Abstract
Animals normally respond to stressful environmental stimuli by releasing glucocorticoid hormones. We investigated whether baseline corticosterone (CORT), handling-induced corticosterone concentration(s), and body condition indices of members of willow tit (Poecile montanus) groups differed while wintering in old growth forests and managed young forests in mild weather conditions and during cold spells. Willow tits spend the winter season in non-kin groups in which dominant individuals typically claim their priority to access resources, while subordinate individuals may experience greater levels of stress and higher mortality, especially during cold spells. We captured birds to measure baseline CORT and levels of handling-induced CORT secretion after 20 min of capture. Willow tits in the young forests had higher baseline CORT and a smaller increase in CORT in response to capture than individuals in the old forests. Baseline CORT was higher in females and juvenile birds compared to adult males, whereas handling-induced CORT secretion did not differ between birds of different ages. During cold spells, baseline CORT of willow tits increased and handling-induced CORT secretion decreased, especially in birds in young forests. Willow tits’ survival was higher in the old forests, with dominant individuals surviving better than subordinates. Our results show that changes in CORT secretion reflect responses to habitat quality and climate harshness, indicating young managed coniferous forests as a suboptimal habitat for the willow tit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The decline of biological diversity as a consequence of habitat loss, deterioration, and fragmentation is a substantial concern in conservation biology (Hanski 1999; Fahrig 2003). Many species depend on particular features of their habitats that are necessary for reproduction and survival (Wiens 1989; Wingfield et al. 2015). High-quality habitats are regarded as areas with a high concentration of important resources and where individual fitness, survival, or other measures of performance are highest. Habitat destruction may create serious difficulties for organisms, potentially leading to local population extinction (Peres 2001; Fahrig 2003). Habitat destruction has recently been suggested to constitute a physiological stressor for animals (Homan et al. 2003; Romero 2004; Wikelski and Cooke 2006; Dantzer et al. 2014).
Free-living organisms face multiple stressors in their natural environments (Romero 2004; Romero et al. 2009) that can challenge homeostasis (Ots et al. 1998; Boonstra 2013). In vertebrates, the autonomic nervous system (Herman et al. 2016) and the hypothalamic–pituitary–adrenal (HPA) axis are among the principal mechanisms that evoke adaptive reactions when organisms face noxious environmental stimuli (Schulkin et al. 2005; Hõrak et al. 2006). Adaptive responses include alterations in life history strategy (Wingfield et al. 1998; Boonstra 2005) during which glucocorticoid hormones are secreted from the adrenal cortex (Sapolsky et al. 2000; Creel et al. 2013). If the stressor is brief and infrequent (Sapolsky et al. 2000; Uchoa et al. 2014; Ketterson et al. 2015), the adrenal gland increases glucocorticoid hormone production, and this boosts energy availability to respond properly to the presence of the stressor (Romero et al. 1997, 1998; Sapolsky et al. 2000; Landys et al. 2006; Ketterson et al. 2015). However, when there are insufficient reserves to satisfy the physiological cost of stress, resources must be shifted away from other biological functions such as reproduction, maintenance, growth, and immunity due to trade-offs between life history traits (Stearns 1992). Chronic stress and the accompanying sustained high concentration of circulating glucocorticoids may have significant effects on immunity and growth due to induction of severe protein loss in muscle and neural tissues, thus impairing health condition and reproductive function (Sapolsky and Pulsinelli 1985; McEwen 2000; Bergeon Burns et al. 2014).
The fragmentation and deterioration of old growth forests by modern forestry have become serious threats to species diversity. In northern Europe, for example, the apparent population decline of some forest parid species resulted from the loss of high-quality wintering and breeding habitats (Virkkala and Liehu 1990; Niemi et al. 1998), where the old, natural forests had decreased in area and become fragmented (Järvinen 1982; Virkkala 1987; Veistola et al. 1997). Willow tits establish their territories in high-quality habitats, while they are absent in habitats of intermediate quality (Ekman 1998). Wintering willow tits have been observed to enter the sapling areas in their flock territories but only for short feeding bouts (Krams 1996). To survive the winter in the higher latitudes of northern Europe with severe continental climate, the physiological capacity of birds must be sufficient to withstand the lowest ambient temperature that may occur. Because of the higher energy requirements or the inability to tolerate reduced body temperature (Reinertsen and Haftorn 1986), wintering individuals may experience greater levels of physiological stress and higher mortality, especially during sudden cold spells (Krams et al. 2010a, 2013). It has been shown that restricted food availability and accessibility (Rowher and Wingfield 1981; Wingfield et al. 1983; Wingfield 1985; Rogers 1987; Rogers et al. 1993; Smith et al. 1994; Raouf et al. 2006; Jenni-Eiermann et al. 2008), habitat harshness (Wingfield et al. 1995; Addis et al. 2011; Krause et al. 2015; Walker et al. 2015), or sudden snowstorms and temperature declines (de Bruijn and Romero 2011, 2013; Krause et al. 2016a, b) affect behavioral decisions (Glądalski et al. 2014; Senner et al. 2015; Briedis et al. 2017) and elicit secretion of plasma corticosterone (CORT), a main glucocorticoid of birds involved in regulation of the immune system, energy, and stress responses (Kitaysky et al. 2001). It has also been demonstrated that different bird species may differ considerably in their capacity to survive under low temperatures (Saarela et al. 1995). Importantly, in studies on dominance-structured groups of wintering birds, higher plasma CORT was found in subordinate group members (Silverin et al. 1984; Pravosudov et al. 2001; Holberton and Able 2002; Poisbleau et al. 2005; but see Pravosudov et al. 2003). It has been shown that subordinate individuals have restricted access to food sources, while the availability of food might be a crucial factor of survival in winter (Ekman and Askenmo 1984; Krams et al. 2010a). Arthropods dwelling in the tree canopy are among the most important food sources for the forest wintering passerines. These arthropods prefer branches with needles within canopies of coniferous trees (Gunnarsson 1990), while the canopies of mature coniferous trees contain more arthropods than canopies of younger trees (Krams 1998a; Krams et al. 2001). This makes old growth forests a better wintering habitat for willow tits than young managed forests (Krams et al. 2001).
Physiological stress caused by low ambient temperatures may interact with habitat quality to undermine the survival of birds wintering in forests affected by anthropogenic changes. For example, the American redstart (Setophaga ruticilla), a migratory songbird, had higher baseline CORT when wintering in low-quality habitats than when occupying high-quality habitats (Marra and Holberton 1998). Redstarts wintering in low-quality habitats exhibited a reduction in their ability to release CORT in response to capture stress (handling-induced CORT). This may suggest a reduction in the sensitivity of the HPA axis to environmental signals (Schwabl et al. 1991; Wingfield et al. 1992, 1994 a, b; Holberton et al. 1996; Silverin 1997), meaning that elevated baseline CORT levels minimized the redstarts’ reactive homeostasis (Romero et al. 2009). While baseline CORT and handling-induced CORT secretion in American redstarts was not age-dependent, in many other forest-dwelling birds wintering in dominance-structured groups, the subordinate individuals (often young birds) occupy habitats of lower quality which negatively affects their survival (Ekman and Askenmo 1984; Suhonen 1993; Krams 2001).
In this field study, we investigated whether three indices of condition (CORT concentration, muscle score, and body fat reserves) in members of willow tit (Poecile montanus) flocks differ across dominance hierarchies, weather conditions (ambient temperature), and between semi-natural old growth and young managed forests. The willow tit is a small hoarding sedentary resident passerine that is a widespread and common resident breeder throughout temperate and subarctic Europe and northern Asia (Snow and Perrins 1998). This species has undergone significant population declines during recent decades (Harrap and Quinn 1996). In northern Europe, willow tits prefer coniferous stands composed of pines and spruces. Willow tits form heterospecific flocks with non-kin conspecifics and other members of the Paridae guild during the non-reproductive season (Ekman 1998). These flocks remain stable in membership and space as members of flocks and jointly defend their territory from other flocks from mid-summer till the next breeding season in early spring. Several studies have shown that the flocks are dominance-structured, that male willow tits always dominate females and, within the sexes, adults dominate juveniles (e.g., Krams 1996, 1998b). In this study, natural temperature regimes ranged from mild to extremely low ambient temperature occurring during sudden cold spells.
Based on the willow tit groups’ social structure, habitat structure, and possible substantial changes in weather conditions, we had four predictions related to CORT levels and body condition of individual willow tits of different age, sex, and social rank. To test our predictions, each bird was captured twice: we took the first sample under mild conditions and the second one under low ambient temperatures. On each occasion, we took two CORT samples: the first within 2 min of capture to obtain baseline CORT and the second after 20 min of capture to obtain levels of handling-induced CORT secretion of individual birds (Marra and Holberton 1998). We hypothesized (i) lower levels of willow tit baseline CORT, higher handling-induced CORT secretion, and a better condition of the pectoral muscle in the birds captured in old forests than in the young forests under conditions of low ambient temperatures where chronic physiological stress can lead to suppressed stress responsiveness. Since rank-related access to resources often results in better winter survival among dominants (Ekman and Askenmo 1984; Koivula et al. 1995; Krams et al. 2001, 2010a), we expected (ii) higher mortality in subordinate willow tits. We also predicted (iii) a positive correlation between dominance rank and the condition of the pectoral muscle, while higher baseline CORT and lower levels of handling-induced CORT secretion were expected in subordinate individuals in the young managed forests under conditions of extremely low ambient temperatures. Finally, we tested whether (iv) willow tits wintering in high-quality territories have higher baseline CORT, lower pectoral muscle scores, and body fat scores because of higher energetic demands required to defend their territories more actively than birds wintering in low-quality territories (Mazerolle and Hobson 2002).
Materials and methods
Study site and birds
The study was conducted near the town of Krāslava in southeastern Latvia (55° 87′ N, 27° 23′ E) in December 2009 and January 2012. The study area covers approximately 12 km2 of mainly coniferous forests of different ages, from open clear-cut areas and bogs to closed forests, dominated by Scots pine (Pinus sylvestris) and Norwegian spruce (Picea abies) (Rytkönen and Krams 2003). The data were obtained from willow tits of 12 mixed-species flocks (6 flocks in 2009 consisting of 6 adult males, 6 adult females, 6 juvenile males, and 6 juvenile females; 6 flocks in 2012 consisting of 6 adult males, 6 adult females, 6 juvenile males, and 6 juvenile females) in young 35–55-year-old managed pine plantations with a sparse understory. We also obtained the data from willow tits of 11 mixed-species flocks (6 flocks in 2009 containing 6 adult males, 6 adult females, 9 juvenile males, and 7 juvenile females; 5 flocks in 2012 containing 5 adult males, 5 adult females, 7 juvenile males, and 5 juvenile females) in unmanaged 105–155-year-old mixed forests dominated by Norwegian spruce. The mixed-species flocks contained 4–5 willow tits (mean number of individuals 4.26 ± 0.45, mean ± SD; 98 individuals in total; all flocks consisted of one adult male, one adult female, one to two juvenile males, and one juvenile female). The flocks also consisted of other species such as crested tits (Lophophanes cristatus), coal tits (Periparus ater), great tits (Parus major), marsh tits (Poecile palustris), blue tits (Cyanistes caeruleus), treecreepers (Certhia familiaris), and nuthatches (Sitta europaea). Aggressive behavior of more dominant crested tits, nuthatches, and great tits may make flocking less attractive to subordinate willow tits, and they often forage as members of smaller conspecific sub-flocks around midday under mild weather conditions (Hogstad 1988a, 1988b).
Each flock of willow tits inhabits a territory of about 9 ha (Krams 1996). To detect boundaries of territories of the flocks, we used a Magellan GPS receiver (MiTAC Digital Corporation, Santa Clara, CA, USA). An observer recorded the flock’s coordinates every 5 min while following adult willow tits (Krama et al. 2015). This was done to ensure that willow tits inhabit only one type of forest.
The birds were trapped by mist nets (Ecotone, Sopot, Poland) at temporary feeders baited with sunflower seeds. Each bird was banded with metal and a unique combination of colored plastic rings in September. Willow tits were sexed and aged (as adult or juvenile). The shape of the rectrices of willow tits was used to determine age (Laaksonen and Lehikoinen 1976), while sexual dimorphism in wing and tarsus length was used to sex individuals (Koivula and Orell 1988). We also used a method developed by Vinogradova et al. 1976: willow tits with wings shorter than 61 mm were identified as females, and those with wings longer than 67 mm were considered males. In addition, the sex of adult willow tits was known from previous breeding seasons. Finally, we continued observations of individual willow tits also outside the wintering season. Observed fights and territorial behavior, singing behavior and parental roles during the breeding season were used to determine the sex of those individuals with overlapping biometrical parameters. In this study, only flocks with all individuals properly sexed were included into analyses.
Dominance ranks
Dominance order was measured within each flock using pairwise interactions between birds at the temporary feeders in the beginning of November. An individual was dominant over another if it chased the other away from the food, caused the withdrawal of the other by approaching, or forced the other to wait by occupying the feeder (Koivula and Orell 1988; Krams et al. 2010a). The dominant won more interactions than the subordinate within each dyad (two-tailed sign test, P < 0.001). Adult males had the highest rank, and males were dominant over females in both age groups. We assigned rank 1 to the highest-ranking individual (adult male), while juvenile females were assigned rank 4 or 5 dependent on whether the flock consisted of four or five willow tits. The dominance hierarchy was linear in the flocks.
Weather conditions
The first sampling of birds was done before a cold spell arrived. The second set of samples was obtained from the same willow tits during a cold spell when the ambient temperature decreased to −35 °C within some days, and remained extremely cold for at least 2 weeks. Two to four days before the weather forecast predicted the onset of a cold spell in mid-December 2009 and mid-January 2012, we captured willow tits from six mixed-species flocks in mid-December 2009 and six flocks in mid-January 2012 in the young managed forests, and from six flocks in mid-December 2009 and five flocks in mid-January 2012 in the old forests. Before the cold spell arrived, the weather was mild with the average temperature in the night within the range of 5 to −3 °C while the mean daytime temperatures varied from 8 to 0 °C. The average night-time temperature during the cold spell was within the range of −28 to −32 °C, with mean daytime temperatures from −18 to −24 °C. January 2012 was colder than December 2009.
Measurements and samples
We captured the birds using mist nets located at permanent feeders provided with ad libitum sunflower seeds between 10:00 and 13:30 h. Within 1–2 min of capture, ca. 100 μl of whole blood was collected from the tarsal vein into heparinized microcapillary tubes (Saerstedt AG & Co, Nümbrecht, Germany). This sample provided the baseline level of plasma CORT. We identified each bird and took basic morphological measurements of wing and tarsus length, muscle score, and body mass. The birds were weighed to the nearest 0.5 g using a 30-g Pesola spring balance. Muscle score was assessed by visually inspecting the pectoral muscle around the keel bone on a 0–3 scale according to Bairlein (1995). Fat score was obtained when checking the amount of fat in the furcular hollow on a 0–4 scale (Krams 2002; Krams et al. 2010a, 2013).
The birds were kept in bird holding bags (Ecotone, Gdynia, Poland) individually. We took a second blood sample 20 min after capture to provide the profile of handling-induced CORT secretion, and then released the bird. It has previously been shown that CORT of wintering willow tits peaks within ca. 20 min of capture-related stress (Silverin et al. 1989). Plasma was separated from blood cells by 10-min centrifugation at 10,000 rpm and stored at −35 °C until analyzed. Correlate EIA kit (Cat No. 900-097; Assay Designs Inc., Ann Arbor, MI) was used for measurements of plasma CORT levels. Plasma samples were diluted 1:3 with assay buffer in the kit, and standard protocol was followed (see online manual: http://www.assaydesigns.com/objects/catalog//product/extras/900-097.pdf). Samples were randomly assigned to microplate wells along with blanks and five standards (32–200,000 pg ml−1 CORT). Plates were read on a Multiscan FC microplate reader (ThermoFisher Scientific, Waltham, MA, USA) at 405 nm. The samples, standards, and controls were assayed in duplicate. The average recovery of the assay was uniformly high (mean ± SD = 86 ± 2.4%; n = 10) and we did not correct for it. The sensitivity for CORT detection was 27 pg/ml, according to the manufacturer’s protocol. The repeatability of CORT measurements was high (r = 0.88, n = 25 pairs of repeated samples), calculated as intraclass correlation coefficients from one-way ANOVA according to Lessells and Boag (1987). The intra- and interassay variations were 8.24 and 10.41%, respectively, which is typical in this kind of study (Tilgar et al. 2009). We obtained CORT measurements from 23 adult males, 23 adult females, 29 juvenile males, and 23 juvenile females.
Survival
We estimated the survival of the willow tits at the end of February 2010 and 2012. We checked for the presence/absence of flock members for 4–5 days by observing color-banded individuals with binoculars (using 10× magnification) at bird feeders baited with sunflower seeds (Krams et al. 2001; Krama et al. 2015). The birds that disappeared in the course of winter but were found again outside the study area in spring were included in the analysis as survivors.
Statistics
We used linear mixed effects models to account for possible correlations between the data points that either come from the same individual or from different individuals from the same flock. Individual IDs nested under flock number were used as random effects. For testing the significance of model parameters corresponding to fixed effects, Satterthwaite correction was used to estimate the degrees of freedom for the F-distribution corresponding to the null hypothesis. Interactions up to the third order were included in the models. If third-order interactions were not significant, models were refit without them. For all models, model residuals were screened to detect possible departures from the model assumptions (normally distributed residuals with constant variance) and no violations were detected. In the case of CORT concentrations (dependent variable), separate models were made for baseline and for handling-induced CORT concentrations. Habitat, age, year, sex, weather conditions, fat score, and muscle score were included as fixed effects. For models where fat scores and muscle scores were analyzed as dependent variables, habitat, age, year, sex, and weather conditions were used as fixed factors. We used R 3.4.0. software. Models were fitted using the lme4 package and P values were calculated by lmerTest package. Post hoc tests for significant interactions were performed with lsmeans packages (Bates et al. 2015; Kuznetsova et al. 2016; Lenth 2016; R Core Team 2017).
Generalized linear mixed effects model with binomial error structure was used to test survival probability of willow tits. Baseline CORT concentration, year, sex, habitat, fat score, and dominance rank were used as fixed factors. The flock number was used as a random effect. Calculations of a possible relationship between survival and social dominance were based on the following categories assigned to each member of the flock: the number 1 was assigned to the highest-ranking individual and the numbers 4 or 5, based on variation in flock size, were assigned to the most subordinate willow tits. Only young birds (n = 52) were included in this analysis because there was no mortality observed among adult individuals.
Results
CORT values
The overall model for the handling-induced CORT concentration showed significant interaction only between habitat and weather (Table 1). In contrast, there were five significant second- and third-order interactions for the baseline CORT concentration (Table 2). The concentrations of baseline CORT of adult willow tits (t 169.6 = −46.1, P < 0.001, Fig. 1) and males (t 170.9 = 5.2, P < 0.001, Fig. 1) were lower than baseline CORT of juveniles and females, respectively. However, handling-induced CORT concentrations did not significantly differ between sexes or age groups (all Ps > 0.05, Table 2). The birds had lower baseline and handling-induced CORT under mild ambient temperatures (16.22 ± 5.03 and 29.54 ± 2.06 ng/ml, respectively; mean ± SD) than in the cold weather (21.38 ± 4.94 and 32.25 ± 1.99 ng/ml). The willow tits’ baseline and handling-induced CORT levels under mild and cold weather were significantly lower in the old forest (17.58 ± 5.27 and 29.72 ± 1.77 ng/ml) than in the young forest condition (20.07 ± 5.68 and 32.13 ± 2.42 ng/ml). In 2009, handling-induced CORT concentrations were higher than in 2012 but there were no significant difference for the baseline CORT across the years (Tables 1 and 2). Fat score and muscle score were not associated with baseline and handling-induced CORT concentrations (P > 0.05).
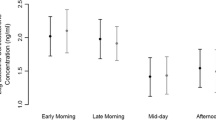
Concentration of plasma CORT (mean and SD) at the time of capture (0) (baseline CORT) and 20 min after capture (20) (handling-induced CORT) in willow tits in a old/young forests and b in willow tit juveniles/adults under conditions of mild weather and during a cold spell. Numbers below error-bars indicate the sample size of each group. There was a significant difference between conditions (P < 0.05) in each combination of duration and sex, age, or habitat type
Body condition: fat reserves and muscle score
Three second-order interactions and one third-order interaction were significant for the fat reserves model. Under mild weather, males were leaner than females but the difference was smaller than during cold weather (F 1,93.0 = 13.4, P < 0.001, Fig. 2). Overall, adults had lower body fat reserves than juvenile birds, but the difference was smaller in mild compared to cold temperatures (F 1,93.0 = 14.8, P < 0.001; Table 3). Furthermore, a significant interaction between sex and age showed that for adult birds the difference between sexes was greater than for juvenile birds (F 1,70.0 = 8.6, P = 0.005; Table 3). No other significant interactions were revealed.
Muscle scores were higher under mild compared to cold weather (F 1,94.3 = 18.5, P < 0.001, Fig. 3). Males had higher scores than females (F 1,71.9 = 10.6, P = 0.002), older birds had higher scores than juvenile birds (F 1,71.6 = 32.6, P < 0.001), and birds in the old forests had higher scores than in the young forests (F 1,20.2 = 4.7, P = 0.043). The only significant interaction was between age, sex, and weather conditions (F 1,94.3 = 5.9, P = 0.017; Table 4). Older females had higher scores than young females under both weather conditions, but males showed difference only under the cold weather.
Survival
The survival of willow tits in the old forests (91.50%) was significantly higher than in the young forests (72.92%) (Chisq = 7.3334, df = 1, P = 0.0068, Table 5) between the beginning of November and the end of February. Birds with higher dominance rank had significantly higher survival probability (Chisq = 4.3236, df = 1, P = 0.0376) (86.4% for dominance rank 2 and only 40% for dominance rank 4). There was no relationship between fat score (Chisq = 1.2252, df = 1, P = 0.2683) or baseline CORT concentration (Chisq = 0.0135, df = 1, P = 0.9074) and survival rate.
Discussion
The main finding of this study was that willow tits wintering as members of dominance-structured flocks differed in levels of baseline CORT and capture handling-induced CORT across habitats, weather, sex, and age. Prior studies on forest passerines show that individuals living in old forests are often in a better physiological condition than those inhabiting heavily managed forests, suggesting a link between habitat and the level of physiological stress in birds (Krams et al. 2010b; Saari et al. 1994; Suorsa et al. 2003, 2004; Grava et al. 2013). Greater stress levels and lower survival in birds inhabiting young, highly managed and fragmented forests may be due to a lower availability of food resources and a higher risk of predation. Frequent changes of habitats may also increase predation risk especially around habitat edges, where birds have to change their anti-predator strategies, which are different in young and old forests (Krams 1996). Food abundance is crucial for the survival of parids wintering in forests (Jansson et al. 1981; Krams et al. 2001). The preference for mature habitats is due to a higher availability of arthropods to foliage-gleaning passerines in older and larger trees (Krams et al. 2001). The number of some parids has recently decreased manifoldly (Virkkala 1988; Virkkala 1990; Virkkala and Liehu 1990; Fuller et al. 2005; Hewson et al. 2007; Eaton et al. 2009), probably because of a year-round food shortage in managed, highly deteriorated and often young forests (Virkkala 1987; Krams et al. 2010b).
It has previously been shown that a long-term lack of food may cause chronic physiological stress (Romero and Wikelski 2001), often resulting in a suppressed immune system and decreased disease resistance (Suorsa et al. 2003), all of which are detrimental to survival (Wasser et al. 1997; Romero and Wikelski 2001; Griesser et al. 2007). We found higher baseline CORT levels in willow tits inhabiting young forests under extremely low temperatures, while the increase in CORT in response to handling-induced stress was significantly lower in the birds in young forests compared to the birds in old forests under mild and cold weather. Young forests might therefore represent the worst habitat for willow tits, especially under extremely low ambient temperatures. The finding that willow tit handling-induced CORT secretion increased less in 2012 (a more severe winter) than in 2009 also highlights the role of habitat quality in facilitating survival during cold weather. The higher baseline CORT in the young forests during cold weather may indicate a physiological response to meet higher energy requirements to compensate for decreased food availability, while the reduced handling-induced CORT secretion may be a response to avoid the deleterious effects that high CORT concentrations have on the physiological condition of individuals (Kitaysky et al. 2003; Blas et al. 2007; Spencer and Verhulst 2007, 2008; Müller et al. 2009), as suggested by the lower body mass of birds in the young forest.
We suggest that levels of handling-induced CORT concentrations revealed in this study and by Silverin et al. (1989) reflect the maximum secretion capability in willow tits. This is supported by the significant differences in handling-induced CORT found in willow tits between habitats and weather conditions. Thus, the most likely explanation for our results is a reduction in the responsiveness of the HPA axis. This can occur through inhibiting the hypothalamic-releasing hormone, suppressing adrenocorticotropin, or diminishing levels of both hormones, which can be followed by a decline in the capacity of the adrenocortical tissue to react to those hormones (Sapolsky et al. 2000; Romero 2004; Charmandari et al. 2005; Smith and Vale 2006). These responses allow an organism to avoid the impairment that high concentrations of CORT can have on the immune system, nerve cell function, and muscle catabolism (e.g., Sapolsky 1993; Belden et al. 2005). Such modulation of the CORT stress response has been observed during activities characterized by extremely high levels of CORT secretion, which require considerable energy investment (Wingfield et al. 1983; Wingfield and Silverin 1986; Holberton et al. 1996; Marra and Holberton 1998; Fokidis et al. 2011, 2012; Angelier and Wingfield 2013) and may be associated with protein sparing (Cherel et al. 1988a; Cherel et al. 1988b). In addition, high concentrations of CORT often cause immune suppression which may decrease the memory of the adaptive immune system (Uchoa et al. 2014) and affect skin inflammatory responses and immune cell influx to wounds (Dhabhar and McEwen 1999; Dhabhar 2002). This is especially relevant to wintering birds, because the skin acts as a barrier against the influences of the biotic and abiotic environments such as invading pathogens and subzero temperatures (Dhabhar and McEwen 1999).
Thus, the quality of a winter habitat is crucial: better habitats might lessen interference competition for food resources and provide cover against attacking predators (Ekman 1998). These effects are relevant to parids, because skin wounds may be a common consequence of failed predation attempts and territorial conflicts, which are common stressors experienced by prey living in groups (Dhondt 2011). Future research should test whether the decline in handling-induced CORT secretion in willow tits wintering in suboptimal habitats (i.e., young forests and cold conditions) is an adaptation to avoid using skeletal muscle protein to cover metabolic requirements or to facilitate skin inflammatory responses and immune cell influx to wounds.
In the present study, we found that subcutaneous fat reserves increased in subordinate birds and remained the same in alpha males under cold weather conditions, while muscle scores decreased for all willow tits. The decrease in the muscle score and the increase in handling-induced CORT suggest a response to stressors which promotes gluconeogenesis, resulting in a source of glucose substrates from non-carbohydrate sources such as skeletal muscle during considerable transitions in weather conditions (Cherel et al. 1988a; Cherel et al. 1988b). In high latitudes, thermoregulatory adjustments of birds should be sufficient to ensure survival under extreme ambient temperatures, which cause great levels of stress and higher mortality under conditions of unexpected cold spells. This is especially true in the case of dominance-structured groups where the competitive superiority of dominant individuals may add uncertainty to feeding opportunities and survival prospects of subordinate flock members. However, in non-hoarding great tits, dominants usually respond to low temperatures by increasing their subcutaneous fat reserves, whereas subdominant birds tend to decrease the amount of their underskin fat (Krams et al. 2010a, 2013). The substantial differences in fattening strategies of food hoarding willow tit and great tit, a non-hoarding species, may indicate the influence of caching behavior and hoarded food reserves on survival strategies and stress responses. All these possibilities need to be addressed in future research.
Overall, our study provides evidence for the role that sociality and dominance hierarchies within the groups have on winter energetics and survival of subordinate individuals. However, we did not find any significant interaction between age and sex in willow tit CORT secretion. This was what we expected, since juvenile males usually dominate adult females in parids, with the effects of age and sex consequently canceling each other out (Saitou 1979; Gosler 1996; Krams 1998b). The lack of interaction between age and sex in CORT secretion may be further explained by mate protection in parids (Hogstad 1995; Krams et al. 2006). Because alpha pairs of willow tits (consisting of adults in this study) stay within their territory year-round and remain mated across years (Hogstad 2015), these individuals have close relationships, with males protecting their mates and females receiving less aggression from other flock members (Hogstad 1992). The alpha females also have a higher foraging rate when accompanied by their mate (Hogstad 2015).
Elevated levels of baseline CORT of subordinate willow tits may have adverse effects on their immune system, metabolic mechanisms, and behavior, especially in suboptimal habitats in years with cold winters (Saino et al. 2003; Spencer and Verhulst 2007, 2008; Müller et al. 2009). However, higher baseline CORT levels may allow subordinate birds to modify their behavioral and physiological reactions to cope with unexpected environmental changes (Angelier and Wingfield 2013). For example, elevated baseline CORT levels may enhance foraging activity of subordinate individuals under low temperatures while it might be less important for dominant individuals in the flock because they always have an optimal access to food resources. Such glucocorticoid stress responses may be partly heritable and their mechanism is likely selected to optimize survival and fitness of individual birds (Breuner et al. 2008; Angelier et al. 2011; Krause et al. 2016b; Lattin et al. 2016). To distinguish between positive and negative effects of high baseline CORT levels, it would be important to find out (among other things) if there is a link between environmentally increased secretion of baseline CORT and shortening of telomeres via oxidative damage, which has already been observed in domestic (Haussmann et al. 2012; Tissier et al. 2014) and wild birds (Quirici et al. 2016).
Taken together, the observed changes in survival and CORT secretion in wintering willow tits reflect responses to habitat quality and suggest young managed coniferous forests to be a suboptimal habitat for this species. If habitat degradation acts as a stressor and leads to negative long-term consequences for individual birds, it may also affect bird populations. Across the distribution range of willow tits, previously continuous forests have been turned into managed forests, where small forested patches are usually separated by clear-cuts and young successional forests (Gustafson and Parker 1992). These changes may result in food shortage for forest dwellers (Zanette et al. 2000; Krams et al. 2001), explaining the dramatic decline of willow tit populations (Lewis et al. 2007).
References
Addis EA, Davis JE, Miner BE, Wingfield JC (2011) Variation in circulating corticosterone levels is associated with altitudinal range expansion in a passerine bird. Oecologia 167:369–378
Angelier F, Ballentine B, Holberton RL, Marra PP, Greenberg R (2011) What drives variation in the corticosterone stress response between subspecies? A common garden experiment of swamp sparrows (Melospiza Georgiana). J Evol Biol 24:1274–1283
Angelier F, Wingfield JC (2013) Importance of the glucocorticoid stress response in a changing world: theory, hypotheses and perspectives. Gen Comp Endocrinol 190:118–128
Bairlein F (1995) European-African songbird migration network. Manual of field methods. European Science Foundation, Wilhelmshaven
Bates D, Mächler, M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Belden LK, Moore IT, Wingfield JC, Blaustein AR (2005) Corticosterone and growth in Pacific treefrog (Hyla regilla) tadpoles. Copeia 2:424–430
Bergeon Burns CM, Rosvall KA, Hahn TP, Demas GE, Ketterson ED (2014) Examining sources of variation in HPG axis function among individuals and populations of the dark-eyed junco. Horm Behav 65:179–187
Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA (2007) Stress response during development predicts fitness in a wild, long-lived vertebrate. Proc Natl Acad Sci USA 104:8880–8884
Boonstra R (2005) Equipped for life: the adaptive role of the stress axis in male mammals. J Mammal 86:236–247
Boonstra R (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27:11–23
Breuner CW, Patterson SH, Hahn TP (2008) In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol 157:288–295
Briedis M, Hahn S, Adamík P (2017) Cold spell en route delays spring arrival and decreases apparent survival in a long-distance migratory songbird. BMC Ecol 17:11
Charmandari E, Tsigos C, Chrousos G (2005) Endocrinology of the stress response. Annu Rev Physiol 67:259–284
Cherel Y, Leloup J, Le Maho Y (1988a) Fasting in the king penguin. II. Hormonal and metabolic changes during molt. Am J Phys 23:R178–R184
Cherel Y, Robin JP, Walch O, Karmann H, Netchitailo P, Le Maho Y (1988b) Fasting in the king penguin. I. Hormonal and metabolic changes during breeding. Am J Phys 23:R170–R177
Creel S, Dantzer B, Goymann W, Rubenstein DR (2013) The ecology of stress: effects of the social environment. Funct Ecol 27:66–80
Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2:cou023
de Bruijn R, Romero LM (2011) Behavioral and physiological responses of wild-caught European starlings (Sturnus vulgaris) to a minor, rapid change in ambient temperature. Comp Biochem Physiol A Mol Integr Physiol 160:260–266
de Bruijn R, Romero LM (2013) Artificial rain and cold wind act as stressors to captive molting and non-molting European starlings (Sturnus vulgaris). Comp Biochem Physiol A Mol Integr Physiol 164:512–519
Dhabhar FS (2002) A hassle a day may keep the doctor away: stress and the augmentation of immune function. Integr Comp Biol 42:556–564
Dhabhar FS, McEwen BS (1999) Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A 96:1059–1064
Dhondt AA (2011) Interspecific competition in birds. Oxford University Press, Oxford
Eaton MA, Brown AF, Noble DG, Musgrove AJ, Hearn RD, Aebischer NJ, Gibbons DW, Evans A, Gregory RD (2009) Birds of conservation concern 3: the population status of birds in the United Kingdom, Channel Islands and Isle of Man. Br Birds 102:296–341
Ekman J (1998) Ecology of non-breeding social systems of Parus. Wilson Bull 10:263–288
Ekman JB, Askenmo CEH (1984) Social rank and habitat use in willow tit groups. Anim Behav 32:508–514
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fokidis HB, des Roziers MB, Sparr R, Rogowski C, Sweazea K, Deviche P (2012) Unpredictable food availability induces metabolic and hormonal changes independent of food intake in a sedentary songbird. J Exp Biol 215:2920–2930
Fokidis HB, Hurley L, Rogowski C, Sweazea K, Deviche P (2011) Effects of captivity and body condition on plasma corticosterone, locomotor behavior, and plasma metabolites in curve-billed thrashers. Physiol Biochem Zool 84:595–606
Fuller RJ, Noble DG, Smith KW, Vanhinsbergh D (2005) Recent declines in populations of woodland birds in Britain: a review of possible causes. Br Birds 98:116–143
Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J (2014) Extreme weather event in spring 2013 delayed breeding time of great tit and blue tit. Int J Biometeorol 58:2169–2173
Gosler AG (1996) Environmental and social determinants of winter fat storage in the great tit Parus major. J Anim Ecol 65:1–17
Grava T, Fairhurst GD, Avey MT, Grava A, Bradley J, Avis JL, Bartolotti GR, Sturdy CB, Otter KA (2013) Habitat quality affects early physiology and subsequent neuromotor development of juvenile blackcapped chickadees. PLoS One 8:e71852
Griesser M, Nystrand M, Eggers S, Ekman J (2007) Impact of forestry practices on fitness correlates and population productivity in an open-nesting bird species. Conserv Biol 21:767–774
Gunnarsson B (1990) Vegetation structure and the abundance and size distribution of spruce-living spiders. J Anim Ecol 59:743–752
Gustafson EJ, Parker GR (1992) Relationship between landcover proportion and indices of landscape spatial pattern. Landsc Ecol 7:101–110
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Harrap S, Quinn D (1996) Chickadees, tits, nuthatches, and treecreepers. Princeton University Press, Princeton
Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM (2012) Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B 279:1447–1456
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 6:603–621
Hewson CM, Amar A, Lindsell JA, Thewlis RM, Butler S, Smith K, Fuller RJ (2007) Recent changes in British woodland bird populations derived from the Repeat Woodland Bird Survey. Ibis 149(Suppl. 2):14–28
Hogstad O (1988a) Advantages of social foraging of willow tits Parus montanus. Ibis 130:275–283
Hogstad O (1988b) Social rank and antipredator behaviour of willow tits Parus montanus in winter flocks. Ibis 130:45–56
Hogstad O (1992) Mate protection in alpha pairs of wintering willow tits, Parus montanus. Anim Behav 43:323–328
Hogstad O (1995) Alarm calling by willow tits, Parus montanus, as mate investment. Anim Behav 49:221–225
Hogstad O (2015) Social behaviour in the non-breeding season in great tits Parus major and willow tits Poecile montanus: differences in juvenile birds’ route to territorial ownership, and pair-bond stability and mate protection in adults. Ornis Norv 38:1–8
Holberton RL, Able KP (2002) Differential migration and an endocrine response to stress in wintering dark-eyed juncos (Junco hyemalis). Proc R Soc Lond B 268:1889–1896
Holberton RL, Parrish JD, Wingfield JC (1996) Modulation of the adrenocortical stress response in Neotropical migrants during autumn migration. Auk 113:558–564
Homan RB, Regosin JV, Rodrigues DM, Reed JM, Windmiller BS, Romero LM (2003) Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim Conserv 6:11–18
Hõrak P, Tummeleht L, Talvik H (2006) Predator threat, copulation effort and immunity in male rats (Rattus norvegicus). J Zool 268:9–16
Jansson C, Ekman J, von Brömssen A (1981) Winter mortality and food supply in tit Parus spp. Oikos 37:313–322
Järvinen A (1982) Ecology of the Siberian tit Parus cinctus in NW Finnish Lapland. Ornis Scand 13:47–55
Jenni-Eiermann S, Glaus E, Grüebler M, Schwabl H, Jenni L (2008) Glucocorticoid response to food availability in breeding barn swallows (Hirundo rustica). Gen Comp Endocrinol 155:558–565
Ketterson ED, Fudicker AM, Atwell JW, Greives TJ (2015) Seasonal timing and population divergence: when to breed, when to migrate. Curr Opin Behav Sci 6:50–58
Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC (2003) Benefits and costs of increased levels of corticosterone in seabird chicks. Horm Behav 43:140–149
Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF (2001) Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J Comp Physiol B 8:701–709
Koivula K, Orell M (1988) Social rank and winter survival in the willow tit Parus montanus. Ornis Fenn 65:114–120
Koivula K, Welling P, Rytkönen S (1995) Differences in mate guarding between age classes in the willow tit, Parus montanus. Anim Behav 49:852–854
Krama T, Krams R, Cīrule D, Moore FR, Rantala MJ, Krams IA (2015) Intensity of haemosporidian infection of parids positively correlates with proximity to water-bodies, but negatively with host survival. J Ornithol 156:1075–1084
Krams IA (1996) Predation risk and shifts of foraging sites in mixed willow and crested tit flocks. J Avian Biol 27:153–156
Krams I (1998a) Rank-related fattening strategies of willow tit Parus montanus and crested tit P. cristatus mixed flock members. Ornis Fenn 75:19–26
Krams I (1998b) Individuals adjust their body reserves to dominance position within mixed flocks of the willow tit (Parus montanus) and the crested tit (P. cristatus): a field experiment. Polish. J Ecol 46:207–216
Krams I (2001) Seeing without being seen: a removal experiment with mixed flocks of willow and crested tits Parus montanus and cristatus. Ibis 143:476–481
Krams I (2002) Mass-dependent take-off ability in wintering great tits (Parus major): comparison of top-ranked adult males and subordinate juvenile females. Behav Ecol Sociobiol 51:345–349
Krams I, Cīrule D, Krama T, Hukkanen M, Rytkönen S, Orell M, Iezhova T, Rantala MJ, Tummeleht L (2010b) Effect of forest management on haematological parameters, blood parasites, and reproductive success of the Siberian tit Poecile cinctus in northern Finland. Ann Zool Fenn 47:335–346
Krams I, Cīrule D, Suraka V, Krama T, Rantala MJ, Ramey G (2010a) Fattening strategies of wintering great tits support the optimal body mass hypothesis under conditions of extremely low ambient temperature. Funct Ecol 24:172–177
Krams I, Cīrule D, Vrublevska J, Nord A, Rantala MJ, Krama T (2013) Nocturnal loss of body reserves reveals high survival risk for subordinate great tits wintering at extremely low ambient temperatures. Oecologia 172:339–346
Krams I, Krama T, Igaune K (2006) Alarm calls of wintering great tits Parus major: warning of mate, reciprocal altruism or a message to the predator? J Avian Biol 37:132–136
Krams IA, Krams T, ČernihoviČs J (2001) Selection of foraging sites in mixed willow and crested tit flocks: rank-dependent strategies. Ornis Fenn 78:1–11
Krause JS, Chmura HE, Pérez JH, Quach LN, Asmus A, Word KR, McGuigan MA, Sweet SK, Meddle SL, Gough L, Boelman N, Wingfield JC (2015) Breeding on the leading edge of a northward range expansion: differences in morphology and the stress response in the arctic Gambel's white-crowned sparrow. Oecologia 180:33–44
Krause JS, Pérez JH, Chmura HE, Meddle SL, Hunt KE, Gough L, Boelman N, Wingfield JC (2016a) The stress response is attenuated during inclement weather in parental, but not in pre-parental, Lapland longspurs (Calcarius lapponicus) breeding in the Low Arctic. Horm Behav 83:68–74
Krause JS, Pérez JH, Chmura HE, Sweet SK, Meddle SL, Hunt KE, Gough L, Boelman N, Wingfield JC (2016b) The effect of extreme spring weather on body condition and stress physiology in Lapland longspurs and white-crowned sparrows breeding in the Arctic. Gen Comp Endocrinol 237:10–18
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: tests in linear mixed effects models. R package version 2.0–30. http://CRAN.R-project.org/package=lmerTest
Laaksonen M, Lehikoinen E (1976) Age determination of willow and crested tit Parus montanus and P.cristatus. Ornis Fenn 53:9–14
Landys MM, Ramenofsky M, Wingfield JC (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148:132–149
Lattin CR, Breuner CW, Romero LM (2016) Does corticosterone regulate the onset of breeding in free-living birds?: the CORT-flexibility hypothesis and six potential mechanisms for priming corticosteroid function. Horm Behav 78:107–120
Lenth RV (2016) Least-squares means: the R Package lsmeans. J Stat Softw 69:1–33
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lewis AJG, Amar A, Cordi-Piec D, Thewlis RM (2007) Factors influencing willow tit Poecile montanus site occupancy: a comparison of abandoned and occupied woods. Ibis 149(Suppl. 2):205–213
Marra PP, Holberton RL (1998) Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116:284–292
Mazerolle DF, Hobson KA (2002) Physiological ramifications of habitat selection in territorial male ovenbirds: consequences of landscape fragmentation. Oecologia 130:356–363
McEwen BS (2000) The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886:172–189
Müller C, Jenni-Eiermann S, Jenni L (2009) Effects of a short period of elevated circulating corticosterone on postnatal growth in free-living Eurasian kestrels Falco tinnunculus. J Exp Biol 212:1405–1412
Niemi G, Hanowski J, Helle P, Howe R, Mönkkönen M, Venier L, Welsh D (1998) Ecological sustainability of birds in boreal forests. Conserv Ecol 2:17
Ots I, Murumagi A, Hõrak P (1998) Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Funct Ecol 12:700–707
Peres CA (2001) Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv Biol 15:1490–1505
Poisbleau M, Fritz H, Guillon N, Chastel O (2005) Linear social dominance hierarchy and corticosterone responses in male mallards and pintails. Horm Behav 47:485–492
Pravosudov VV, Kitaysky AS, Wingfield JC, Clayton NS (2001) Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli). Gen Comp Endocrinol 123:324–331
Pravosudov VV, Mendoza SP, Clayton NS (2003) The relationship between dominance, corticosterone, memory, and food caching in mountain chickadees (Poecile gambeli). Horm Behav 44:93–102
Quirici V, Guerrero CJ, Krause JS, Wingfield JC, Vásquez RA (2016) The relationship of telomere length to baseline corticosterone levels in nestlings of an altricial passerine bird in natural populations. Front Zool 13:1
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL https://www.R-project.org/
Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR (2006) Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav 71:39–48
Reinertsen RE, Haftorn S (1986) Different metabolic strategies of northern birds for nocturnal survival. J Comp Physiol B 156:655–663
Rogers CM (1987) Predation risk and fasting capacity: do wintering birds maintain optimal body mass? Ecology 68:1051–1061
Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galápagos marine iguanas during el Ninõ events. PNAS 98:7366–7370
Rogers CM, Ketterson ED, Nolan V Jr (1993) Geographic variation in winter fat of dark-eyed juncos: displacement to a common environment. Ecology 74:1183–1190
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Romero LM, Ramenofsky M, Wingfield JC (1997) Season and migration alters the corticosterone response to capture and handling in an arctic migrant, the white crowned sparrow (Zonotrichia leucophrys gambelii). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 116:171–177
Romero LM, Soma KK, Wingfield JC (1998) Changes in pituitary and adrenal sensitivities allow the snow bunting (Plectrophenax nivalis), an Arctic-breeding song bird, to modulate corticosterone release seasonally. J Comp Physiol B Biochem Syst Environ Physiol 168:353–358
Rowher S, Wingfield JC (1981) A field study of social dominance, plasma levels of luteinizing hormone and steroid hormones in wintering Harris' sparrows. Z Tierpsychol 57:173–183
Rytkönen S, Krams I (2003) Does foraging behaviour explain the poor breeding success of great tits Parus major in northern Europe? J Avian Biol 34:288–297
Saarela S, Klapper B, Heldmaier G (1995) Daily rhythm of oxygen consumption and thermoregulatory responses in some European winter- or summer-acclimatized finches at different ambient temperatures. J Comp Physiol B 165:366–376
Saari L, Pulliainen E, Hildén O, Järvinen A, Mäjusalo I (1994) Breeding biology of the Siberian tit Parus Cinctus in Finland. J Ornithol 135:549–575
Saino N, Suffritti C, Martinelli R, Rubolini D, Møller AP (2003) Immune response co-varies with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica). Behav Ecol 14:318–325
Saitou T (1979) Ecological study of social organization in the great tit, Parus major L. III. Home range of the basic flocks and dominance relationships of the members in a basic flock. Misc Rep Yamashina Inst Orn 11:149–171
Sapolsky RM (1993) Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D (eds) Behavioral endocrinology. MIT Press, Cambridge, Massachusetts, pp 287–324
Sapolsky RM, Pulsinelli WA (1985) Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229:1397–1400
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol Rev 21:55–89
Schulkin J, Morgan MA, Rosen JB (2005) A neuroendocrine mechanism for sustaining fear. Trends Neurosci 28:629–635
Schwabl H, Bairlein F, Gwinner E (1991) Basal and stress-induced corticosterone levels of garden warblers, Sylvia Borin, during migration. J Comp Physiol 161:576–580
Senner NR, Verhoeven MA, Abad-Gómez JM, Gutiérrez JS, Hooijmeijer JCEW, Kentie R, Masero JA, Tibbitts TL, Piersma T (2015) When Siberia came to the Netherlands: the response of continental black-tailed godwits to a rare spring weather event. J Anim Ecol 84:1164–1176
Silverin B (1997) The stress response and autumn dispersal behaviour in willow tits. Anim Behav 53:451–459
Silverin B, Viebke P-A, Westin J (1984) Plasma levels of luteinizing hormone and steroid hormones in free-living winter groups of willow tits (Parus montanus). Horm Behav 18:367–379
Silverin B, Viebke PA, Westin J (1989) Hormonal correlates of migration and territorial behavior in juvenile willow tits during autumn. Gen Comp Endocrinol 75:148–156
Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8:383–395
Smith GT, Wingfield JC, Veit RR (1994) Adrenocortical response to stress in the common diving petrel, Pelecanoides urinatrix. Physiol Zool 67:526–537
Snow DW, Perrins CM (1998) The birds of the Western Palearctic. Concise edition. Oxford University Press, Oxford
Spencer KA, Verhulst S (2007) Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata). Horm Behav 51:273–280
Spencer KA, Verhulst S (2008) Post-natal exposure to corticosterone affects standard metabolic rate in the zebra finch (Taeniopygia guttata). Gen Comp Endocrinol 159:250–256
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Suhonen J (1993) Predation risk influences the use of foraging sites by tits. Ecology 74:1197–1203
Suorsa P, Helle H, Koivunen V, Huhta E, Nikula A, Hakkarainen H (2004) Effects of forest patch size on physiological stress and immunocompetence in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris): an experiment. Proc R Soc B 271:435–440
Suorsa P, Huhta E, Nikula A, Nikinmaa M, Jäntti A, Helle H, Hakkarainen H (2003) Forest management is associated with physiological stress in an old-growth forest passerine. Proc R Soc B 270:963–969
Tilgar V, Saag P, Moks K (2009) Development of stress responses in nestling pied flycatchers. J Comp Physiol A 195:799–803
Tissier ML, Williams TD, Criscuolo F (2014) Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata). Plos One 9:e97705
Uchoa ET, Aguilera G, Herman JP, Fiedler JL, Deak T, de Sousa MB (2014) Novel aspects of glucocorticoid actions. J Neuroendocrinol 26:557–572
Veistola S, Lehikoinen E, Eeva T (1997) Weather and breeding success at high latitudes—the pied flycatcher Ficedula hypoleuca and the Siberian tit Parus cinctus. Ornis Fenn 74:89–98
Vinogradova HV, Dolnik VR, Jefremov VD, Paevskii VA (1976) Identification of sex and age of passerine birds of the Fauna of the USSR. Nauka, Moscow
Virkkala R (1987) Effects of forest management on birds breeding in northern Finland. Anns Zool Fenn 24:281–294
Virkkala R (1988) Foraging niches of foliage-gleaning birds in the northernmost taiga in Finland. Ornis Fenn 65:104–113
Virkkala R (1990) Ecology of the Siberian tit Parus cinctus in relation to habitat quality: effects of forest management. Ornis Scand 21:139–146
Virkkala R, Liehu H (1990) Habitat selection by the Siberian tit Parus cinctus in virgin and managed forests in northern Finland. Ornis Fenn 67:1–12
Walker BG, Meddle SL, Romero LM, Landys MM, Reneerkens J, Wingfield JC (2015) Breeding on the extreme edge: modulation of the adrenocortical response to acute stress in two high arctic passerines. J Exp Zool A Ecol Genet Physiol 323:266–275
Wasser SK, Bevis K, King G, Hanson E (1997) Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv Biol 11:1019–1022
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21:38–46
Wingfield JC (1985) Influences of weather on reproductive function in male song sparrows, Melospiza melodia. J Zool 205:525–544
Wingfield JC, Silverin B (1986) Effects of corticosterone on territorial behaviour of free-living male song sparrows, Melospiza melodia. Horm Behav 20:405–417
Wingfield JC, Moore MC, Farner DS (1983) Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk 100:56–62
Wingfield JC, Vleck CM, Moore MC (1992) Seasonal changes in the adrenocortical response to stress in birds of the Sonoran desert. J Exp Zool 264:419–428
Wingfield JC, Suydam R, Hunt K (1994a) Adrenocortical responses to stress in snow buntings and Lapland longspurs at barrow, Alaska. Comp Biochem Physiol 108:299–306
Wingfield JC, Deviche P, Sharbaugh S, Astheimer LB, Holberton R, Suydam R, Hunt K (1994b) Seasonal changes of the adrenocortical responses to stress in redpolls, Acanthis Flammea, in Alaska. J Exp Zool 270:372–380
Wingfield JC, Kubokawa K, Ishida K, Ishii S, Wada M (1995) The adrenocortical response to stress in male bush warblers, Cettia diphone: a comparison of breeding populations in Honshu and Hokkaido, Japan. Zool Sci 12:615–621
Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn SE, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone–behavior interactions: the “emergency life history stage”. Am Zool 38:191–206
Wingfield JC, Krause JS, Perez JH, Chmura HE, Németh Z, Word KR, Calisi RM, Meddle SL (2015) A mechanistic approach to understanding range shifts in a changing world: what makes a pioneer? Gen Comp Endocrinol 222:44–53
Zanette L, Doyle P, Trémont SM (2000) Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology 81:1654–1666
Acknowledgements
We thank Todd M. Freeberg for valuable comments which greatly improved the manuscript.
Funding
The study was supported by two grants of the Latvian Council of Science (No. 07.2100 and 290/2012) and a grant of the Estonian Research Council (No. PUT1223) to I.A.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All animal manipulations were carried out in accordance with the legal and ethical standards of the Republic of Latvia. The project had the permission of the Nature Conservation Agency of the Republic of Latvia (No. 16/2012).
Additional information
Communicated by: Alxandre Roulin
Rights and permissions
About this article
Cite this article
Cīrule, D., Krama, T., Krams, R. et al. Habitat quality affects stress responses and survival in a bird wintering under extremely low ambient temperatures. Sci Nat 104, 99 (2017). https://doi.org/10.1007/s00114-017-1519-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-017-1519-8