Abstract
Superfoetation is the ability of females to simultaneously bear multiple broods of embryos at different developmental stages. Most studies on the phylogenetic distribution of superfoetation and on the factors that potentially promote superfoetation ignore variation within species. Here, we studied 11 populations of two species of viviparous fishes of the family Poeciliidae (Poeciliopsis gracilis and Poeciliopsis infans) and document wide variation in superfoetation and in three related life history traits: brood size, individual embryo mass and total reproductive allotment. We found significant differences in the average number of simultaneous broods among populations of P. gracilis but not among populations of P. infans. In addition, we found even greater variation between months within populations for both species, although no specific pattern of temporal variation was evident. Instead of the expected consistency of seasonal differences in superfoetation across populations, we found that large variation among months within seasons and the amount and direction of this monthly variation differed widely between populations. Our results emphasize the importance of including intraspecific variation in superfoetation and other life history traits in studies that aimed at finding general explanations of life history trait evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Superfoetation is the ability of females to simultaneously bear multiple broods of offspring at different developmental stages (Turner 1937; Scrimshaw 1944; Roellig et al. 2011). This reproductive strategy has been documented in many taxa, including angiosperm plants (Kennedy 1978), viviparous fishes (Scrimshaw 1944; Reznick et al. 2007) and in at least ten different orders of mammals (Roellig et al. 2011), including humans (Pape et al. 2008; Lantieri et al. 2010). In altricial birds, clutch overlap represents a similar phenomenon because the parents provide care to distinct sets of offspring in different stages of development (Burley 1980). Superfoetation is common in viviparous fishes (Turner 1937; Scrimshaw 1944; Thibault and Schultz 1978) and has been reported in at least three unrelated families: Clinidae (Gunn and Thresher 1991), Zenarchopteridae and Poeciliidae (Reznick and Miles 1989; Reznick et al. 2007). Within the Poeciliidae, superfoetation occurs in several genera such as Poeciliopsis, Heterandria, Neoheterandria (Reznick and Miles 1989; Reznick et al. 1992; Pollux et al. 2009) and Poecilia (subgenus Micropoecilia; Pires et al. 2010), whereas other genera do not include superfoetating species (e.g. Belonesox, Brachyrhaphis and Xiphophorus; Reznick and Miles 1989; Pollux et al. 2009). This phylogenetic distribution suggests that, in fishes and particularly within Poeciliidae, superfoetation has evolved multiple times independently (Reznick and Miles 1989; Reznick et al. 2007; Pollux et al. 2009).
The number of broods present within a female (i.e. degree of superfoetation) varies substantially among species. For instance, female Poeciliopsis baenschi bear two to three simultaneous broods (Molina-Moctezuma 2011), whereas female Heterandria formosa can bear up to eight broods in different stages of development (Travis et al. 1987). Additionally, a few studies have documented intraspecific variation in superfoetation (Johnson and Bagley 2011). In the laboratory, Travis et al. (1987) found that female H. formosa experimentally given high food levels had a significantly greater incidence of superfoetation than females given restricted amounts of food, whereas Pires et al. (2007) found differences in the degree of superfoetation exhibited by two distinct populations of Poeciliopsis prolifica in captivity. In the field, population differences in superfoetation have been reported in P. baenschi (Molina-Moctezuma 2011), Poecilia branneri (Pires et al. 2010) and Poeciliopsis turrubarensis, which produce more simultaneous broods (and are thus more streamlined) in fast- than in slow-flowing streams (Zúñiga-Vega et al. 2007), whereas seasonal intrapopulation differences in superfoetation have been documented only in H. formosa (Travis et al. 1987; Leips and Travis 1999). As the limited number of the above examples indicates, evidence of intraspecific variation in superfoetation is scarce (Johnson and Bagley 2011).
The comparative method has been used to investigate the evolution of superfoetation (Meredith et al. 2011; Pollux et al. 2009, 2014). However, sampling errors in classification have led to erroneous mapping of the trait onto phylogenies. For example, Pires et al. (2010) recently found superfoetation in the lineage composed of Poecilia bifurca, P. branneri and P. parae, and this represents an additional independent origin of superfoetation that was previously unknown. In addition, more information is needed to clarify the spatial and temporal variation in this reproductive trait. A simple classification of species as either having or not having superfoetation, without assessment of intraspecific variation, may also obscure our inferences about the mode by which superfoetation may respond to the environment or its relation to other phenotypic traits.
Indeed, theoretical models posit that superfoetation should be selected for in particular environments/conditions. Several hypotheses have been proposed to explain the adaptive significance of this reproductive strategy (reviewed in Zúñiga-Vega et al. 2010). One hypothesis contends that superfoetation is beneficial in environments where a streamlined body shape is needed, such as in fast-flowing streams or in habitats where fish must swim fast to escape from predators. Under these situations, superfoetation may allow females to produce a relatively high number of offspring without large increases in body mass or volume (Thibault and Schultz 1978; Zúñiga-Vega et al. 2007). A second hypothesis suggests that superfoetation reduces peak reproductive demand and, therefore, should be favoured in environments where reproduction is costly and resources are scarce. The reasoning behind this hypothesis is that superfoetation may spread reproduction more evenly over time (e.g. superfoetating females produce two or more small broods spaced in time instead of a single large brood), reducing the total reproductive investment made by the female at any particular time (Downhower and Brown 1975; Thibault and Schultz 1978). A third hypothesis proposes that superfoetation increases the rate of offspring production because females overlap different broods (Burley 1980; Travis et al. 1987). For example, during a certain time period, a female without superfoetation may produce a single brood of, say, four newborns, whereas a superfoetating female may overlap two smaller broods of three embryos each, which results in a total of six newborns. According to this hypothesis, if natural selection favours higher fecundity, then females will use any additional amount of resources to produce more newborns, presumably by means of increased superfoetation (Travis et al. 1987).
Given the marked seasonal changes in water flow—and thus in ecology—of streams and rivers (Allan and Castillo 2007), we predict substantial variation in the incidence of superfoetation associated with seasonal changes in the fluvial regime. The predicted changes in superfoetation could result from at least one of the mechanisms proposed by the three hypotheses mentioned above. The first hypothesis predicts that superfoetation should increase during the rainy season, because water flow increases dramatically, and therefore, more superfoetation may result in smaller body mass and volume and improved ability to deal with fast currents. The second hypothesis predicts that superfoetation should decrease when reproduction becomes less costly, which in rivers coincides with those months when primary productivity, and thus food availability, are higher. In subtropical latitudes, during the late dry season, temperatures are warmer, and water volume is low (Allan and Castillo 2007). These circumstances promote productivity (Moss 2013), making reproduction a less costly process and, hence, superfoetation less necessary. We inferred productivity from water physicochemical parameters, since productivity is positively correlated with temperature, and with the concentration of nitrogen in the form of nitrites and nitrates and phosphorous in the form of phosphates. Finally, and contrary to the second hypothesis, the third hypothesis predicts higher superfoetation during the late dry season, because greater amounts of food should be used to increase the number of offspring.
In this study, we examine temporal (monthly) and spatial (among 11 populations) variation in superfoetation and related life history traits (brood size, individual embryo mass and reproductive allotment [RA]) of Poeciliopsis gracilis and Poeciliopsis infans, two native Mexican poeciliids (Miller et al. 2005). We aim to find seasonal patterns, consistent across populations, which could provide support for one of the hypotheses that attempt to explain the adaptive significance of superfoetation.
Material and methods
Study species
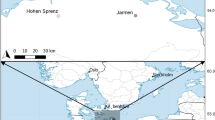
P. gracilis is native to basins in the Atlantic (Gulf) slope of Mexico, mainly those of rivers Coatzacoalcos and Papaloapan, in the Mexican states of Veracruz and Oaxaca, although it has been introduced and successfully colonized some basins of rivers in western and central Mexico (Gutiérrez-Cabrera et al. 2005; Miller et al. 2005). It is found in most types of water bodies of varying turbidity and water flow (Miller et al. 2005). In one of the localities where it has been introduced, Gómez-Márquez et al. (2008) found that only 25 % of the females bore simultaneous broods. We collected female P. gracilis from eight different localities within its original geographic range and from three newly colonized localities north of its native range (sites 1–3; Fig. 1; Table 1).
Poeciliopsis infans is native to basins of the Pacific slope of Mexico, mostly found in the Lerma-Santiago catchment, but also in the basins of the Ameca, Armeria, Coahuayana and Balsas rivers (Mateos et al. 2002; Galindo-Villegas and Sosa-Lima 2002; Miller et al. 2005). It is also found in a variety of water bodies of varying turbidity and water flow. It has been reported that females can bear two simultaneous broods (Turner 1937). Females were collected from 11 different populations throughout the states of Jalisco and Zacatecas (Fig. 1; Table 1).
Field methods
Collections of both species and of water chemistry were made during the dry (November–May) and rainy (June–October) seasons (see Table 1). Every effort was made to collect at least 20 mature females per locality on each visit using seine nets (1.3-m depth × 5-m length, 8-mm mesh). Captured fish were anaesthetized with 3-amenobenzoic acid ethyl ester (MS-222™), sacrificed by immersion in 95 % ethanol and taken to the laboratory, where they were stored in 70 % ethanol. We did not assess productivity directly, but at each site collected data on water physicochemistry which are correlated with it (Moss 2013). At each visit, we measured in situ temperature (°C), pH, salinity (g/Kg), acidity (mg/L) and the concentration of phosphorus, phosphates, nitrites, nitrates and ammonium (mg/L) using a multiparameter “HI 83200”, (Hanna Instruments). All field and laboratory procedures were approved by the Mexican fisheries and environmental agencies (Comisión Nacional de Acuacultura y Pesca and Secretaría de Medio Ambiente y Recursos Naturales). Sample sizes are shown in Table 1. Lack of collections in some months in particular populations was due to logistical constraints such as lack of field crew or flooded (inaccessible) rivers after hurricanes (e.g. most rivers in September 2013; Table 1).
Quantifying life history traits
All females were dissected, and if found pregnant, we quantified superfoetation (number of broods in different developmental stages) and brood size (number of developing embryos per brood), measured individual embryo mass and calculated RA following Reznick and Endler (1982) and Zúñiga-Vega et al. (2007). Embryos which shared developmental stage (as per Haynes 1995) were counted to obtain the number of embryos per brood. Individual embryo mass was measured by drying the entire brood for 24–48 h at 55 °C, weighing it (Sartorius™ LA120S, ±0.05 mg) and dividing brood dry mass by the number of embryos. RA was calculated as the total dry weight of all the broods borne by the female. Additionally, we measured the female dry mass (24–48 h at 55 °C) excluding the digestive tract.
Statistical analyses
To estimate variation among populations and between months within populations in life history traits, we applied general linear models with “population” and “month” (nested within population) as the two main factors and number of simultaneous broods (superfoetation), brood size, individual embryo mass and RA as response variables. We conducted one model per response variable per species using STATISTICA™ 7.0 (StatSoft). All models included female dry mass as a covariate, and the models to evaluate variation in embryo mass had as an additional covariate the stage of development. In addition, we included in all models the interaction between “month” (nested within “population”) and female dry mass to account for temporal and spatial differences in the way that the studied life history traits covary with female size. Since estimates from simultaneous broods are not independent, we randomly choose one brood from each superfoetating female, thus ensuring that each female was represented only once in the analyses of brood size and individual embryo mass. Only data from pregnant females were used.
Number of simultaneous broods (superfoetation) and brood size were square-root transformed, and individual embryo mass and RA were log-transformed to meet assumptions of normality and homogeneity of variances. An additional set of equivalent linear models were conducted on untransformed data to generate graphs and least-square means in the original scale of the variables in order to facilitate interpretation. Least-square means derived from general linear models represent values adjusted for the effect of the covariates (Sokal and Rohlf 2012). Hence, hereafter, we report mean values per month, population and species adjusted for the effect of female mass (all traits) and developmental stage (individual embryo mass).
Finally, we searched for statistical associations between superfoetation and physicochemical parameters of the rivers by means of Spearman rank correlation coefficients. We used the mean value per population of superfoetation (adjusted for female mass) and of each physicochemical parameter. Correlation coefficients were calculated using JMP™ 7.0 (SAS Institute Inc.).
Results
Both species had similar overall incidence of superfoetation (pregnant P. gracilis, means adjusted for female mass ± SE = 1.9 ± 0.02; range 1–4; P. infans, 1.8 ± 0.02; range 1–4 simultaneous broods across all months and populations). The mean percentages of pregnant females bearing two or more simultaneous broods were 68, 73 and 65 % in non-native populations of P. gracilis (sites 1–3), native populations of P. gracilis and all populations of P. infans, respectively. Pregnant females of both species were found in all months, but the proportion of gravid females varied between months within populations (Table 1). The standard length (SL) of the smallest gravid females was 18.7 (P. gracilis) and 15.4 mm (P. infans).
Spatial and temporal variation in superfoetation
Variation in mean degree of superfoetation between populations was large and substantial for P. gracilis (F 10,861 = 3.53, P = 0.0001; Table 2), with population means ranging from (means adjusted for female mass ± SE) 1.46 ± 0.12 broods per female in population 8 to 2.14 ± 0.08 broods in population 5 (Fig. 2a). Although significant, the effect size of population was weak (partial η2 = 0.04; Table 2). In contrast, for P. infans, variation in superfoetation between populations was not significant (F 10,774 = 1.75, P = 0.07; Table 2), although mean values varied from 1.39 ± 0.11 broods in population 8 to 1.97 ± 0.10 broods in population 2 (Fig. 3a). Female dry mass covaried positively with superfoetation (P. gracilis, β = 3.44 ± 0.66; P. infans, β = 14.65 ± 2.51).
Interpopulation variation in superfoetation and life history traits of Poeciliopsis gracilis. Population means were adjusted for female mass (all traits) and stage of development (individual embryo mass). a Superfoetation, b brood size, c individual embryo mass, d reproductive allotment. Population numbers as in Table 1. Error bars represent ±1 SE
Interpopulation variation in superfoetation and life history traits of Poeciliopsis infans. Population means were adjusted for female mass (all traits) and stage of development (individual embryo mass). a Superfoetation, b brood size, c individual embryo mass, d reproductive allotment. Population numbers as in Table 1. Error bars represent ±1 SE
Superfoetation also varied temporally within populations of P. gracilis (F 49,861 = 3.51, P < 0.0001; Table 2), but we cannot discern any clear temporal pattern in this variation. Neither during particular months nor during the rainy (June–October) or late dry (Mar–May) season was superfoetation consistently higher or lower across populations (Fig. 4). The smallest number of simultaneous broods (1.03 ± 0.25) was found in June among females from population 8, whereas the highest occurred at population 6 in November (2.55 ± 0.16; Fig. 4). The effect size of month was the largest among those of all the factors tested (η2 = 0.17; Table 2).
Temporal variation in superfoetation of Poeciliopsis gracilis and P. infans. Monthly means were adjusted for female mass. Population numbers are shown at the top right corner of each panel. Dotted lines indicate rainy months. Error bars represent ±1 SE. Sampled months for P. gracilis March (Mar), April (Apr), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar) in 2013. Sampled months for P. infans May (May), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar), September (Sep) in 2013
Temporal variation in superfoetation within populations was also significant in P. infans (F 43,774 = 4.79, P < 0.0001; Table 2), but again, variation did not seem be associated with seasons or with rainfall patterns (Fig. 4). The smallest number of simultaneous broods (1.02 ± 0.16) was found in January among females from population 11, whereas the highest occurred at population 10 in September (2.50 ± 0.13; Fig. 4). The effect size of month was the second largest (η2 = 0.21), only after that of female mass (η2 = 0.41; Table 2).
The interaction between month (nested within populations) and female mass had a significant effect on superfoetation of P. infans (F 6,774 = 2.21, P = 0.04) but not P. gracilis (F 6,861 = 1.73, P = 0.11; Table 2). The effect size of this significant interaction affecting superfoetation of P. infans was weak (η2 = 0.02). Variation among rivers in psychochemical parameters could not explain the observed variation in superfoetation as indicated by non-significant correlation coefficients (Table S1).
Spatial and temporal variation in additional life history traits
There was substantial interpopulation variation in brood size, individual embryo mass and RA of P. gracilis (Table 2; Fig. 2b–d), and female dry mass was positively correlated with these three variables (brood size, β = 32.91 ± 4.14; individual embryo mass, β = 0.003 ± 0.0004; RA, β = 0.21 ± 0.01). Females from population 8 produced the smallest broods (means adjusted for female mass ± SE 3.33 ± 0.76 embryos), whereas females from population 1 had the largest (17.72 ± 0.94 embryos; Fig. 2b). Such a large mean brood size was due to the presence in our April sample of three particularly large females (>46 mm SL) bearing broods with more than 50 embryos. Variation in individual embryo mass among populations ranged between (means adjusted for female mass and stage of development) 0.87 ± 0.05 mg in population 4 and 1.27 ± 0.05 mg in population 3 (Fig. 2c). RA ranged between (means adjusted for female mass) 9.26 ± 1.04 mg in population 11 and 22.93 ± 1.93 mg in population 1, again due to the presence of those three very large females (Fig. 2d).
Interpopulation variation in brood size, embryo mass and RA of P. infans was also large (Table 2; Fig. 3b–d), and as with P. gracilis, all three variables were positive functions of female dry mass (brood size, β = 92.28 ± 20.73; individual embryo mass, β = 0.002 ± 0.0002; RA, β = 0.23 ± 0.03). Variation in brood size among populations ranged between 3.08 ± 0.94 embryos in population 6 and 10.64 ± 1.08 embryos in population 5 (Fig. 3b), whereas embryo mass ranged from 0.61 ± 0.11 mg in population 5 to 1.03 ± 0.09 mg in population 8 (Fig. 3c). Females from population 6 made the smallest RA (7.51 ± 1.29 mg), whereas those from population 5 made the largest (14.40 ± 1.48 mg; Fig. 3d). We found a trade-off between number and size of embryos in both species as evidenced by significant negative correlations between average values per population of brood size and individual embryo mass (Fig. 5).
Life history traits of P. gracilis also varied between months within populations (Table 2), but again, this variation was neither consistent across populations nor linked with seasons in any obvious way (Figs. 6 and 7). Mean brood size and RA of females from population 1 were notably larger in April 2012 (Fig. 7), when the three large females were collected. As with its congener, life history traits of P. infans varied between months within populations (Table 2), and once more, no consistent pattern of temporal variation was evident (Figs. 6 and 7). Month (nested within population) was the strongest predictor of the three traits for both P. gracilis (brood size, partial η2 = 0.37; individual embryo mass, partial η2 = 0.17; RA, partial η2 = 0.40) and P. infans (brood size, partial η2 = 0.26; individual embryo mass, partial η2 = 0.16; RA, partial η2 = 0.37; Table 2).
Temporal variation in brood size and individual embryo mass of Poeciliopsis gracilis and P. infans. Monthly means were adjusted for female mass (both traits) and stage of development (individual embryo mass). Population numbers are shown at the top right corner of each panel. Dotted lines indicate rainy months. Error bars represent ±1 SE. Non-visible error bars are contained within the symbols. Sampled months for P. gracilis March (Mar), April (Apr), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar) in 2013. Sampled months for P. infans May (May), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar), September (Sep) in 2013
Temporal variation in reproductive allotment of Poeciliopsis gracilis and P. infans. Monthly means were adjusted for female mass. Population numbers are shown at the top right corner of each panel. Dotted lines indicate rainy months. Error bars represent ±1 SE. Non-visible error bars are contained within the symbols. Sampled months for P. gracilis March (Mar), April (Apr), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar) in 2013. Sampled months for P. infans May (May), June (Jun), September (Sep), November (Nov) in 2012, January (Jan), March (Mar), September (Sep) in 2013
The relationships between female size and life history traits varied between months within populations for P. gracilis as indicated by significant female dry mass × month interactions (Table 2). However, the differences between months in the slopes of these relationships were small (brood size, partial η2 = 0.02; individual embryo mass, partial η2 = 0.02; RA, partial η2 = 0.03). In contrast, these interactions were not significant for P. infans, revealing that the effect of female mass on life history traits was consistent across months.
Discussion
Spatial and temporal variation in superfoetation
Our data reveal a substantial amount of variation in the degree of superfoetation among and within populations of P. gracilis as well as within populations of P. infans. This adds to the still small number of studies reporting population variation in the number of simultaneous broods that females bear (Johnson and Bagley 2011). In spite of substantial spatial variation in water physicochemistry, we did not find any association between the correlates of water productivity (temperature, phosphorous, phosphates, nitrites, nitrates) and superfoetation in either species. This is unlikely to indicate that productivity is irrelevant for superfoetation and may instead be consequence of individual variation/plasticity in the strategic reproductive responses of females. In addition, spatial variation in water temperature could promote the observed interpopulation differences in superfoetation not only through its effect on primary productivity but also through its potential effect on the average size of adult females (Vondracek et al. 1988). In other words, warmer rivers may result in larger females, and as our results indicate, larger females of both species bear more simultaneous broods. However, variation among populations in female mass was not statistically associated with variation among rivers in water temperature (Fig. S1). Further work should evaluate the possibility that the observed local variation in superfoetation is linked to differences among rivers in water velocity (e.g. Zúñiga-Vega et al. 2007), in food availability (e.g. Travis et al. 1987) or in age-specific mortality rates (e.g. due to differences in predation; Downhower and Brown 1975).
Monthly differences in superfoetation—and in the additional life history traits—were larger in both species than those observed among populations (Table 2). We anticipated that temporal consistence between populations would help inferring the underlying causes of variation in superfoetation. According to the first hypothesis that contends that superfoetation is beneficial in environments where a streamlined body shape is needed (Thibault and Schultz 1978; Zúñiga-Vega et al. 2007), increased superfoetation was expected during the rainy season. However, a detailed examination of Fig. 4 reveals that, although rivers carried a greater water volume in the rainy months, females collected in this season did not bear, on average, more broods simultaneously than females captured during the dry season. The second hypothesis suggests that superfoetation reduces peak reproductive demand for pregnant females (Downhower and Brown 1975; Thibault and Schultz 1978). Hence, less superfoetation was expected during the late dry season when food availability is highest and reproductive costs are lowest. Our data did not support this hypothesis either because females did not bear, on average, less simultaneous broods during these dry months when reproduction is presumably less costly (Fig. 4). Thus, superfoetation does not appear to be the result of reproductive costs. The third hypothesis suggests that superfoetation increases the rate of offspring production (Burley 1980; Travis et al. 1987). Therefore, given the higher food availability during the late dry season, females should use these additional resources to produce more offspring by means of increased superfoetation. Again here, we found no support for this hypothesis. We did not find consistently higher superfoetation during these late dry months (Fig. 4).
Our (rather standard) methods to quantify temporal variation in superfoetation being destructive, we are unable to address the question of whether this lack of temporal consistency across populations is the result of (1) phenotypic plasticity, (2) maternal effects priming the breeding strategy of females of the subsequent cohorts or (3) different genotypes breeding in different seasons. The lifespan of similar-sized poeciliids (up to 5 years in captivity) seems long enough to allow the same females to breed in different seasons (even years), but the estimates are from laboratory (e.g. Carey and Judge 2000; Tacutu et al. 2013) and should be taken with caution. If wild females live throughout a single year, our results would indicate that they produce different numbers of simultaneous broods in different seasons (i.e. the degree of superfoetation would be a plastic response to the environmental conditions). In addition, genotype × environment interactions could be expected, with individual females differing in their reaction norms. The complex variation that we observed among months within populations is likely the result of a complex interaction between phenotypic plasticity and genetic differences among individuals.
Variability in superfoetation and comparative studies
The comparative studies that have attempted to explain the evolution and maintenance of superfoetation and its relationships with other phenotypic traits have assumed time invariance or lack of variation among populations (Pires et al. 2007; Zúñiga-Vega et al. 2007; Johnson and Bagley 2011). For instance, in their comprehensive summary of life histories within the family Poeciliidae, Reznick and Miles (1989) classified species as either superfoetating or non-superfoetating. Yet, ignoring intraspecific variation did lead to incorrect classifications; these authors put down Poecilia parae as non-superfoetating, but a subsequent study showed that females sometimes bear simultaneous broods (Pires et al. 2010), a finding that revealed an additional independent evolutionary origin of superfoetation (Meredith et al. 2011). Even repeated sampling, if limited, can lead to an underestimate of the number of species that undergo superfoetation. As shown by our data, no superfoetating females were found in our June 2012 sample of populations 1 and 7 of P. gracilis, or in the May 2012 and January 2013 samples of populations 8 and 9 of P. infans, respectively. If these were our only samples, we should have concluded that these species are non-superfoetating (Table 1; Fig. 4).
Adaptive explanations have also ignored temporal variation in superfoetation. Zúñiga-Vega et al. (2007) demonstrated that differences in the degree of superfoetation among populations of P. turrubarensis are partially due to differences in water flow between rivers, as females inhabiting fast-flowing waters produce more simultaneous broods and are more streamlined—and hence their swimming is more energetically efficient—than females inhabiting slow-flowing waters. That study was based, however, on samples taken only in the dry season. As our results of congeneric P. infans and P. gracilis demonstrate, the number of simultaneous broods can vary substantially between months. Thus, the association found between stream flow, body shape and superfoetation in P. turrubarensis might not hold during the wet season, when water flow is greatest. Indeed, physical constraints for reproduction should increase during rainy months also in more lentic habitats, such as the sites that Zúñiga-Vega et al. (2007) classified as “slow-water environments” during the dry season. Therefore, we recommend investigating the evolution and possible adaptive consequences of superfoetation by repeated sampling encompassing all seasons, preferably in different localities. In addition, controlled experiments, in which putative selective agents (e.g. water flow or food availability) are modified, would also provide insight on the adaptive significance of superfoetation.
Spatial and temporal variation in additional life history traits
Substantial intraspecific variation in brood size, individual embryo mass and RA has been reported in several poeciliid species (Reznick et al. 1992; Zúñiga-Vega et al. 2007; Johnson and Bagley 2011), a list to which we add P. gracilis and P. infans. Ultimate causal factors of such variation may include differences in temperature (McManus and Travis 1998; Karayucel et al. 2008), predation (Reznick and Endler 1982; Jennions and Telford 2002), population density (Leips and Travis 1999; Soucy and Travis 2003; Schrader and Travis 2012) and the physico-chemical composition of the water bodies (Riesch et al. 2010). We cannot assign the observed variation in our samples to fish density (as patterns of variation are unrelated to season, a major correlate of density), and we are currently assessing the possible role of predation. The above ecological factors may have led through selection to different, relatively stable phenotypes or combinations of phenotypes in each population (Plath et al. 2010), or to the evolution of different reaction norms within and among populations (Green 2008; Aubin-Horth and Renn 2009). It is also possible that the observed variation is solely due to the same genotypes responding plastically to spatial and temporal variation in ecology (Reznick and Yang 1993), but we find this possibility unlikely given the diversity of strategies shown by females within the same locality and in the same month.
The several life history traits measured here are likely to be intercorrelated. For instance, Reznick and Miles (1989) and Pollux et al. (2009) suggested that more superfoetation could entail the production of smaller broods. Hence, we expected smaller broods in those populations with the highest degree of superfoetation, a prediction that was not borne by our data (Figs. 2a, b and 3a, b). Instead, we found evidence of a trade-off between number and size of embryos in both P. gracilis and P. infans that apparently is independent of the degree of superfoetation (Fig. 5). Indeed, such negative association can be found in taxa where there is no superfoetation or an equivalent (Charnov et al. 1995; Jennions and Telford 2002). A potential association between superfoetation and RA was not evident either (Figs. 2a, b and 3a, b).
Superfoetation may also be associated with larger embryos given the proposed relationship between matrotrophy and superfoetation (Reznick and Miles 1989; Pires et al. 2011) and the potential effect of matrotrophy on offspring size (Schrader and Travis 2009). Matrotrophy is defined as the mode of reproduction in which females transfer nutrients to embryos during development as opposed to lecithotrophy in which females provide nutrients to embryos before fertilization in the form of yolk (Wourms 1981). Matrotrophy may result in a conflict between mother and embryos with respect to the amount of nutrients that must be transferred, and this in turn may promote competition between embryos, high abortion rates and fewer larger embryos per brood (Schrader and Travis 2009). Given that a large number of species with superfoetation are matrotrophic (Pires et al. 2011), we expected larger embryos in those populations where females produce on average more simultaneous broods. This hypothesis was not supported in our data as can be seen in (Figs. 2a, c and 3a, c). The likely reason for this lack of association between superfoetation and mean embryo mass is that both P. gracilis and P. infans are predominantly lecithotrophic (Reznick et al. 2002), and hence, the conflict between mother and embryos in the amount of nutrient transfer must be small or even inexistent because females provide most nutrients before fertilization.
Given that our samples are substantial and encompass a variety of habitats and seasons and are essentially the same for two allopatric yet widely distributed species, we are confident that our finding that brood size, individual embryo mass and RA do not covary with superfoetation is robust. This suggests that genetic integration of those traits is weak, permitting the evolution of a wider variety of life history traits than might have been expected.
References
Allan JD, Castillo MM (2007) Stream ecology. Structure and function of running waters, 2nd edn. Spring, The Netherlands
Aubin-Horth N, Renn SCP (2009) Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol Ecol 18:3763–3780. doi:10.1111/j.1365-294X.2009.04313.x
Burley N (1980) Clutch overlap and clutch size: alternative and complementary reproductive tactics. Am Nat 115:223–246
Carey JR, Judge DS (2000) Longevity records: life spans of mammals, birds, amphibians, reptiles, and fish. Odense University Press, Odense Denmark
Charnov EL, Downhower JF, Brown LP (1995) Optimal offspring sizes in small litters. Evol Ecol 9:57–6.3. doi:10.1007/BF01237697
Downhower JF, Brown L (1975) Superfoetation in fishes and the cost of reproduction. Nature 256:345. doi:10.1038/256345a0
Galindo-Villegas J, Sosa-Lima E (2002) Gonopodial system review and a new fish record of Poeciliopsis infans (Cyprinodontiformes: Poeciliidae) for Lake Patzcuaro, Michoacan, central Mexico. Rev Biol Trop 50:1151–1157
Gómez-Márquez JL, Peña-Mendoza B, Salgado-Ugarte IH, Sánchez-Herrera AK, Sastré-Baez L (2008) Reproduction of the fish Poeciliopsis gracilis (Cyprinodontiformes: Poeciliidae) in Coatetelco, a tropical shallow lake in Mexico. Rev Biol Trop 56:1801–1812
Green BS (2008) Maternal effects in fish populations. Adv Mar Biol 54:1–105. doi:10.1016/S0065-2881(08)00001-1
Gunn JS, Thresher RE (1991) Viviparity and the reproductive ecology of clinid fishes (Clinidae) from temperate Australian waters. Environ Biol Fish 31:323–344. doi:10.1007/BF00002357
Gutiérrez-Cabrera AE, Pulido-Flores G, Monks S, Gaytán-Oyarzún JC (2005) Presencia de Bothriocephalus acheilognathi Yamaguti, 1934 (Cestoidea: Bothriocephalidae) en peces de Metztitlán, Hidalgo, México. Hidrobiológica 15:283–288
Haynes JL (1995) Standardized classification of poeciliid development for life-history studies. Copeia 1995:147–154. doi:10.2307/1446809
Jennions M, Telford S (2002) Life-history phenotypes in populations of Brachyrhaphis episcopi (Poeciliidae) with different predator communities. Oecologia 132:44–50. doi:10.1007/s00442-002-0942-4
Johnson JB, Bagley JC (2011) Ecological drivers of life-history divergence. In: Evans JP, Pilastro A, Schlupp I (eds) Ecology and evolution of poeciliid fishes. University of Chicago Press, Chicago, pp 38–49
Karayucel I, Orhan AK, Karayucel S (2008) Effect of temperature on some reproductive parameters of gravid females and growth of newly hatched fry in guppy, Poecilia reticulta (Peters, 1860). J Anim Vet Adv 7:1261–1266
Kennedy H (1978) Systematics and pollination of the “closed-flowered” species of Calathea (Marantaceae). Univ Calif Publ Bot 71:1–90
Lantieri T, Revelli A, Gaglioti P, Menato G, Gennarelli G, Delle Piane L, Massobrio M (2010) Superfetation after ovulation induction and intrauterine insemination performed during an unknown ectopic pregnancy. Reprod Biomed Online 20:664–666. doi:10.1016/j.rbmo.2010.01.017
Leips J, Travis J (1999) The comparative expression of life‐history traits and its relationship to the numerical dynamics of four populations of the least killifish. J Anim Ecol 68:595–616. doi:10.1046/j.1365-2656.1999.00311.x
Mateos M, Sanjur OI, Vrijenhoek RC (2002) Historical biogeography of the livebearing fish genus Poeciliopsis (Poeciliidae: Cyprinodontiformes). Evolution 56:972–984. doi:10.1111/j.0014-3820.2002.tb01409.x
McManus MG, Travis J (1998) Effects of temperature and salinity on the life history of the sailfin molly (Pisces: Poeciliidae): lipid storage and reproductive allocation. Oecologia 114:317–325. doi:10.1007/s004420050453
Meredith RW, Pires MN, Reznick DN, Springer MS (2011) Molecular phylogenetic relationships and the coevolution of placentotrophy and superfetation in Poecilia (Poeciliidae: Cyprinodontiformes). Mol Phylogenet Evol 59:148–157. doi:10.1016/j.ympev.2011.01.014
Miller RR, Mincley WL, Norris SM (2005) Freshwater fishes of Mexico. University of Chicago Press, Illinois
Molina-Moctezuma A (2011) Influencia de la depredación sobre las características de historias de vida y la dinámica poblacional de Poeciliopsis baenschi.. Dissertation, Facultad de Ciencias Universidad Nacional Autónoma de México
Moss BR (2013) Ecology of fresh waters: a view for the twenty-first century, 4th edn. Wiley-Blackwell, Oxford
Pape O, Winer N, Paumier A, Philippe HJ, Flatrès B, Boog G (2008) Superfetation: case report and review of the literature. J Gynecol Obstet Biol Reprod 37:791–795. doi:10.1016/j.jgyn.2008.06.004
Pires MN, McBride KE, Reznick DN (2007) Interpopulation variation in life‐history traits of Poeciliopsis prolifica: implications for the study of placental evolution. J Exp Zool 307A:113–125. doi:10.1002/jez.a.356
Pires MN, Arendt J, Reznick DN (2010) The evolution of placentas and superfetation in the fish genus Poecilia (Cyprinodontiformes: Poeciliidae: subgenera Micropoecilia and Acanthophacelus). Biol J Linn Soc 99:784–796. doi:10.1111/j.1095-8312.2010.01391.x
Pires MN, Banet AI, Pollux BJA, Reznick DN (2011) Variation and evolution of reproductive strategies. In: Evans JP, Pilastro A, Schlupp I (eds) Ecology and evolution of Poeciliid fishes. The University of Chicago Press, Chicago, pp 28–37
Plath M, Hermann B, Schröder C, Riesch R, Tobler M, García de León FJ, Schlupp I, Tiedemann R (2010) Locally adapted fish populations maintain small-scale genetic differentiation despite perturbation by a catastrophic flood event. BMC Evol Biol 10:256. doi:10.1186/1471-2148-10-256
Pollux BJA, Pires MN, Banet AI, Reznick DN (2009) Evolution of placentas in the family Poeciliidae: an empirical study of macroevolution. Annu Rev Ecol Evol Syst 40:271–289. doi:10.1146/annurev.ecolsys.110308.120209
Pollux BJA, Meredith RW, Springer MS, Reznick DN (2014) The evolution of the placenta drives a shift in sexual selection in livebearing fish. Nature 513:233–236. doi:10.1038/nature13451
Reznick DN, Endler JA (1982) The impact of predation on life history evolution in trinidadian guppies (Poecilia reticulata). Evolution 36:160–177. doi:10.2307/2407978
Reznick DN, Miles DB (1989) Review of life history patterns in Poeciliid fish. In: Meffe GK, Snelson FF Jr (eds) Ecology and evolution of livebearing fishes (Poeciliidae). Pretice Hall, Englewood Cliffs, pp 125–148
Reznick DN, Yang AP (1993) The influence of fluctuating resources on life history: patterns of allocation and plasticity in female guppies. Ecology 74:2011–2019. doi:10.2307/1940844
Reznick DN, Miles BD, Winslow S (1992) Life History of Poecilia picta (Poeciliidae) from the Island of Trinidad. Copeia 1992:782–790
Reznick DN, Mateos M, Springer MS (2002) Independent origins and rapid evolution of the placenta in the fish genus Poeciliiopsis. Science 298:1018–1020. doi:10.1126/science.1076018
Reznick DN, Meredith R, Collette BB (2007) Independent evolution of complex life history adaptations in two families of fishes, live-bearing halfbeaks (Zenarchopteridae, Beliformes) and Pociliidae (Cyprinodontiformes). Evolution 61:2570–2583. doi:10.1111/j.1558-5646.2007.00207.x
Riesch R, Plath M, García de Leon FJ, Schlupp I (2010) Convergent life-history shifts: toxic environments result in big babies in two clades of poeciliids. Naturwissenschaften 97:133–141. doi:10.1007/s00114-009-0613-y
Roellig K, Menzies BR, Hildebrandt TB, Goeritz F (2011) The concept of superfetation: a critical review on a ‘myth’in mammalian reproduction. Biol Rev 86:77–95. doi:10.1111/j.1469-185X.2010.00135.x
Schrader M, Travis J (2009) Do embryos influence maternal investment? Evaluating maternal-fetal coadaptation and the potential for parent-offspring conflict in a placental fish. Evolution 63:2805–2815. doi:10.1111/j.1558-5646.2009.00763.x
Schrader M, Travis J (2012) Assessing the roles of population density and predation risk in the evolution of offspring size in populations of a placental fish. Ecol Evol 2:1480–1490. doi:10.1002/ece3.255
Scrimshaw NS (1944) Superfetation in poeciliid fishes. Copeia 1944:180–183
Sokal RR, Rohlf FJ (2012) Biometry, 4th edn. W.H. Freeman and Company, New York
Soucy S, Travis J (2003) Multiple paternity and population genetic structure in natural populations of the poeciliid fish, Heterandria formosa. J Evol Biol 16:1328–1336. doi:10.1046/j.1420-9101.2003.00608.x
Tacutu R, Craig T, Budovsky A., Wuttke D, Lehmann G, Taranukha D., Costa J, Fraifeld VE, de Magalhaes JP. (2013) Human Ageing Genomic Resources: Integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res gks:1155. doi: 10.1093/nar/gks1155
Thibault RE, Schultz RJ (1978) Reproductive adaptations among viviparous fishes (Cyprynodontiformes: Poeciliidae). Evolution 32:320–333
Travis J, Farr JA, Henrich S, Cheong RT (1987) Testing theories of clutch overlap with the reproductive ecology of Heterandria formosa. Ecology 68:611–623. doi:10.2307/1938466
Turner CL (1937) Reproductive cycles and superfetation in poeciliid fishes. Biol Bull 72:145–164
Vondracek B, Wurtsbaugh WA, Cech JJ (1988) Growth and reproduction of the mosquitofish, Gambusia affinis, in relation to temperature and ration level: consequences for life history. Environ Biol Fish 21:45–57. doi:10.1007/BF02984442
Wourms JP (1981) Viviparity: the maternal-fetal relationship in fishes. Am Zool 21:473–515
Zúñiga-Vega JJ, Reznick D, Johnson JB (2007) Habitat predicts reproductive superfetation and body shape in the livebearing fish Poeciliopsis turrubarensis. Oikos 116:995–1005. doi:10.1111/j.0030-1299.2007.15763.x
Zúñiga-Vega JJ, Macias-Garcia C, Johnson JB (2010) Hypotheses to explain the evolution of superfetation in viviparous fishes. In: Urine MC, Grier HJ (eds) Viviparous fishes II. New Life Publications, Homestead, pp 241–253
Acknowledgments
This study was supported by the Mexican Research Council (Consejo Nacional de Ciencia y Tecnología, CONACYT) through the grant no. 129675 and through a doctorate scholarship awarded to PFA and is a partial fulfillment of the requirements for the doctoral degree (Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México) of PFA under the supervision of JJZV. Fieldwork was conducted under permits SDPA/DGVS/03492, DGOPA. 07010.210612.1749 and PPF/DGOPA-223/2013. D. Piñero gave extensive advice, and logistic assistance was provided by E. Ávila-Luna, J. L. Bortolini-Rosales, H. Espinosa-Pérez, M. Hernández-Quiroz, I. A. Morales-Salas, P. Mendoza-Hernández, M. E. Muñiz-Díaz de León, M. E. Pérez-Cruz and B. Zúñiga-Ruiz. We also thank the following people for field and laboratory assistance: P. García-Avilés, A. Hernández-Rosas, A. Molina-Moctezuma, C. Olivera-Tlahuel, H. Pérez-Mendoza, D. Robledo, N. Saleh-Bubaie, H. Salinas-Matus, T. Sandoval, R. Vega-Trejo, D. Villa-Meza and I. Zapata-Morán.
Ethical standards
The study reported in this paper conform to the laws in the country in which they were performed.
Conflict of interest
We have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Frías-Alvarez, P., Macías Garcia, C., Vázquez-Vega, L.F. et al. Spatial and temporal variation in superfoetation and related life history traits of two viviparous fishes: Poeciliopsis gracilis and P. infans . Naturwissenschaften 101, 1085–1098 (2014). https://doi.org/10.1007/s00114-014-1247-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-014-1247-2