Abstract
Predation is an unavoidable and dangerous fact in the lifetime of prey animals and some sign of the proximity of a predator may be enough to trigger a response in the prey. We investigated whether different degrees of predation risk by red foxes (Vulpes vulpes) evoke behavioural and physiological stress responses in wood mice (Apodemus sylvaticus). We examined the variation in mice responses due to individual factors (sex and reproductive status) and related them to the concentration of the volatile compounds from fox faeces over time. In our experiment, we introduced predation cues into four plots, each subjected to a different concentration treatment (0, 10, 50 and 100% concentration of fresh faeces of red fox), based on the following outline: initial odourless phase 0, phase1 in which predation treatment was renewed daily, and phase 2 in which we renewed the treatment only on the first day. Wood mice were live trapped during all three phases and the physiological response was measured non-invasively by analysing faecal corticosterone metabolites (FCM) in freshly collected faeces. Data were analysed by Generalized Linear Mixed Models. Overall, males were trapped less often than females, and reproductively active individuals from both sexes avoided traps more than non-reproductively active individuals, especially in medium- and high- concentration plots. Variations in FCM concentrations were explained by plot, the interaction between plot and treatment phase, and the interaction between the treatment phase and the reproductive status. During phase 1, we detected a significant rise in FCM levels that increased with predator faecal odour concentration. Additionally, reproductively active individuals showed a strong physiological response during both phases 1 and 2 in all plots, except the control plot. Our results indicated that wood mice are able to discriminate different degrees of predation risk, which allows them to trigger gradual changes in their behavioural and physiological stress responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life is not easy for prey species, which daily face a wide range of threats and dangers. Among threatening factors, predation poses a serious risk for small mammals acting directly on prey ecology by reducing local population density, and also indirectly by shaping prey behaviour, physiology and morphology (Lima and Dill 1990; Apfelbach et al. 2005; Yin et al. 2011; Hegab et al. 2014b; Sánchez-González et al. 2017). Successful predation usually involves at least five steps: detection, identification, approach, subjugation and consumption (Endler 1986; Sherbrooke 2008; Schmitz et al. 2013). From a prey’s perspective, avoiding detection by a predator before a possible encounter is the most important step to avoid being eaten. Thus, prey species have developed different mechanisms to prevent primary detection, such as behavioural changes or morphological defences (Lima 1998; Huffard 2006), but also secondary mechanisms when a predator is nearby and has already detected its prey (e.g. escape behaviours or distasteful flavours) (Huffard 2006; Luttbeg and Trussell 2013).
In mammals, auditory, visual and chemosensory signals play a particularly important role in most intraspecific or interspecific interactions (Gorman 1990), with scent-marking as one of the main means of exchanging information at night (Eisenberg and Eisenberg 1981; Macdonald 1985; Kats and Dill 1998; Wyatt 2003). Further, it has been widely demonstrated that carnivore-derived scents from urine (Jorgenson et al. 1978; Barja and de Miguel 2004), faeces (Gese and Ruff 1997; Hutchings and White 2000; Barja et al. 2011; Piñeiro and Barja 2012; Barja and List 2014) and odorous glandular secretions (Albone and Perry 1976; Asa et al. 1985) also induce a broad variety of defensive responses in small mammals (Dickman and Doncaster 1984; Jędrzejewski et al. 1993; Yin et al. 2011; Hegab et al. 2014b; Navarro-Castilla and Barja 2014a; Tortosa et al. 2015). Thus, chemical cues seem to provide accurate information about when and where the predator may be a threat, allowing prey to adapt their responses (Kusch et al. 2004; Mirza et al. 2006).
According to Apfelbach et al. (2005), behavioural responses seem to be the most common antipredatory defence and they mainly include modifications in prey daily activities, such as foraging (Verplancke et al. 2010), reproduction (Creel et al. 2007) and use of space (Creel et al. 2005). For instance, besides reducing the time invested on foraging, the presence of a possible predator also changes rodents’ foraging behaviour spatially and/or temporally (Fenn and Macdonald 1995; Díaz et al. 2005; Navarro-Castilla et al. 2017a). However, under certain circumstances, prey animals will also display a physiological response to predation pressure (Masini et al. 2005; Hegab et al. 2014b). Thereby, when an animal is subjected to the presence of a predator, the endocrine stress response enhances the activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, stimulating the secretion of glucocorticoids (cortisol or corticosterone depending on the species) (Sapolsky et al. 2000; Melmed and Kleinberg 2003). The increase of glucocorticoids has different effects depending on the duration of exposure to the stressor. Thereby, short-term stress has been related to an adaptive response for improving the prey’s fitness (Wingfield et al. 1998), whereas long-term glucocorticoid secretion may cause important pathologies (e.g. reproductive failure, endocrine disruption, suppression of the immune system and/or gastrointestinal ulcerations) (Munck et al. 1984; Stewart 2003).
The behavioural and physiological changes in the prey’s life also involve some costs (Lima and Dill 1990); therefore, the prey should balance daily activities in relation to the risk of predation perceived in each moment (Lima and Bednekoff 1999; Dielenberg and McGregor 2001; Kavaliers and Choleris 2001). Recent experimental studies have confirmed the threat-sensitive predator avoidance hypothesis described by Helfman (1989). According to this hypothesis, preys are able to use some predator cue characteristics (e.g. differences between fresh/old faeces (Hegab et al. 2014a) to evaluate the situation.
The aim of the present study was to test whether wood mice (Apodemus sylvaticus) are able to modulate their behavioural and physiological stress responses according to different degrees of predation risk by red fox (Vulpes vulpes). We examined the variation in both behavioural and physiological responses due to individual factors, such as sex and mice reproductive status, and related them to concentration and degradation of the volatile compounds from predator faeces over time. We predicted that wood mice would show avoidance behaviour as well as a physiological response, i.e. an activation of the HPA axis, in response to an increased concentration of predator faecal odour. In addition, we predicted a reduction of these responses with the degradation of volatile faecal compounds over time.
Material and methods
Study area
Field work was performed in Valdelatas (Madrid, Spain), a Mediterranean forest situated at an altitude of 650 m a.s.l. The study area is constituted predominantly by dense forests of holm oak (Quercus ilex ballota) and pine reforestations (Pinus pinea and Pinus pinaster). Scrubland vegetation was mostly composed by gum rock roses (Cistus ladanifer), umbel-flowered sun roses (Halimium umbellatum) and thyme (Thymus zygis). The selected study area presented a continuous suitable shrub matrix and it was divided into four plots with similar vegetation cover and height characteristics. During the experiment, climate was characterised by a mean rainfall of 0.2 mm and mean temperature was 9.3 °C (www.opendata.aemet.es). Climatic conditions were the same for the four plots.
Live-trapping and data collection
To minimise observer bias, blinded methods were used during the study. Each faecal sample collected was firstly labelled in the field by two researchers and later on, in the laboratory, it was newly encoded by a third researcher to protect any information regarding its origin. This way, physiological stress levels were analysed in each faecal sample without knowing the identity of the individual.
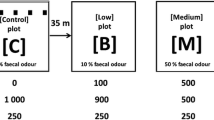
Live-trapping was performed between February and March 2014. The study area was divided into four plots 35 m apart from each other to avoid capturing the same mouse in two different plots. In each plot, we placed 20 Sherman® live traps shaping a 4 × 5 grid with 7 m of distance among them. All traps were placed under vegetation to buffer extreme environmental conditions and they were oriented against the slope in order to allow correct closing. Total trapping effort was 2400 traps per night (20 traps per grid × 4 plots × 15 nights × 2 trapping sessions).
The experiment was divided into three different phases: preliminary (phase 0), phase 1 and phase 2. Each phase took place during 5 consecutive days. During phase 0, no faecal odour was brought out in any plot to determine the basal behavioural and physiological responses of wood mice without red-fox scent. In phase 1, one plot was used as control with no experimentally added faecal odour and the other three plots were subjected to increasing concentrations of faecal odour: 10% (low-concentration plot), 50% (medium-concentration plot) and 100% (high-concentration plot) to simulate different degrees of predation risk. In this phase, 10 g of faecal material (see section Simulation of different degrees of predation risk by faecal odour) was placed outside each trap. Faecal material was renewed every day at sunset to ensure correct odour effectiveness at the maximum activity time of mice, i.e. 2 or 4 h after dusk (Montgomery and Gurnell 1985). Finally, in phase 2, we only brought new faecal material out on the first day, in order to evaluate the effect of volatile compounds loss over time. All traps during the three study phases were baited with 4 g of toast corn as a positive stimulus.
Moonlight affects small mammal behaviour (Kaufman and Kaufman 1982; Díaz 1992; Navarro-Castilla and Barja 2014a); so in order to avoid any potential influence of moonlight on our experiments, we selected the nearest days to new moon to carry out our study. Further, Wróbel and Bogdziewicz (2015) recently demonstrated that increased cloud cover enhanced activity of yellow-necked mice (Apodemus flavicollis), but this effect tended to be weaker during the full moon. Therefore, we recorded the percentage of cloud cover to control for its possible effect. Traps were checked every 10–12 h, i.e. at dawn and dusk, to minimise the time that animals were kept.
Each captured individual was identified to species level based on external morphology. Sex and reproductive status of individuals were assessed following Gurnell and Flowerdew (2006). Sex was determined using the anal-genital distance, which is longer in males than in females. In the same way, reproductively active females were classified on the basis of the presence of nipples and perforated vaginal membranes, whereas reproductive active males were identified due to the increased size of their testicles that usually descended into the scrotal sac. Body weight was measured employing a 100-g hand-held scale (PESNET, 100 g) and individuals were classified into age classes according to their body weight as previously described by Lewis (1968): juveniles (< 13 g), subadults (13–20 g) and adults (≥ 20 g). However, only adults and subadults (i.e. weighing > 13 g) were included in this study, as the number of juveniles captured was insufficient for comparative analyses. All captured animals were marked on specific body areas (paws, inner ear area, tail) with harmless waterproof paints (Marking stick DFV, www.divasa-farmavic.com) to identify possible recaptures in each phase and avoid pseudoreplication. All captured animals were immediately released at the same place of capture.
Simulation of different degrees of predation risk by faecal odour
To simulate different degrees of predation risk, we used red-fox faecal odour, one of the main predators of wood mice (Serafini and Lovari 1993; Padial et al. 2002), and because of the well-demonstrated effectiveness of fox faeces to trigger antipredatory responses in small mammals (Dickman and Doncaster 1984; Navarro-Castilla and Barja 2014a, b). Thus, we collected fresh faeces from a semi captive couple of red foxes (one male and one female) from the Cañada Real Opennature Center (Peralejo, Madrid, Spain). Samples were considered fresh on the characteristics previously defined by Liu et al. (2006) (i.e. those which presented a layer of mucus, a high level of hydration and strong odour). These foxes had a carnivorous diet throughout the experiment similar to what they eat in the wild. Collected faeces were frozen at − 20 °C until used in the experiments to avoid the degradation of volatile compounds (Martín et al. 2010).
All red-fox collected faeces were thawed and homogenised for 1 h and a half.
During the experiment, different degrees of predation risk by red fox were simulated by mixing faecal material with soil and water in three different concentrations, one for each plot: low-concentration plot (10% of faecal material), medium-concentration plot (50% of faecal material) and high-concentration plot (100% of faecal material). Additionally, for the control odourless plot (0% of faecal material), only a mix of soil and water was prepared. Specific composition of faecal treatments were the following: control (1000 g of soil from the study area and 250 mL of distillate water), low (100 g of faeces, 900 g of soil from the study area and 250 mL of distillate water), medium (500 g of faeces, 500 g of soil from the study area and 250 mL of distillate water) and high concentration plot (1000 g of faeces and 250 mL of distillate water). Final faecal material was similar in shape and texture to actual faeces in nature in order to avoid a possible bias due to visual cues.
Mice faeces collection and quantification of faecal corticosterone metabolites
Measuring faecal glucocorticoid levels is a powerful noninvasive method to assess the physiological response to stress in wild animals (Möstl and Palme 2002; Touma and Palme 2005; Sheriff et al. 2011; Barja et al. 2012). Fresh faeces from captured mice were collected from traps each day at sunrise, avoiding the possible influence of circadian rhythms in excretion patterns (Touma et al. 2003), and therefore, in faecal corticosterone measurements. A recent study revealed that wood mice were trapped on average 5–7 h after trap activation at dusk (Navarro-Castilla et al. 2017a); thus, we assumed that trapping was not likely influencing the physiological stress response since captured mice spent similar times inside traps. Only faeces without presence of urine were sampled to avoid potential cross-contamination effects in our results. Faeces were stored at − 20 °C until being processed.
FCM extraction was done following the modified method of Touma et al. (2003). Faeces were dried in a heater (90 °C, 3 h) and then 0.05 g were weighed and mixed with 500 μl of 80% methanol and 500 μl of phosphate buffer. Then, samples were vortexed by hand for 15 s and in a multivortex for 16 h, followed by 15 min of centrifugation (2500g). The quantification of FCM levels was done with a commercial corticosterone enzyme immunoassay (DEMEDITEC Diagnostics GmbH, D-24145 Kiel, Germany) previously used in this and other rodent species (Navarro-Castilla et al. 2017a, b). The cross-reactivity of the antibodies with other substances according to the manufacturer was 2.4% for 11-deoxycorticosterone, while cross-reactivity was less than 1% with any other substance (aldosterone, cortisol, progesterone). Since validation of the enzyme immunoassay (parallelism, accuracy and precision tests) is needed (Goymann et al. 1999; Young et al. 2004), we carried out parallelism test with serial dilutions of faecal extracts (1:32, 1:16, 1:8, 1:4, 1:2, 1:1) which resulted in a curve parallel to the one created with the standards provided in the kit. Accuracy (recovery) was 118.6 ± 31.7% (n = 6). The intra- and inter-assay coefficients of variation for three biological samples were 4.7% (n = 6) and 8.2% (n = 3), respectively. In each assay, we used a standard, whose corticosterone metabolite concentration was known. FCM levels are calculated as nanograms per gram dry faeces.
Biological validation carried out by Navarro-Castilla et al. (2017a) has confirmed the suitability of the enzyme immunoassay for analysing faecal corticosterone metabolites in wood mouse faecal samples.
Statistical analysis
Both behavioural and physiological stress responses were analysed by Generalized Linear Mixed Models (GLMMs). Behavioural response was analysed using capture frequency as response variable in a model with Poisson error distribution. Explanatory variables included in the model were the following: phase (0/1/2), plot (control/low/medium/high), sex (male/female), reproductive status (active/non-active), cloud cover (%) and the interactions phase × plot, reproductive status × sex, phase × reproductive status, plot × reproductive status, sex × plot and sex × phase. Regarding the physiological stress response, since weight of individuals has been shown to have a significant influence on FCM in this rodent species (Navarro-Castilla et al. 2014b), we previously corrected FCM by dividing it by the weight of individuals (g) to avoid any possible influence. Later, we used a model with Gaussian error distribution to analyse the concentration of FCM (ng/g). For both cases, the explanatory variables were phase (0/1/2), plot (control/low/medium/high), sex (male/female), reproductive status (active/non-active), cloud cover (%) and the interactions phase × plot, reproductive status × sex, phase × reproductive status and plot × reproductive status. In both models, the potential temporal effect due to the consecutive sampling was controlled by including day as a random factor in the analyses. Assumptions of error distribution and homoscedasticity were checked in the residuals.
We used likelihood ratio tests to estimate the p value of the explanatory variables in the models. We used α < 0.05 as the criterion level for significance. Statistical data analyses were done in R 3.3.3 software (R Core Team 2017), using libraries lme4 (Bates et al. 2015) for GLMM, and afex (Singmann et al. 2017) for obtaining the p values of explanatory variables.
Results
Behavioural response
During the present study, 156 different individuals were captured (Table 1). Among the experimental phases, a decrease in the overall numbers of captured mice was observed, with the exception of reproductively active males that were captured in similar numbers throughout (Table 1). Results of the GLMM analysis for capture frequency showed significant effects of plot, reproductive status and the interaction of reproductive status and sex (Table 2). Looking closer at the effects of the different levels of each analysed factor (supplementary material, Table S1), males were captured less often than females (β = − 1.298 ± 0.458, p = 0.005). Moreover, reproductively active animals were also captured less often than non-reproductive individuals (β = − 0.722 ± 0.422, p = 0.087), although more reproductively active males were captured than reproductively active females (β = 0.968 ± 0.379, p = 0.011). Captures were higher in the highest concentration plot (Fig. 1); however, reproductively active mice significantly avoided traps from the medium and high plots (βMedium plot = − 1.167 ± 0.530, p = 0.028; βHigh plot = − 1.114 ± 0.475, p = 0.019) (Fig. 1, dark area).
Physiological response
The results of the GLMM are shown in Table 3 and Table S2 in supplementary material. Factors which most contributed to the stress response were sex and reproductive status, although some differences between plots and the interaction of plot and treatment phase could also be detected. Thus, although in control plot basal FCM levels were greater than in the rest of plots (βLow plot = − 0.751 ± 0.286, p = 0.010; βMedium plot = − 0.789 ± 0.245, p = 0.002; βHigh plot = − 0.938 ± 0.219, p < 0.001), during phase 1, a significant rise of FCM levels in mice captured in plots with predator odour was detected, increasing with the increase of predator faecal odour concentration (βLow plot = 0.799 ± 0.402, p = 0.049; βMedium plot = 0.960 ± 0.344, p = 0.006; βHigh plot = 0.861 ± 0.325, p = 0.009) (Fig. 2). Additionally, reproductively active individuals showed a significant increase in stress levels during both phase 1, with renewal of predator faecal odour (β = 0.707 ± 0.258, p = 0.007), and phase 2, without the replenishment (β = 0.696 ± 0.334, p = 0.039) (Fig. 3).
Discussion
Behavioural response
In accordance with the threat-sensitive predator avoidance hypothesis (Helfman 1989), our study showed evidence that wood mice are able to display a distinctive behavioural response after exposure to different faecal odour concentrations of red fox. This result is also in accordance with other studies where prey species were able to discriminate among different concentrations of chemical predator cues by adjusting the intensity of their responses to match the predation risk perceived (Kusch et al. 2004; Hegab et al. 2014c).
Overall, captures were influenced by sex and reproductive status. Specifically, we found a risk avoidance pattern in reproductive individuals, both males and females, in the plots with medium and high concentrations of predator faecal odour. This is an interesting result, as during the breeding season animals require more resources than in other times of the year (Gittleman and Thompson 1988; Speakman 2008; Dantzer et al. 2010). This implies that they tend to increase foraging to maximise the input of resource to reproduction, even if it means increased exposure to predators. Further, males usually have high testosterone levels during the breeding season so they are expected to have higher risk-taking behaviours. Additionally, males with elevated testosterone also tend to exhibit greater space use and higher copulation frequency, all of which may have reduced the probability of being captured more often. Our results suggest that reproductively active individuals could be more careful under risky situations to ensure survival for mating in the case of males, while females have to bring up their offspring. In both cases, they must balance obtaining food with the risk of predation. Our results pointed out that when the concentration exceeds the acceptable limit, from 50% of faecal odour in this case, and the trade-off between costs and benefits is unbalanced, wood mice avoid taking risk in order to prioritise breeding and mating and ensure offspring’s success (Montgomery et al. 1991).
Sex was also an important factor influencing risk-taking behaviour and males were less likely to be captured than females. There might be two potential explanations for this result. Our capture rates may reflect the composition of the population, which could contain more females than males, then making males less probable to be captured. Alternatively, this result could be also explained by the higher reliance of females on energy resources (Montgomery et al. 1991; Penn and Smith 2007), and thus they could be prone to take more risks than males to obtain the food placed inside the traps during the experiment. In any case, we did not find different predator avoidance strategies specifically related to the sex of individuals. However, reproductive status could inversely influence this response, since reproductively active males were trapped more than reproductively active females. This type of response appears to be related to the fitness costs involved during reproduction in each sex. While males’ investment is usually limited to fecundation, females have to ensure the protection of the offspring (Penn and Smith 2007). This essential task of females entails biological costs (e.g. increase mothers’ mortality) which have to be solved without taking more risks than necessary.
Physiological response
The threat-sensitive predator avoidance hypothesis (Helfman 1989) was also corroborated by the physiological results observed. Significant differences found in FCM levels were explained by plot and interaction between plot and treatment phase, in addition to sex and reproductive status of mice. Basal FCM concentrations were higher in the control plot than in the rest of the plots, which may be because of some intrinsic factors (e.g. animal transit). However, during phase 1, wood mice exhibited a significant rise of FCM in low-, medium- and high-concentration plots where the predator faecal odour was placed. This antipredatory response due to predation risk has been already found in previous studies performed in different species of mammals (Boonstra et al. 1998; Eilam et al. 1999; Monclús et al. 2006). More interestingly, hormonal secretion increased with increasing concentration of the faecal odour. Our results showed that mice are able to discriminate among at least three different situations of predation risk (no risk, low risk and medium-high risk), setting the threshold for the antipredator response activation when the concentration reaches 10% and modulating the intensity of this response between low and medium-high risk situations. A positive correlation between the strength of physiological stress response and the concentration of the chemical cue of a predator has been found previously in Brandt’s voles (Lasiopodomys brandtii) (Hegab et al. 2014c). According to these studies, the prey could evaluate chemical cues left by predators triggering the hormonal response only when the perceived signal overcomes a threshold, as posed by the signal detection theory (Forward et al. 2003; Dupuch et al. 2004). Signal detection theory and threat-sensitive predator avoidance hypotheses complement each other; whilst the first one explains how a prey can set a threshold level of response, the latter analyses the modulation of this response considering different levels of predation risk perceived. Furthermore, wood mice physiological stress response during phase 2 was not as strong as during phase 1, which could be likely explained by the decreased intensity of predator faecal cues over time due to the degradation of volatile compounds and the loss of sulphurous metabolites by evaporation (Sullivan and Sullivan 1982). Thus, decreased volatile compound concentrations could indicate that a predator is neither present nor close, and therefore, this would be perceived by the prey as a less risky situation.
The higher stress hormone levels found in reproductively active individuals in both phases 1 and 2 showed that reproductive status has also a huge influence on the physiological stress response. This result is consistent with other works (Dantzer et al. 2010; Navarro-Castilla et al. 2014a) and supports the important role of glucocorticoids during the reproductive season likely due to pregnancy and lactation in females (Tataranni et al. 1996) as well as the social interaction and competition between males (Navarro-Castilla et al. 2014a). In addition, despite the degradation of faecal volatile compounds over time, reproductively active individuals still showed significantly higher FCM levels during phase 2. Thus, the reproductive season could be considered as a critical period during which individuals are more sensitive to predation cues.
In summary, we can conclude that wood mice under field conditions seem to be able to discriminate among different degrees of predation risk, modulating their behavioural and physiological stress responses according to the specific situation. In addition, our results suggest that these antipredatory and physiological responses seem to be modulated by individual factors, highlighting reproductive status. But further research would be necessary to fully understand how individual factors influence prey decision-making process and physiological stress responses under predation risk.
References
Albone ES, Perry GC (1976) Anal sac secretion of the red fox, Vulpes vulpes; volatile fatty acids and diamines: implications for a fermentation hypothesis of chemical recognition. J Chem Ecol 2(1):101–111. https://doi.org/10.1007/BF00988029
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29(8):1123–1144. https://doi.org/10.1016/j.neubiorev.2005.05.005
Asa CS, Peterson EK, Seal US, Mech LD (1985) Deposition of anal-sac secretions by captive wolves (Canis lupus). J Mammal 66(1):89–93. https://doi.org/10.2307/1380960
Barja I, List R (2014) The role of spatial distribution of faeces in coyote scent marking behaviour. Pol J Ecol 62(2):373–384. https://doi.org/10.3161/104.062.0215
Barja I, Escribano G, Lara C, Virgós E, Benito J, Rafart E (2012) Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim Biol 62(4):419–432. https://doi.org/10.1163/157075612X642914
Barja I, Silván G, Martínez-Fernández L, Illera JC (2011) Physiological stress responses, fecal marking behavior, and reproduction in wild European pine martens (Martes martes). J Chem Ecol 37(3):253–259. https://doi.org/10.1007/s10886-011-9928-1
Barja I, de Miguel FJ (2004) Variation in stimulus, seasonal context, and response to urine marks by captive Iberian wolves (Canis lupus signatus). Acta ethologica 7(2):51–57. https://doi.org/10.1007/s10211-004-0101-5
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Boonstra R, Hik D, Singleton GR, Tinnikov A (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 68(3):371–394.
Creel S, Christianson D, Liley S, Winnie JA (2007) Predation risk affects reproductive physiology and demography of elk. Science 315(5814):960. https://doi.org/10.1126/science.1135918
Creel S, Winnie J Jr, Maxwell B, Hamlin K, Creel M (2005) Elk alter habitat selection as an antipredator response to wolves. Ecology 86(12):3387–3397. https://doi.org/10.1890/05-0032
Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S, Humphries MM, Boonstra R (2010) Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167(2):279–286. https://doi.org/10.1016/j.ygcen.2010.03.024
Díaz M, Torre I, Peris A, Tena L (2005) Foraging behavior of wood mice as related to presence and activity of genets. J Mammal 86(6):1178–1185. https://doi.org/10.1644/04-MAMM-A-127R1.1
Díaz M (1992) Rodent seed predation in cereal crop areas of central Spain: effects of physiognomy, food availability, and predation risk. Ecography 15(1):77–85. https://doi.org/10.1111/j.1600-0587.1992.tb00011.x
Dickman CR, Doncaster CP (1984) Responses of small mammals to red fox (Vulpes vulpes) odour. J Zool 204:521–531
Dielenberg RA, McGregor IS (2001) Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev 25(7-8):597–609. https://doi.org/10.1016/S0149-7634(01)00044-6
Dupuch A, Magnan P, Dill LM (2004) Sensitivity of northern redbelly dace, Phoxinus eos, to chemical alarm cues. Can J Zool 82(3):407–415. https://doi.org/10.1139/z04-003
Eilam D, Dayan T, Ben-Eliyahu S, Schulman I, Shefer G, Hendrie CA (1999) Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim Behav 58(5):1085–1093. https://doi.org/10.1006/anbe.1999.1224
Eisenberg JF, Eisenberg JF (1981) The mammalian radiations: an analysis of trends in evolution, adaptation, and behaviour. Evolution, Adaptation, and Behavior. University of Chicago Press, Chicago, In
Endler JA (1986) Natural selection in the wild. Princeton University Press
Fenn MGP, Macdonald DW (1995) Use of middens by red foxes: risk reverses rhythms of rats. J Mammal 76(1):130–136. https://doi.org/10.2307/1382321
Forward R, Tankersley R, Smith K, Welch J (2003) Effects of chemical cues on orientation of blue crab, Callinectes sapidus, megalopae in flow: implications for location of nursery areas. Mar Biol 142(4):747–756. https://doi.org/10.1007/s00227-002-0966-7
Gese EM, Ruff RL (1997) Scent-marking by coyotes, Canis latrans: the influence of social and ecological factors. Anim Behav 54(5):1155–1166. https://doi.org/10.1006/anbe.1997.0561
Gittleman JL, Thompson SD (1988) Energy allocation in mammalian reproduction. Am Zool 28(3):863–875. https://doi.org/10.1093/icb/28.3.863
Gorman ML (1990) Scent marking strategies in mammals. Rev Suisse Zool 97:3–29. https://doi.org/10.5962/bhl.part.79722
Goymann W, Möstl E, Van't Hof T, East ML, Hofer H (1999) Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. Gen Comp Endocrinol 114(3):340–348. https://doi.org/10.1006/gcen.1999.7268
Gurnell J, Flowerdew JR (2006) Live trapping small mammals. A practical guide, Mammal Society, London
Hegab IM, Shang G, Ye M, Jin Y, Wang A, Yin B, Yang S, Wei W (2014a) Defensive responses of Brandt’s voles (Lasiopodomys brandtii) to chronic predatory stress. Physiol Behav 126:1–7. https://doi.org/10.1016/j.physbeh.2013.12.001
Hegab IM, Wang A, Yin B, Yang S, Wanhong W (2014b) Behavioral and neuroendocrine response of Brandt’s voles, Lasiopodomys brandtii, to odors of different species. Eur J Wildl Res 60(2):331–340. https://doi.org/10.1007/s10344-013-0790-z
Hegab IM, Jin Y, Ye M, Wang A, Yin B, Yang S, Wei W (2014c) Defensive responses of Brandt’s voles (Lasiopodomys brandtii) to stored cat feces. Physiol Behav 123:193–199. https://doi.org/10.1016/j.physbeh.2013.10.030
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol 24(1):47–58. https://doi.org/10.1007/BF00300117
Huffard CL (2006) Locomotion by Abdopus aculeatus (Cephalopoda: Octopodidae): walking the line between primary and secondary defenses. J Exp Biol 209(19):3697–3707. https://doi.org/10.1242/jeb.02435
Hutchings MR, White PCL (2000) Mustelid scent-marking in managed ecosystems: implications for population management. Mamm Rev 30(3-4):157–169. https://doi.org/10.1046/j.1365-2907.2000.00065.x
Jędrzejewski W, Rychlik L, Jędrzejewska B (1993) Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator-vole relationships. Oikos 68(2):251–257. https://doi.org/10.2307/3544837
Jorgenson JW, Novotny M, Carmack M, Copland GB, Wilson SR, Katona S, Whitten WK (1978) Chemical scent constituents in the urine of the red fox (Vulpes vulpes L.) during the winter season. Science 199(4330):796–798. https://doi.org/10.1126/science.199.4330.796
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5(3):361–394. https://doi.org/10.1080/11956860.1998.11682468
Kaufman DW, Kaufman GA (1982) Effect of moonlight on activity and microhabitat use by Ord’s kangaroo rat (Dipodomys ordii). J Mammal 63(2):309–312. https://doi.org/10.2307/1380644
Kavaliers M, Choleris E (2001) Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci Biobehav Rev 25(7-8):577–586. https://doi.org/10.1016/S0149-7634(01)00042-2
Kusch RC, Mirza RS, Chivers DP (2004) Making sense of predator scents: investigating the sophistication of predator assessment abilities of fathead minnows. Behav Ecol Sociobiol 55(6):551–555. https://doi.org/10.1007/s00265-003-0743-8
Lewis JW (1968) Studies on the helminth parasites of the long-tailed field mouse, Apodemus sylvaticus sylvaticus from Wales. J Zool 154:287–312
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153(6):649–659. https://doi.org/10.1086/303202
Lima SL (1998) Stress and decision-making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Advances in the Study of Behavior 27:215–290. https://doi.org/10.1016/S0065-3454(08)60366-6
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68(4):619–640. https://doi.org/10.1139/z90-092
Liu J, Chen Y, Guo L, Gu B, Liu H, Hou A, Liu X, Sun L, Liu D (2006) Stereotypic behavior and fecal cortisol level in captive giant pandas in relation to environmental enrichment. Zoo Biol 25(6):445–459. https://doi.org/10.1002/zoo.20106
Luttbeg B, Trussell GC (2013) How the informational environment shapes how prey estimate predation risk and the resulting indirect effects of predators. Am Nat 181(2):182–194. https://doi.org/10.1086/668823
Macdonald DW (1985) The carnivores: order Carnivora. Clarendon Press, Oxford
Martín J, Barja I, López P (2010) Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus). Biochem Syst Ecol 38(6):1096–1102. https://doi.org/10.1016/j.bse.2010.10.014
Masini C, Sauer S, Campeau S (2005) Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci 119(1):280–292. https://doi.org/10.1037/0735-7044.119.1.280
Melmed S, Kleinberg D (2003) Anterior pituitary. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds) Williams textbook of endocrinology. WB Saunders Co, Philadelphia, pp 177–279
Mirza RS, Mathis A, Chivers DP (2006) Does temporal variation in predation risk influence the intensity of antipredator responses? A test of the risk allocation hypothesis. Ethology 112(1):44–51. https://doi.org/10.1111/j.1439-0310.2006.01111.x
Monclús R, Rödel HG, Palme R, Von Holst D, de Miguel J (2006) Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16(1):25–29. https://doi.org/10.1007/s00049-005-0324-6
Montgomery WI, Wilson WL, Hamilton R, McCartney P (1991) Dispersion in the wood mouse, Apodemus sylvaticus: variable resources in time and space. J Anim Ecol 60(1):179–192. https://doi.org/10.2307/5453
Montgomery WI, Gurnell J (1985) The behaviour of Apodemus. In: Flowerdew JR, Gurnell J, Gipps JHW (eds) The ecology of woodland rodents bank voles and wood mice. Symp. Zool. Soc. Lond, London, pp 89–115
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrinol 23(1-2):67–74. https://doi.org/10.1016/S0739-7240(02)00146-7
Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5(1):25–44. https://doi.org/10.1210/edrv-5-1-25
Navarro-Castilla Á, Barja I, Díaz M (2017a) Foraging, feeding, and physiological stress responses of wild wood mice to increased illumination and common genet cues. Curr Zool:zox048
Navarro-Castilla Á, Díaz M, Barja I (2017b) Does ungulate disturbance mediate behavioral and physiological stress responses in Algerian mice (Mus spretus)? A wild exclosure experiment. Hystrix:https://doi.org/10.4404/hystrix-28.2-12332
Navarro-Castilla Á, Barja I (2014a) Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice (Apodemus sylvaticus)? Behav Ecol Sociobiol 68(9):1505–1512. https://doi.org/10.1007/s00265-014-1759-y
Navarro-Castilla Á, Barja I (2014b) Antipredatory response and food intake in wood mice (Apodemus sylvaticus) under simulated predation risk by resident and novel carnivorous predators. Ethology 120(1):90–98. https://doi.org/10.1111/eth.12184
Navarro-Castilla Á, Barja I, Olea PP, Pineiro A, Mateo-Tomás P, Silván G, Illera JC (2014a) Are degraded habitats from agricultural crops associated with elevated faecal glucocorticoids in a wild population of common vole (Microtus arvalis)? Mammalian Biology-Zeitschrift für Säugetierkunde 79(1):36–43. https://doi.org/10.1016/j.mambio.2013.08.004
Navarro-Castilla Á, Mata C, Ruiz-Capillas P, Palme R, Malo JE, Barja I (2014b) Are motorways potential stressors of roadside wood mice (Apodemus sylvaticus) populations? PLoS One 9(3):e91942. https://doi.org/10.1371/journal.pone.0091942
Padial JM, Avila E, Sanchez JM (2002) Feeding habits and overlap among red fox (Vulpes vulpes) and stone marten (Martes foina) in two Mediterranean mountain habitats. Mammalian Biology-Zeitschrift für Säugetierkunde 67(3):137–146. https://doi.org/10.1078/1616-5047-00021
Penn DJ, Smith KR (2007) Differential fitness costs of reproduction between the sexes. Proc Natl Acad Sci U S A 104(2):553–558. https://doi.org/10.1073/pnas.0609301103
Piñeiro A, Barja I (2012) The plant physical features selected by wildcats as signal posts: an economic approach to fecal marking. Naturwissenschaften 99(10):801–809. https://doi.org/10.1007/s00114-012-0962-9
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Sánchez-González B, Barja I, Navarro-Castilla Á (2017) Wood mice modify food intake under different degrees of predation risk: influence of acquired experience and degradation of predator’s faecal volatile compounds. Chemoecology 27(3):115–122. https://doi.org/10.1007/s00049-017-0237-1
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocr Rev 21(1):55–89. https://doi.org/10.1210/edrv.21.1.0389
Schmitz OJ, Bradford MA, Strickland MS, Hawlena D (2013) Linking predation risk, herbivore physiological stress and microbial decomposition of plant litter. J Vis Exp:e50061
Serafini P, Lovari S (1993) Food habits and trophic niche overlap of the red fox and the stone marten in a Mediterranean rural area. Acta Theriol 38:233–244. https://doi.org/10.4098/AT.arch.93-19
Sherbrooke WC (2008) Antipredator responses by Texas horned lizards to two snake taxa with different foraging and subjugation strategies. J Herpetol 42(1):145–152. https://doi.org/10.1670/07-072R1.1
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166(4):869–887. https://doi.org/10.1007/s00442-011-1943-y
Singmann H, Bolker B, Westfall J, Aust F (2017) afex: analysis of factorial experiments. R package version 0.18-0. https:// CRAN.R-project.org/package=afex. R package version 0.13–145
Speakman JR (2008) The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond Ser B Biol Sci 363(1490):375–398. https://doi.org/10.1098/rstb.2007.2145
Stewart PM (2003) The adrenal cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds) Williams textbook of endocrinology. Saunders, Philadelphia, p 491
Sullivan TP, Sullivan DS (1982) Barking damage by snowshoe hares and red squirrels in lodgepole pine stands in central British Columbia. Can J For Res 12(2):443–448. https://doi.org/10.1139/x82-068
Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E (1996) Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Phys 271:E317–E325
Tortosa FS, Barrio IC, Carthey AJ, Banks PB (2015) No longer naïve? Generalized responses of rabbits to marsupial predators in Australia. Behav Ecol Sociobiol 69(10):1649–1655. https://doi.org/10.1007/s00265-015-1976-z
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046(1):54–74. https://doi.org/10.1196/annals.1343.006
Touma C, Sachser N, Möstl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130(3):267–278. https://doi.org/10.1016/S0016-6480(02)00620-2
Verplancke G, Le Boulengé É, Diederich C (2010) Differential foraging in presence of predator and conspecific odors in bank voles: a field enclosure study. Ecol Res 25(5):973–981. https://doi.org/10.1007/s11284-010-0721-3
Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone—behavior interactions: the “emergency life history stage”. Am Zool 38(1):191–206. https://doi.org/10.1093/icb/38.1.191
Wróbel A, Bogdziewicz M (2015) It is raining mice and voles: which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus? Eur J Wildl Res 61(3):475–478. https://doi.org/10.1007/s10344-014-0892-2
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511615061
Yin B, Fan H, Li S, Hegab I, Lu G, Wei W (2011) Behavioral response of Norway rats (Rattus norvegicus) to odors of different mammalian species. J Pest Sci 84(3):265–272. https://doi.org/10.1007/s10340-011-0351-8
Young K, Walker S, Lanthier C, Waddell W, Monfort S, Brown J (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137(2):148–165. https://doi.org/10.1016/j.ygcen.2004.02.016
Acknowledgements
The authors wish to thank José España, technician director of the Cañada Real Open Center, for furnishing the red-fox faecal material needed to perform the experiments exposed. We also would like to express our thanks to the Autonomous Community of Madrid (Spain) for providing the permits required to conduct this study and to the Autonomous University of Madrid (Spain) for allowing us to carry out this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This research complies with the regulations on the protection of animals used for scientific purposes (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010) and the Spanish legislation (Royal Decree 53/2013). The study had the approval of the Autonomous Community of Madrid (reference number 10/211643.9/13) and favourable reports from both the Ethics Committee of the Autonomous University of Madrid and the Body Enabled (CIS 50-940-A007).
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Sánchez-González, B., Planillo, A., Navarro-Castilla, Á. et al. The concentration of fear: mice’s behavioural and physiological stress responses to different degrees of predation risk. Sci Nat 105, 16 (2018). https://doi.org/10.1007/s00114-018-1540-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-018-1540-6