Abstract
In this study, we aimed to investigate the antitumor effects of trichostatin A (TSA), an antifungal antibiotic that inhibits histone deacetylase (HDAC) family of enzymes, alone or in combination with anyone of the three chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) for the treatment of human urothelial carcinoma (UC). Two high-grade human UC cell lines (T24 and NTUB1) were used. Cytotoxicity and apoptosis were assessed by MTT assay and flow cytometry, respectively. The expression of phospho-c-Raf, phospho-MEK1/2, and phospho-ERK1/2 was measured by western blotting. ERK siRNA knockdown and the specific MEK inhibitor U0126 were used to examine the role of Raf/MEK/ERK signaling pathway in combined cytotoxicity of TSA and chemotherapy. TSA co-treatment with any one of the three chemotherapeutic agents induced synergistic cytotoxicity (combination index < 1) and concomitantly suppressed chemotherapeutic drug-induced activation of Raf-MEK-ERK pathway. Combination of ERK siRNA knockdown and treatment with the specific MEK inhibitor (U0126) enhanced the cytotoxic effects of the chemotherapy on UC cells. These observations were confirmed in a xenograft nude mouse model. Moreover, activated Raf/MEK/ERK pathway was observed in human bladder UC specimens from patients with chemoresistant status. In conclusion, TSA elicits a synergistic cytotoxic response in combination with chemotherapy via targeting the Raf/MEK/ERK pathway. TSA elicits synergistic cytotoxic response in combination with three DNA-damaging drugs (cisplatin, gemcitabine, and doxorubicin). Activated Raf/MEK/ERK pathway is involved in chemoresistant mechanism of UC. Combining chemotherapeutic agents with HDAC inhibitor (TSA) or with targeting Raf/MEK/ERK pathway is promising to circumvent chemoresistance in UCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder urothelial carcinoma (UC) ranks 4th in incidence among cancers in men and 11 in women in the USA [1]. Recurrence and metastases occur in 20–50% of patients after radical surgery. Once bladder UC progresses to metastatic disease, cisplatin-based chemotherapy is the mainstay of treatment in these patients. Despite treatment with the current first-line regimen, including cisplatin, gemcitabine, or other combinations such as methotrexate, vinblastine, doxorubicin, and cisplatin, the overall response rate is approximately 50–60% [2,3,4]. Moreover, the side effects of chemotherapy remain substantial. The newly approved immunotherapy drug PD-1 or PD-L1 inhibitor shows effectiveness and tolerable side effects in patients with metastatic UC, whose cancers had worsened during or after treatment with platinum-containing chemotherapy. However, the response rate was approximately 15–20%. Nearly all patients with metastatic UCs inevitably experience drug resistance and eventually die of disease progression. The search for novel compounds to enhance effectiveness of treatment, reduce chemotherapy-related side effects, and overcome drug resistance are imperative in UC treatment.
Aberrant epigenetic modifications and alterations in chromatin structure without changing DNA sequences commonly exist in human cancers [5]. Histones are the primary protein molecules of chromatin and undergo several types of post-translational modifications. These modifications can influence interactions between DNA and histones and result in alterations of gene transcription, DNA repair, and even DNA replication [6, 7]. Among them, histone acetylation and deacetylation have a critical role in translational activation and gene expression [8, 9]. HDAC inhibitors modulate a wide range of cellular processes. The acetylation status of histones influences chromatin conformation and the accessibility of transcription factors and effector proteins to DNA, thereby regulating gene expression. In addition, HDACs regulate gene expression indirectly by mediating the post-translational acetylation and deacetylation of various non-histone protein substrates, including DNA-binding proteins, transcription factors, signal-transduction molecules, DNA-repair proteins, and chaperone proteins. TSA used in this study is a pan-inhibitor rather than isoenzyme-specific inhibitor of HDAC [10, 11]. Several HDAC inhibitors have been approved for the treatment of cutaneous T cell lymphoma [12, 13].
Bladder UCs are good candidates for HDAC inhibitor treatment. Approximately 76% of all primary bladder tumors display mutations in at least one chromatin regulatory gene [14]. Meanwhile, increased expression of HDACs has been observed in high-grade UC [15]. Some HDAC inhibitors (i.e., TSA and belinostat) showed antitumor effects on bladder cancer cell lines through cell cycle blockade and apoptosis induction [16,17,18,19]. Li et al. found that the HDAC inhibitor AR-42 exhibited synergistic effects with cisplatin against bladder cancer [20]. In 2011, Yoon et al. observed that TSA could exert synergistic antitumor activity with cisplatin and re-sensitize cisplatin treatment in human UC cells [21]. Similarly, Yeh et al. demonstrated that TSA re-sensitized gemcitabine-resistant UC cells [22]. HDAC inhibitors combined with chemotherapeutic agents might be a promising therapeutic strategy to improve therapeutic efficacy or to overcome resistance of UC. Nevertheless, the underlying mechanism involved in the augmented cytotoxicity and re-sensitization of drug resistance in UCs remains unclear.
Systemic chemotherapy is the standard modality to improve survival in patients with metastatic UC. The key agents in the current standard regimens of chemotherapy are DNA-targeting agents, including gemcitabine plus cisplatin (GC) and methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC). The antitumor efficacy of DNA-targeting agents is also limited by tight DNA coiling. Unwinding of tight DNA coiling to facilitate DNA targeting is becoming a promising approach for improving the anticancer effects of these DNA-targeting agents. HDAC inhibitors lead to histone hyper-acetylation and less compacted DNA to enhance the antitumor effects of DNA-targeting agents, such as cisplatin and gemcitabine.
In the in vitro and in vivo studies, we examined whether TSA, an antifungal antibiotic that selectively inhibits class I and II HDAC families of enzymes, enhances the efficacy of current primary chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) in treating human bladder UC. In addition, we aimed to elucidate the mechanism underlying the combination effects of TSA and chemotherapy.
Material and methods
Cell culture
Two human UC cell lines were used in this study. NTUB1 cells, which have been proven to be tumorigenic in nude mice, were derived from the specimen of a patient with high-grade transitional cell carcinoma. T24 cells were separated from highly malignant grade III human urinary bladder carcinoma [23] and purchased from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). The cell lines were cultured in RPMI-1640 medium (for NTUB1) or Dulbecco’s modified Eagle’s medium (for T24) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL of streptomycin (Invitrogen, Carlsbad, CA).
Reagents and chemicals
TSA compounds were obtained from Sigma-Aldrich (St. Louis, MO). Chemotherapeutic agents used in this study were from clinical medications: cisplatin from Abiplatin (Pharmachemie BV, GA Haarlem, the Netherlands), gemcitabine from Gemzar (Lilly, Fegersheim, France), and doxorubicin from Adriblastina Rapid Dissolution Pfizer (New York, NY). All the other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Merck Millipore (Billerica, MA).
Antibodies
For western blotting, antibodies against cleaved PARP, cleaved caspase-3, cleaved caspase-7, phospho-Bcl2, phospho-MEK1/2, phospho-ERK1/2, phospho-p90, phospho-c-Raf, MEK1/2, p90, and c-Raf were purchased from Cell Signaling Technologies (Danvers, MA). The other antibodies against ERK1/2 and ɑ-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-actin and GAPDH antibodies were purchased from Genetex, Inc. (Irvine, CA). For immunohistochemistry (IHC), antibodies against phospho-ERK1/2 and phospho-c-Raf were obtained from Santa Cruz Biotechnology.
Cell viability assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT, Sigma-Aldrich) was used to determine cell viability. The cells were suspended in culture medium, seeded in 96-well microplates (4500 cells/well), and incubated at 37 °C for 48 h before drug treatment. After drug exposure, the cells were further incubated with 0.5 mg/mL MTT in complete medium at 37 °C for 4 h. Then, dimethyl sulfoxide (DMSO) was applied to dissolve the reduced MTT crystals, and the solvents were subjected to a plate reader for detection of absorbance at 570 nm.
Combination index
The combined effects of chemotherapeutic agents and TSA were determined using CalcuSyn software (version 1.1.1, 1996, Biosoft, Cambridge, UK). The combined effects at combination ratios of 1:20 (TSA to cisplatin), 1:5 (TSA to gemcitabine), and 2:1 (TSA to doxorubicin) were subjected to median-effect and combination index (CI) analysis as previously described [24, 25]. The combined dose effects were presented by median, dose, and CI effects as plotted in Fig. 3. CI values of less than 1, equal to 1, and greater than 1 were defined as synergistic, additive, and antagonistic, respectively.
Western blot analysis
To determine protein expression, NTUB1 and T24 cells were lysed with cell lysis buffer (Cell Signaling Technologies) on ice after washing with cold phosphate-buffered saline (PBS). The supernatants were collected after centrifugation of cell lysates at 14,000 rpm for 10 min at 4 °C, and the BCA protein assay (Thermo Scientific Pierce, Rockford, IL) was used to detect the total protein concentrations. Equal protein amounts from each group, which were mixed with TOOLS sample loading buffer (Biotools, Taipei, Taiwan), were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore). After blocking with 5% bovine serum albumin (BSA) in PBS, the membranes were incubated with various primary antibodies in PBS at 4 °C overnight. After washing twice with TBST (TBS containing 0.05% Tween 20), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Genetex) at recommended dilution ratios in PBS at room temperature for 2 h. Antibody-labeled membranes were again washed twice with TBST and visualized by enhanced chemiluminescence (ECL) substrates (Merck Millipore and Biotools) under ImageQuant LAS 4000 (GE Healthcare) system.
Knockdown of ERK using siRNA
To knockdown ERK1/2, NTUB1 and T24 cells were transfected with 10 nM ON-TARGETplus SMARTpool siRNA targeting ERK1/2 (Thermo Scientific Dharmacon, Lafayette, CO) or 10 nM non-targeting scramble siRNA (as control, Thermo Scientific Dharmacon) by DharmaFECT 1 transfection reagent (Thermo Scientific Dharmacon) in accordance with the manufacturer’s instructions for 48 h. After various treatments, the cells were collected for different analyses.
Apoptosis assay
The apoptosis assay was performed by FITC Annexin V Apoptosis Detection Kit (BD Biosciences, Bedford, MA), according to the manufacturer’s protocol. Then, the stained apoptotic cells were examined and quantified using FACS flow cytometry (Becton Dickinson, Cockeysville, MD).
IHC in human UC specimens
Formalin-fixed, paraffin-embedded tissue blocks and fresh UC specimens from 10 patients with metastatic bladder UC were collected from patients who had received systemic chemotherapy with gemcitabine and cisplatin regimens. Five of them were defined as chemoresistant for disease progression during chemotherapy, while five of them were defined as chemosensitive for being responsive to chemotherapy. IHC staining by phospho-ERK1/2 and phospho-c-Raf antibodies was performed as previously described on 5-μm sections of formalin-fixed, paraffin-embedded specimens. Two board-certified pathologists (Lin W.C. and Sun C.T.) who were unaware of the clinical data evaluated the immunoreactivity of phospho-ERK1/2 and phospho-c-Raf. The staining intensity was categorized as 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive).The mean percentage of positively stained tumor cells was determined by counting at least 10 random fields at both 40 and 400 magnification in each section. The IHC score was calculated by multiplying intensity with the mean percentage of positive staining.
In vivo xenograft
In the present study, 80 mice were used in total. NTUB1 or T24 cells (5 × 105) were suspended in 200 μL of serum-free media and mixed with an equivalent volume of Matrigel (BD Biosciences). Eight-week-old nude mice (obtained from the Taiwan National Laboratory Animal Center, Taipei, Taiwan) were injected subcutaneously with the above mixture into the dorsal flanks. The mice were treated with cisplatin, gemcitabine, doxorubicin, TSA, or each combined with TSA after the tumors had grown to approximately 100–150 mm3 and paralleled with the control group mice (n = 5 for each group). The anti-cancer agents cisplatin (10 mg/kg, three times weekly), gemcitabine (15 mg/kg, twice weekly), doxorubicin (10 mg/kg, twice weekly), or TSA (1 mg/kg, three times weekly) in normal saline were intraperitoneally (i.p.) injected into the chemotherapy- and TSA-treated groups, respectively, three times a week for four weeks. Meanwhile, the same doses of both the drug and TSA were applied with the same frequency within the same duration in the combined groups. Mice receiving a mixture of DMSO and normal saline were designated as the non-treated control group. The tumor sizes were measured by calipers, and the volume was calculated as follows: volume = longest tumor diameter × (shortest tumor diameter)2 / 2 every 4 days. The tumors after 4 weeks of treatment were abscised, and pictures were taken before being frozen in liquid nitrogen and stored at − 80 °C. The study that involve human participants and animal experiments have been approved by the institutional research ethics committee (no. 201112136RIC) and National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC) (No. 20160117).
Statistical analysis
Statistical analyses was performed using GraphPad Prism® 5 software. All data were presented as the means ± SD and analyzed by one-way ANOVA. Bonferroni post hoc test was further applied to compare the significance of each set of two groups. P values < 0.05 are considered statistically significant.
Results
TSA reduced cell viability and enhanced cytotoxicity of chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) in human UC cells
We first assessed the impact of TSA alone and in combination with three chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) on the viability of UC cells using MTT assay. Figure 1 shows that TSA alone effectively reduced cell viability in a dose-dependent manner (0–1 μM) in both NTUB1 and T24 cells after 48 h of exposure. We then investigated the cytotoxic effects of TSA (0.1–1 μM) in combination with various concentrations of three chemotherapeutic agents (2.5–30 μM cisplatin, 0.5–20 μM gemcitabine, and 0.05–5 μM doxorubicin) on UC cells after 48 h of exposure. Figure 2 shows that TSA efficiently enhances the cytotoxic effects of the three chemotherapeutic agents on both NTUB1 and T24 cells.
TSA inhibits cell viability and enhances cytotoxicity of chemotherapeutic agents (cisplatin, gemcitabine, or doxorubicin) in human UC cells. a, b NTUB1 (a) and T24 (b) cells were treated with various concentrations of TSA (0.1–1 μM) for 48 h in combination with various concentrations of chemotherapeutic agents (cisplatin 2.5–30 μM, gemcitabine 0.5–20 μM, and doxorubicin 0.05–5 μM). Cell viability was assessed by MTT assay
TSA potentiates the apoptotic effects of three chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) on UC cells. a NTUB1 and T24 cells were treated with 0.5 μM TSA in combination with either cisplatin 10 μM, gemcitabine 2.5 μM, or doxorubicin 0.25 μM. Quantitative analyses of total apoptosis (early and late) population following 48-h treatment are presented. Apoptotic cells were analyzed by FACS flow cytometry with propidium iodide (PI) and Annexin V-FITC staining. *Indicates significant difference (p < 0.05) between untreated and treated groups. b Cell lysates were harvested and analyzed by western blotting with specific antibodies against cleaved caspase-3, caspase-7, and cleaved PARP. Results shown are representative of at least three independent experiments
TSA potentiates the apoptotic effects of three chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) on UC cells
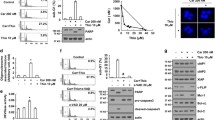
Next, we evaluated the apoptotic effect of TSA alone and in combination with three chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) on NTUB1 and T24 cells using flow cytometry (FACS) with propidium iodide (PI) and Annexin V-FITC staining. TSA (0.5 μM) alone induced apoptosis in NTUB1 and T24 cells after 48 h of exposure (Fig. 2a). TSA treatment significantly increased apoptosis compared to those in untreated cells. TSA significantly potentiates the apoptotic effects of the three chemotherapeutic agents (10 μM cisplatin, 2.5 μM gemcitabine, and 0.25 μM doxorubicin) on UC cells. Western blot showed that TSA in combination with each of the three chemotherapeutic agents further increased cleaved caspase 3, 7, and PARP compared to those induced by chemotherapeutic agent alone (Fig. 2b). Consistently, co-treatment with TSA suppressed chemotherapy-induced activation of phospho-Bcl2, an anti-apoptosis regulator as can be seen in Fig. S1 of supplemental data. These data consistently indicate that TSA potentiates the apoptotic effects of chemotherapy on UC cells.
TSA in combination with chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) synergistically inhibited cell viability in human UC cells
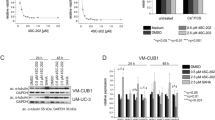
The combination index (CI) was analyzed to clarify the combined effect of TSA and chemotherapeutic agents. The combination index-effect and dose-effect plots are shown in Fig. 3. TSA in combination with each of the three chemotherapeutic agents consistently exhibited synergistic effects (CI < 1) both on NTUB1 and T24 UC cells. These findings indicate that TSA cooperates synergistically with chemotherapy to inhibit cell viability of UC cells.
TSA in combination with chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) synergistically inhibits cell viability in human UC cells (CI < 1). a, b Human UC cells NTUB1 (a) and T24 (b) were treated with TSA alone or in combination with cisplatin, doxorubicin, or gemcitabine (1:20 ratio) for 24 h. Cell viability was determined using MTT assay. The median-effect plot, dose-effect plot, and combination index (CI) plot of TSA and chemotherapeutic agents are presented
TSA suppresses the activation of Raf/MEK/ERK pathway associated with chemotherapeutic agent treatment in human UC cells
Raf/MEK/ERK signaling pathway is involved in the regulation of cell growth proliferation, survival, and apoptosis [26]. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis, and sensitivity to chemo- and targeted therapy and has been reported to be a therapeutic target of cancer [27]. However, the precise role of Raf/MEK/ERK pathway in the combinatorial effects of TSA and chemotherapeutic agents has never been explored. Thus, we examined the expression levels of phospho-c-Raf, phospho-MEK1/2, phospho-ERK1/2 and the downstream target phospho-p90 after treatment with TSA alone, each chemotherapeutic agent alone (10 μM cisplatin, 2.5 μM gemcitabine, and .25 μM doxorubicin), or TSA in combination with each chemotherapeutic agent. We observed that each chemotherapeutic agent alone markedly activated Raf/MEK/ERK pathway and the downstream p90 (Fig. 4). Co-treatment with TSA with chemotherapy suppressed chemotherapy-induced activation of Raf/MEK/ERK pathway and phospho-p90. Raf/MEK/ERK signaling pathway seemed to be involved in TSA and DNA damaging drug synergy.
Expression Raf/MEK/ERK signaling pathway after TSA alone and in combination with chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin) in human UC cells. a, b NTUB1 (a) and T24 (b) cells were treated with 0.5 μM TSA in combination with either cisplatin 10 μM, gemcitabine 2.5 μM, or doxorubicin 0.25 μM for 48 h. Cell lysates were harvested and analyzed by western blotting with specific antibodies against phospho-c-Raf, c-Raf, phospho-MEK1/2, MEK1/2, phospho-ERK1/2, total ERK1/2, phospho-p90 and p90. Results shown are representative of at least three independent experiments
ERK knockdown and an inhibitor of the Raf/MEK/ERK signaling pathway, U0126, augmented the effectiveness of chemotherapy in UC cells
To clarify whether regulation of the Raf/MEK/ERK pathway potentiated chemotherapy efficacy in UC cells, we used U0126, a specific MEK inhibitor, and ERK siRNA knockdown to examine the role of the Raf/MEK/ERK pathway in the combination effects of chemotherapy and TSA on UC cells. Figure 5 shows that U0126 decreased chemotherapy-induced phospho-ERK1/2 activation without changing total ERK1/2 level. ERK knockdown by siRNA decreased ERK1/2 level and enhanced chemotherapy-induced cytotoxicity. Downregulation of Raf/MEK/ERK pathway by U0126 or ERK siRNA significantly enhanced chemotherapy-induced cytotoxicity in UC cells.
Inhibition of Raf/MEK/ERK pathway enhances the cytotoxic effects of chemotherapeutic agents (cisplatin, gemcitabine, doxorubicin) on UC cells. a NTUB1 and T24 cells were treated with cisplatin 10 μM, gemcitabine 2.5 μM, or doxorubicin 0.25 μM for 48 h in combination with U0126 10 μM. Cell viability was assessed by MTT assay. b Cell lysates were harvested and analyzed by western blotting with specific antibodies against phospho-ERK1/2 and ERK1/2. Results shown are representative of at least three independent experiments. c Effects of combination treatment and ERK knockdown on viability of NTUB1 and T24 cells. Cells were transfected with ERK siRNA (10 nM) or scramble siRNA (10 nM) (as a control), followed by treatment with cisplatin 10 μM, gemcitabine 2.5 μM, or doxorubicin 0.25 μM for 48 h. Cell viability was assessed by MTT assay. Data are presented as means ± SD of three independents experiments. *p < 0.05 compared with scramble siRNA and chemotherapeutic agents
TSA enhanced chemotherapy-induced antitumor effects in a xenograft mouse model
We then evaluated the antitumor effects of chemotherapy and TSA alone or in combination in vivo using a xenograft mouse model. NTUB1 or T24 cells were mixed with Matrigel and then injected subcutaneously into the flanks of homozygous nude mice. Figure 6 shows that the combination of chemotherapy and TSA yielded significant antitumor effects on T24 and NTUB1 xenografts compared to chemotherapeutic agent or TSA alone. These results further confirm the in vitro findings that TSA works together with chemotherapeutic agents to improve the antitumor effect on UCs.
Xenograft model demonstrates the efficacy of the combination of chemotherapy and TSA in vivo. a, b Nude mice bearing NTUB1 (a) or T24 (b) xenograft tumors were treated with dimethyl sulfoxide (as control), cisplatin, gemcitabine, doxorubicin, TSA, cisplatin/TSA, gemcitabine/TSA, and doxorubicin/TSA for 4 weeks, respectively. The representative excised tumors from each group are shown in the upper part. The lower part shows the time-dependent tumor volume (mm3) change presented as means ± SD. *p < 0.05 represents a significant difference between the cisplatin group and combination group
Activated Raf/MEK/ERK signaling pathway in UC is associated with chemo-resistance in patients with metastatic UC
We then examined the expression of phospho-ERK1/2 and phospho-c-Raf in bladder UC tissue samples from 10 patients with metastatic UC who had received systemic chemotherapy with gemcitabine and cisplatin regimen by using IHC staining. The immunoreactivities of phospho-ERK1/2 and phospho-c-Raf in 5 chemo-resistant UCs (a-e) were stronger compared to those in 5 chemo-sensitive UCs (f-j) (Fig. 7). The IHC scoring of p-ERK and p-c-Raf in five chemo-sensitive and five chemo-resistant UC tumors are shown in Fig. S2 of supplement data.
Activated Raf/MEK/ERK signaling pathway in UC cells is associated with chemo-resistance in patients with metastatic UC. a-j Immunohistochemical (IHC) staining for analysis of phospho-ERK and phospho-c-Raf ontumors from all 10 patients with metastatic bladder UC, 5 (a–e) with chemo-sensitive and 5 (f–j) with chemo-resistant status. IHC staining of formalin-fixed, paraffin-embedded bladder UC tissues. Upper part shows stronger nuclear and cytoplasmic staining of phospho-ERK1/2 in chemo-resistant cells than that in chemo-sensitive UC cells. Lower part shows stronger nuclear staining of phospho-c-Raf in chemo-resistant cells than that in chemo-sensitive UC cells
Figure S2 shows western blot analysis in two chemo-sensitive (a, b in Fig. 7) and two chemo-resistant samples (f, g in Fig. 7) among ten tumors. The IHC scores for phospho-ERK1/2 and phospho-c-Raf showed stronger in chemo-resistant UCs compared to those in chemo-sensitive UCs (p = 0.01 and 0.14, respectively) (Fig. S2).
Discussion
In the present study, we demonstrated that TSA synergistically enhanced cytotoxic and apoptotic effects of cisplatin, gemcitabine, or doxorubicin on human UC cells. Previous studies have indicated the synergistic effects of similar combinations as we described in introduction [20,21,22]. Moreover, we observed that Raf/MEK/ERK was involved in the combination effects of TSA and chemotherapy. TSA suppressed chemotherapy-induced Raf/MEK/ERK signaling activation. Inhibition of Raf/MEK/ERK pathway by U0126 and ERK knockdown potentiated the cytotoxicity of chemotherapy in UC cells. Raf/MEK/ERK pathway play a role in the drug synergy. Regulation of Raf/MEK/ERK pathway may augment chemotherapy and re-sensitization of drug resistance in UCs.
The Raf/MEK/ERK pathway plays a critical role in many aspects of tumorigenesis and is a promising therapeutic target because it represents a common downstream pathway for several growth factors of tyrosine kinase receptors, which are frequently mutated or overexpressed in human cancers [28]. Raf kinases are a family of serine threonine kinases that phosphorylate and activate MEK1/2, which then phosphorylates and activates ERK1/2. When activated, ERK1/2 phosphorylates various downstream substrates, such as p90 (90–90-kDa Ribosomal S6 kinase) involved in multiple cellular responses from cytoskeletal changes to gene transcription, which are associated with apoptosis, invasion, and metastasis of cancer cells.
Moreover, mutations in RAS genes are the most common mutations found in bladder UC, and up to 13% of all UCs harbor mutations in HRAS, KRAS, or NRAS [29]. Raf was the first identified and most characterized downstream effector kinase of Ras. Intriguing evidence has emerged indicating that the Raf/MEK/ERK pathway plays a critical role in drug resistance. Activated Raf/MEK/ERK pathway renders tumor cells resistant to chemotherapy.
Novel anticancer agents targeting the Raf/MEK/ERK pathway are currently being evaluated and may prove to be more effective and less toxic than conventional cytotoxic therapies. In the present study, Raf/MEK/ERK activation was observed after chemotherapy, and co-treatment with TSA suppressed the activation of Raf/MEK/ERK and enhanced the antitumor effects on UC cells. Furthermore, downregulation or suppression of Raf/MEK/ERK pathway potentially served as a promising therapeutic option to develop a novel strategy for the treatment of metastatic UCs. Our findings indicate the critical role of Raf/MEK/ERK signaling pathway in enhancing susceptibility to chemotherapy in UC cells. In addition, the differential expression of phospho-c-Raf and phospho-ERK between chemo-resistant and chemo-sensitive UC is potentially an indicator of chemotherapy response in clinical practice.
HDAC inhibitors have been studied in pre-clinical investigations as therapeutic or chemopreventive agents in various cancers. In this study, we showed that TSA is capable of inducing apoptosis and cell death in human UC cells. Co-treatment with TSA suppressed chemotherapy-induced Raf/MEK/ERK pathway activation and synergistically enhanced chemotherapy efficacy. To date, a number of natural and synthetic chemical compounds functioning as HDAC inhibitors have been developed. HDAC inhibitors are classified into groups based on their chemical structure, including hydroxamic acids (TSA, vorinostat), carboxylic acids (valproate, butyrate), aminobenzamides (entinostat, mocetinostat), cyclic peptides (apicidin, romidepsin), epoxyketones (trapoxins), and hybrid molecules. Future studies are warranted to investigate and clarify the outcomes by inhibiting specific HDAC-dependent complexes in UC following treatment with various HDAC inhibitors.
Our study has some limitations. First, we use bladder cancer cell lines as a surrogate for evaluating primary bladder cancer behavior. Moreover, the xenograft mouse model may not totally represent the cancer microenvironment. Second, the potential targets of TSA to non-histone proteins have not been well explored. Third, we did not study the alterations of epigenetic modulation after TSA treatment. HDAC inhibitors have broad effects. Similarly, Raf/MEK/ERK cascade regulates the activity of many proteins and is involved in multiple cellular processes. We could not assume that Raf/MEK/ERK cascade is the only or main mechanism of TSA and DNA damaging drug synergy based on the current data; nevertheless, it cannot be ruled out as a factor.
In conclusion, TSA synergistically enhances the cytotoxic effects of three DNA-targeting chemotherapeutic agents (cisplatin, gemcitabine, and doxorubicin). Raf/MEK/ERK pathway is involved in the synergistic effects of TSA and chemotherapeutic agents. These findings are promising for the development of new strategies to circumvent drug resistance in human UC treatment via suppression of Raf/MEK/ERK pathway and combining with HDAC inhibitors.
Change history
11 February 2019
In Fig. 1b, upper part, the cell viability counts after treatment with cisplatin and TSA in T24 cells was by mistake a duplication of the image for NTUB1 on the left. In the corrected version of Fig. 1, the image was replaced appropriately.
11 February 2019
In Fig. 1b, upper part, the cell viability counts after treatment with cisplatin and TSA in T24 cells was by mistake a duplication of the image for NTUB1 on the left. In the corrected version of Fig. 1, the image was replaced appropriately.
Abbreviations
- ERK:
-
extracellular signal-regulated kinase 1 and 2 (ERK1/2)
- UC:
-
urothelial carcinoma
- HDAC:
-
histone deacetylase
- TSA:
-
trichostatin A
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Harker WG, Meyers FJ, Freiha FS, Palmer JM, Shortliffe LD, Hannigan JF, McWhirter KM, Torti FM (1985) Cisplatin, methotrexate, and vinblastine (CMV): an effective chemotherapy regimen for metastatic transitional cell carcinoma of the urinary tract. A northern California oncology group study. J Clin Oncol 3:1463–1470
Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N, Weiselberg L, Rosado K, Smart T, Lin SY, Penenberg D, Fair WR, Whitmore WF (1989) Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 64:2448–2458
von der MH HSW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Marsh DJ, Shah JS, Cole AJ (2014) Histones and their modifications in ovarian cancer—drivers of disease and therapeutic targets. Front Oncol 4:144
Hunt CR, Ramnarain D, Horikoshi N, Iyengar P, Pandita RK, Shay JW, Pandita TK (2013) Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat Res 179:383–392
Abmayr SM, Workman JL (2012) Holding on through DNA replication: histone modification or modifier? Cell 150:875–877
Kamieniarz K, Izzo A, Dundr M, Tropberger P, Ozretic L, Kirfel J, Scheer E, Tropel P, Wisniewski JR, Tora L, Viville S, Buettner R, Schneider R (2012) A dual role of linker histone H1.4 Lys 34 acetylation in transcriptional activation. Genes Dev 26:797–802
Zhang Z, Liu D, Murugan AK, Liu Z, Xing M (2014) Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer 21:161–173
Ropero S, Esteller M (2007) The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 1:19–25
New M, Olzscha H, La Thangue NB (2012) HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol 6:637–656
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124:30–39
Prince HM, Dickinson M (2012) Romidepsin for cutaneous T-cell lymphoma. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 18:3509–3515
Giordano TJ (2014) The cancer genome atlas research network: a sight to behold. Endocr Pathol 25:362–365
Poyet C, Jentsch B, Hermanns T, Schweckendiek D, Seifert HH, Schmidtpeter M, Sulser T, Moch H, Wild PJ, Kristiansen G (2014) Expression of histone deacetylases 1, 2 and 3 in urothelial bladder cancer. BMC Clin Pathol 14:10
Buckley MT, Yoon J, Yee H, Chiriboga L, Liebes L, Ara G, Qian X, Bajorin DF, Sun TT, Wu XR, Osman I (2007) The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med 5:49
Vallo S, Xi W, Hudak L, Juengel E, Tsaur I, Wiesner C, Haferkamp A, Blaheta RA (2011) HDAC inhibition delays cell cycle progression of human bladder cancer cells in vitro. Anti-Cancer Drugs 22:1002–1009
Qu W, Kang YD, Zhou MS, Fu LL, Hua ZH, Wang LM (2010) Experimental study on inhibitory effects of histone deacetylase inhibitor MS-275 and TSA on bladder cancer cells. Urol Oncol 28:648–654
Ozawa A, Tanji N, Kikugawa T, Sasaki T, Yanagihara Y, Miura N, Yokoyama M (2010) Inhibition of bladder tumour growth by histone deacetylase inhibitor. BJU Int 105:1181–1186
Li DR, Zhang H, Peek E, Wang S, Du L, Li G, Chin AI (2015) Synergy of histone-deacetylase inhibitor AR-42 with cisplatin in bladder cancer. J Urol 194:547–555
Yoon CY, Park MJ, Lee JS, Lee SC, Oh JJ, Park H, Chung CW, Abdullajanov MM, Jeong SJ, Hong SK, Byun SS, Lee ES, Lee SE (2011) The histone deacetylase inhibitor trichostatin a synergistically resensitizes a cisplatin resistant human bladder cancer cell line. J Urol 185:1102–1111
Yeh BW, Li WM, Li CC, Kang WY, Huang CN, Hour TC, Liu ZM, Wu WJ, Huang HS (2016) Histone deacetylase inhibitor trichostatin A resensitizes gemcitabine resistant urothelial carcinoma cells via suppression of TG-interacting factor. Toxicol Appl Pharmacol 290:98–106
Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H, Donner J (1973) Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer 11:765–773
Ho IL, Kuo KL, Liu SH, Chang HC, Hsieh JT, Wu JT, Chiang CK, Lin WC, Tsai YC, Chou CT, Hsu CH, Pu YS, Shi CS, Huang KH (2015) MLN4924 synergistically enhances cisplatin-induced cytotoxicity via JNK and Bcl-xL pathways in human urothelial carcinoma. Sci Rep 5:16948
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Sridhar SS, Hedley D, Siu LL (2005) Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther 4:677–685
Abrams SL, Steelman LS, Shelton JG, Wong EW, Chappell WH, Basecke J, Stivala F, Donia M, Nicoletti F, Libra M et al (2010) The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle 9:1781–1791
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A et al (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–1284
Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA (2005) FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24:5218–5225
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of Taiwan (104-2314-B-002-164-MY3 and 103-2314-B-002-161-MY3), National Taiwan University Hospital (103-S2349, 104-M2868, 105-S2978,105-28, 106-3415, and 107-S3784), and New Taipei City Hospital.
Funding
We also thank the personnel of the Second, Third, and Sixth Core Laboratories of National Taiwan University Hospital.
Author information
Authors and Affiliations
Contributions
Wei-Chou Lin, Kuan-Lin Kuo, and Kuo-How Huang conceived of the presented idea and study design.
Kuan-Lin Kuo and Kuo-How Huang wrote the manuscript with support from Fu-Shun Hsu and Wei-Chou Lin.
Kuan-Lin Kuo, Shih-Ming Liao, Jo-Yu Hong, and Shao-Ping Yang carried out the experiment and performed the computations.
Kuo-How Huang, Shing-Hwa Liu, Chung-Sheng Shi, Hong-Chiang Chang, Fu-Shun Hsu, Wei-Chou Lin, Yu-Chieh Tsai, and June-Tai Wu verified the analytical methods and helped supervise the project.
Wei-Chou Lin and Chia-Dong Shun interpreted the results of immunostaining.
All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
The study that involve human participants and animal experiments have been approved by the institutional research ethics committee (no. 201112136RIC) and National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC) (No. 20160117).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
a, b NTUB1 (a) and T24 (b) cells were treated with 0.5 μM TSA alone or in combination with either cisplatin 10 μM, gemcitabine 2.5 μM, or doxorubicin 0.25 μM. Cell lysates were harvested and analyzed by western blotting with specific antibodies against phospho-Bcl2. (JPG 44 kb)
Fig. S2
a Western blot analysis by phospho-ERK1/2 and phospho-c-Raf antibodies on proteins extracted from UC tumors of two patients in chemo-sensitive and two patients in chemo-resistant status. b Comparative results of IHC scores in UC tumors from 5 patients in chemo-sensitive and 5 patients in chemo-resistant status.*p < 0.05 represents a significant difference between chemo-resistant and chemo-sensitive groups. (JPG 35 kb)
Rights and permissions
About this article
Cite this article
Lin, WC., Hsu, FS., Kuo, KL. et al. Trichostatin A, a histone deacetylase inhibitor, induces synergistic cytotoxicity with chemotherapy via suppression of Raf/MEK/ERK pathway in urothelial carcinoma. J Mol Med 96, 1307–1318 (2018). https://doi.org/10.1007/s00109-018-1697-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-1697-7