymenoptera, Ibaliidae) to the fungus Cerrena unicolor. J Hymenopt Res 68:37–48. https://doi.org/10.3897/jhr.68.30372
Longair RW (2004) Tusked males, male dimorphism and nesting behavior in a subsocial afrotropical wasp, Synagris cornuta, and weapons and dimorphism in the genus (Hymenoptera: Vespidae: Eumeninae). J Kansas Entomol Soc 77:528–557. https://doi.org/10.2317/E-38.1
Low M (2006) The energetic cost of mate guarding is correlated with territorial intrusions in the New Zealand stitchbird. Behav Ecol 17:270–276. https://doi.org/10.1093/beheco/arj025
Miller AK, Miller PL, Siva-Jothy MT (1984) Pre-copulatory guarding and other aspects of reproductive behaviour in Sympetrum depressiusculum (Selys) at rice fields in southern France (Anisoptera: Libellulidae). Odonatologica 13:407–414
Murai M, Backwell PRY (2005) More signalling for earlier mating: conspicuous male claw waving in the fiddler crab, Uca perplexa. Anim Behav 70:1093–1097. https://doi.org/10.1016/j.anbehav.2005.02.019
Nordlander G, Liu Z (1994) Review of the family Ibaliidae (Hymenoptera: Cynipoidea) with keys to genera and species of the world. Insect Syst Evol 25:377–392
Quicke DLJ (2014) Phylogeny and systematics of the Ichneumonidae. In: The braconid and Ichneumonid parasitoid wasps: biology, systematics, evolution and ecology. John Wiley & Sons, Chichester, pp 341–449
Romani R, Ishidoro N, Riolo P, Bin F, Fortunato A, Turillazzi S, Beani L (2005) A new role for antennation in paper wasps (Hymenoptera, Vespidae): antennal courtship and sex dimorphic glands in antennomeres. Insect Soc 52:96–102. https://doi.org/10.1007/s00040-004-0780-y
Ruther J (2013) Novel insights into pheromone-mediated communication in parasitic hymenopterans. In: Wajnberg E, Colazza S (eds) Chemical ecology of insect parasitoids. Wiley-Blackwell, Chichester, pp 112–144. https://doi.org/10.1002/9781118409589.ch6
Schubert M, Schradin C, Rödel HG, Pillay N, Ribble DO (2009) Male mate guarding in a socially monogamous mammal, the round-eared sengi: on costs and trade-offs. Behav Ecol Sociobiol 64:257–264. https://doi.org/10.1007/s00265-009-0842-2
Spradbery J (1974) The responses of Ibalia species (Hymenoptera: Ibaliidae) to the fungal symbionts of siricid woodwasp host. J Entomol Ser A, Gen Entomol 48:217–222. https://doi.org/10.1111/j.1365-3032.1974.tb00058.x
van den Assem J, Gijswijt MJ, Nübel BK (1980) Observations on courtship - and mating strategies in a few species of parasitic wasps (Chalcidoidea). Netherlands J Zool 30:208–227. https://doi.org/10.1163/002829679X00386
Wada S, Ashidate M, Goshima S (1997) Observations on the reproductive behavior of the spiny king crab Paralithodes brevipes (Anomura: Lithodidae). Crustac Res 26:56–61. https://doi.org/10.18353/crustacea.26.0_56
Wada S, Tanaka K, Goshima S (1999) Precopulatory mate guarding in the hermit crab Pagurus middendorffii (Brandt) (Decapoda: Paguridae): effects of population parameters on male guarding duration. J Exp Mar Biol Ecol 239:289–298. https://doi.org/10.1016/S0022-0981(99)00045-3
Walker LA, Holwell GI (2018) The role of exaggerated male chelicerae in male–male contests in New Zealand sheet-web spiders. Anim Behav 139:29–36. https://doi.org/10.1016/j.anbehav.2018.02.020
Wilson F (1961) Adult reproductive behaviour in Asolcus basalis (Hymenoptera: Scelionidae). Aust J Zool 9:739–751
Acknowledgments
We are grateful to the members of staff at the Tsukuba Experimental Forest, University of Tsukuba, Japan for their permission to conduct this study on their land. We are also grateful to Dr. Takuya Uehara (National Agriculture and Food Research Organization, Japan) and Mr. Ryousuke Matsushima (University of Tsukuba, Japan) for providing information on the mating behavior of insects. We are grateful to Mr. Teruhito Ishihara (The University of Melbourne) for helping to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Lars Koerner
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kuramitsu, K., Yooboon, T., Tomatsuri, M. et al. First come, first served: precopulatory mate-guarding behavior and male–male contests by a hymenopteran saproxylic parasitoid. Sci Nat 106, 23 (2019). https://doi.org/10.1007/s00114-019-1616-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-019-1616-y