Abstract
Neutrophil granulocytes possess a large arsenal of pro-inflammatory substances and mechanisms that empower them to drive local acute immune reactions to invading microorganisms or endogenous inflammatory triggers. The use of this armory needs to be tightly controlled to avoid chronic inflammation and collateral tissue damage. In gout, inflammation arises from precipitation of uric acid in the form of needle-shaped monosodium urate crystals. Inflammasome activation by these crystals in local immune cells results in a rapid and dramatic recruitment of neutrophils. This neutrophil influx is accompanied by the infamously intense clinical symptoms of inflammation during an acute gout attack. Neutrophilic inflammation however is equipped with a built-in safeguard; activated neutrophils form neutrophil extracellular traps (NETs). At the very high neutrophil densities that occur at the site of inflammation, NETs build aggregates that densely pack the monosodium urate (MSU) crystals and trap and degrade pro-inflammatory mediators by inherent proteases. Local removal of cytokines and chemokines by aggregated NETs explains how acute inflammation can stop in the consistent presence of the inflammatory trigger. Aggregated NETs resemble early stages of the typical large MSU deposits that constitute the pathognomonic structures of gout, tophi. Although tophi contribute to muscosceletal damage and mortality in patients with chronic gout, they can therefore be considered as a payoff that is necessary to silence the intense inflammatory response during acute gout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutrophil granulocytes constitute the most abundant leukocytes in the blood and are recruited in large numbers to entry sites of invading microorganisms or in response to other inflammatory stimuli. Neutrophils kill pathogens by either phagocytic uptake or degradation in intracellular vesicles or by the release of anti-microbial cytotoxic molecules from secretory granules. In 1996, a novel form of neutrophil suicide was described that takes several hours and comprises a stepwise progression of chromatin decondensation, nuclear swelling, spilling of the nucleoplasm in the cytoplasm, and membrane perforation [1]. In 2004, this phenomenon was identified as other anti-microbial mechanism neutrophils that are capable of the formation of neutrophil extracellular traps (NETs) [2]. In the process called NETosis, neutrophils release nuclear DNA into the extracellular milieu. During externalization, the DNA is decorated with anti-microbial peptides, granular enzymes, and DNA-associated proteins such as histones.
Citrullination of histones and the presence on chromatin of proteases normally confined to intracellular granules distinguishes NETosis from other forms of cell death. Citrullination and chromatin decondensation are mediated by the enzyme peptidylarginine deiminase 4 (PAD4) that is highly expressed in neutrophils. PAD4 removes positive charges from core histones by converting arginine residues to citrullines, thereby weakening the interaction between histones and DNA. Inhibition of PAD4 was reported to decrease NET formation and PAD4-deficient mice to display impaired NETosis [3, 4]. The neutrophil-specific serine proteases neutrophil elastase (NE) and myeloperoxidase (MPO) are involved in the later stages of NETosis. NE migrates from azurophilic granules to the nucleus where it degrades histones. This contributes to chromatin decondensation which is further enhanced by the binding of MPO to chromatin. Both enzymes seem to be essential for NETosis since NE knockout mice and MPO-deficient humans exhibit a reduced ability to produce NETs after stimulation with phorbol myristate acetate [5, 6]. The canonical NETosis pathway also depends on the production of reactive oxygen species (ROS) during the oxidative burst [7]. In particular, ROS are essential for the release of NE and MPO from azurophilic granules [5, 8].
More recently, a non-suicidal pathway of NET formation was described, where the cell remains intact and normal cellular functions of neutrophils, such as chemotaxis and phagocytosis, are still carried out [9, 10]. During this so-called vital NETosis, DNA is released by vesicular trafficking of the DNA from the nucleus to the extracellular space. This process occurs more quickly than an oxidative burst can be mounted and is therefore independent of ROS production [11].
Pro-inflammatory role of NETs in autoimmune diseases
Apart from invading microorganisms, also endogenous molecules trigger formation of NETs; pro-inflammatory cytokines and chemokines, activated platelets and endothelial cells, nitric oxide, monosodium urate (MSU) crystals, anti-neutrophil cytoplasmic antibodies, and immune complexes were all reported to induce NETosis [2, 12–17]. Since the discovery of NETs, there has been a renewed interest in the neutrophil as a potential driver of systemic autoimmune diseases. Despite the beneficial effects for host defense, NETs occur at the expense of tissue injury to the host, since NET constituents, especially histones, can cause bystander injury on endothelia [14, 18]. The tissue-damaging properties of NETs have particularly been observed in the lungs and the circulation in severe sepsis [19, 20]. In addition, excessive NETs in the vasculature system provide a scaffold and stimulus for deep vein thrombosis [21].
Apart from that, NETs provide a source of autoantigens in SLE, small vessel vasculitis, and other autoimmune diseases and are prime candidates for the initiation or enhancement of autoimmunity. For example, SLE patients produce autoantibodies against a range of nuclear antigens associated with NETs, including dsDNA, histones, and anti-microbial peptides [22, 23], and patients with defective NET degradation tend to have higher levels of anti-NET and anti-dsDNA autoantibodies and suffer renal damage [24]. Tight control of the formation and clearance of NETs is therefore crucial to prevent an autoimmune reaction and host damage.
Somewhat confusingly, however, it has recently been shown that decreased production of ROS is associated with a higher risk to develop lupus in humans and mice [25] [8]. Furthermore, human individuals that cannot produce ROS due to mutations in subunits of the NADPH oxidase 2 complex and are therefore unable to form NETs develop the clinical picture of chronic granulomatous disease which, apart from persistent infections, often also includes autoimmune disorders resembling SLE, Crohn’s disease, and inflammatory arthritis [26–29]. Thus, the role of NETosis in the pathogenesis of autoimmune diseases is more complex than what meets the eye at first glance.
Aggregation of NETs for resolution of inflammation
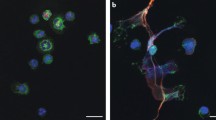
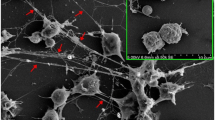
Activated neutrophils at the site of inflammation release large amounts of pro-inflammatory mediators that attract and activate further granulocytes and other immune cells. To avoid a positive feedback loop of cell activation and mediator release, stringent control of these processes is required. This control was thought to be exerted mainly by induction of neutrophil apoptosis [30, 31] followed by phagocytosis by macrophages, leading to neutrophil clearance and release of anti-inflammatory cytokines such as TGF-β. However, the concept of the mere “fizzling out” of an ongoing neutrophilic inflammation was recently undermined by reports providing evidence for an active role of neutrophils in the resolution of inflammation; in a murine model of gout, we observed that the depletion of neutrophils led to chronification of joint inflammation [32]. In this study, we further noted that under conditions of high neutrophil densities, NETs induced by MSU crystals form larger aggregates. The size of these so-called aggNETs depends on the density of neutrophils in the culture and the amount of added MSU crystals but was not influenced by the addition of peripheral blood mononuclear cells. AggNETs trap and efficiently degrade neutrophil-derived cytokines and chemokines by inherent serine proteases.
NETosis and the aggregation of NETs after exposure to MSU crystals are dependent on the oxidative burst [32]. In the traditional view, ROS have been connected with promotion of inflammation and tissue damage, but in recent years, ROS have also been implicated in regulation of inflammation and protection from autoimmunity [25, 33–35]. Employing mice that are unable to mount an oxidative burst in phagocytes, we showed that lack of ROS results in exacerbation and chronification of MSU-triggered joint inflammation, suggesting that ROS production is crucial for the termination of inflammation elicited by MSU. We observed that the anti-inflammatory action of ROS is mediated by enabling formation of aggNETs that trap and degrade inflammatory mediators. In line with that, DNase I treatment, which leads to disassembly of aggNETs, also restored impaired neutrophil chemokine release.
In conclusion, aggNET formation helps to explain the spontaneous remission of acute inflammatory attacks in gout and might also be of high relevance for other forms of neutrophilic inflammation.
Local situation in gout
In gout, inflammatory flares are evoked by oversaturated concentrations of urate that precipitates in the tissue as needle-shaped MSU crystals. A dramatic neutrophil influx to the synovial fluid and synovium is the hallmark of acute gout, yet neutrophils are absent in the healthy joint. MSU crystals that are newly precipitated or shed from pre-existing MSU pools are therefore first encountered by cells of the synovial lining (type A synoviocytes, i.e., cells of the monocyte-macrophage lineage and fibroblast-like type B synoviocytes), dendritic cells (DC), possibly endothelial cells, and mast cells.
The negatively charged MSU crystals directly interact via electrostatic and hydrogen bonding with lipid membranes and signaling proteins (integrins, FcyR, CD16, CD14, phospholipases, or chemokine receptors) on the cell surface. MSU crystals are also taken up by these cells via phagocytosis, followed by lysosomal fusion, oxidative burst and synthesis of nitric oxide, and the release of inflammatory mediators [36, 37]. Crystal uptake disrupts the phagolysosome acidic compartment and leads to the release of cathepsin B [38]. Activation of the inflammasome is triggered by volume gain of the cells by water influx after massive release of sodium from acidified phagolysosomes. Water influx dilutes intracellular K+ which turns on the inflammasome [39].
Inflammasome activation via MSU crystals may have evolved as a danger signal; upon cell injury, urate and ATP are released into the environment [40]. Locally, concentrations can exceed the solubility limits and crystals may form. Resident cells are then stimulated, with DCs providing a link to the adaptive immune response. The DC-activating properties of MSU depend on the interaction between crystals and membrane cholesterol on DC [41].
As a consequence of inflammasome activation, IL-1β is produced. In macrophages and neutrophils, neither inflammasome activation by MSU nor IL-1β processing are dependent on ROS in vivo, with ROS even negatively regulating caspase-1 activity and IL-1β secretion [42–45]. IL-1β production by macrophages, monocytes, and DCs is a key regulator of gout and stands at the beginning of the inflammatory cascade [46]. During later stages of inflammation, also neutrophils importantly contribute to IL-1β production [44, 47]. Interestingly, the release of IL-1β from neutrophils requires ROS. A recent report has shown that NADPH oxidase complexes are not necessarily the only source of ROS production during inflammasome activation. The NLRP3 inflammasome/IL-1β secretion axis is also susceptible to changes in mitochondrial and intracellular ROS, as well as to overall mitochondrial dysfunction [48].
Processing and release of IL-1β do not have to occur simultaneously, thus giving room to its release during the process of NETosis. Indeed, bioactive IL-1β has been detected on NETs released during inflammatory attacks of familial Mediterranean fever [49]. Moreover, IL-1β can also be created outside of the cell via mechanisms involving caspase-1 or independently of the inflammasome by cleavage of pro-IL-1 β into its bioactive form by neutrophil serine protease 3 [50–52]. Results from mice suggest that this extracellular IL-1β production crucially contributes to the development of inflammatory arthritis.
Since IL-1β is the main trigger for neutrophil influx, the inflammasome is a crucial link between the causal stimulus of gout (MSU crystals) and the subsequent pathological hallmark of the acute attack.
Recruitment of neutrophils is further mediated by CXCR2, CXCL-8, CXCL-1, CXCL-2, and CXCL-3 [53], while CCL-2 recruits monocytes [54]. Blood neutrophils attach to the synovial vascular endothelium through selectins, exit into the synovial capillaries, and migrate through the synovial matrix to the joint cavity, guided by the chemokine concentration gradient.
Pro-inflammatory mediators at the inflammation site prime the neutrophil for an elevated oxidative burst and a higher degranulative response and prolong their survival by inhibiting apoptosis. At the site of inflammation, neutrophils also ingest MSU crystals which trigger NETosis and the release of inflammatory mediators, including TNFα and IL-6, as well as neutrophil attractants (e.g., CXCL-8) and activators (e.g., CCL3 and CSCL10) [32, 55, 56]. Due to the intense local inflammation, cytokines are produced in large quantities and also enter the circulation, resulting in an acute phase response that can trigger fever and leukocytosis.
Continuous recruitment of neutrophils to the site of inflammation results in very high neutrophil densities [57]. After the neutrophil concentration in the tissue exceeds a certain threshold, NETs begin to aggregate and build aggNETs in which the crystals are embedded in a mesh of DNA and proteins from neutrophil granules (Fig. 1). As such, these aggNETs resemble the crystalline core of tophi in patients during established gout [32].
Development and resolution of an acute gout flare. MSU crystals newly formed in situ or shed from pre-existing MSU deposits are taken up by mononuclear phagocytes. Sodium set free in large amounts from acidic phagolysosomes disturbs the osmotic balance of the cells. As a reaction, cells enhance the entry of water through aquaporins. Aside from the dilution of sodium in the cell, also potassium is diluted, which falls below the threshold for the activation of the inflammasome. As a consequence, large amounts of IL-1β are produced. IL-1β can also be produced in the extracellular milieu by the serine proteinase 3 that is released from activated neutrophils and cleaves pro-IL-1β. Bioactive IL-1β recruits neutrophils to the joint space that ingest MSU crystals and undergo a specific form of cell death known as NETosis. During low concentrations of neutrophils, this process releases pro-inflammatory mediators that recruit further cells. Recruitment proceeds until a critical concentration of neutrophils is reached at the inflammation site, and aggregation of NETs sets in. AggNETs then interrupt the inflammatory circle by degrading chemokines and pro-inflammatory cytokines by associated proteases, thus bringing NETosis to a halt and resolving inflammation. AggNETs also densely pack and wall-off MSU crystals and can mature into larger tophi
MSU crystal-induced aggNET formation is augmented by release of ATP and lactoferrin from activated neutrophils. The release of ATP during NET formation is of high importance since extracellular nucleotides initiate anti-inflammatory clearance of dead cells by mononuclear phagocytes [58]. In addition, lactoferrin on NETs abrogates further recruitment of neutrophils and thus contributes to the anti-inflammatory action of NETosis in highly infiltrated tissues [59]. Both ATP and lactoferrin have an additional function in NET aggregation; with more and more neutrophils infiltrating to the site of inflammation, the local concentration of these mediators will increase, gradually fostering aggNET formation. Indeed, addition of both mediators promoted aggNET formation also in low density cultures [32].
The tophus—good, bad, or just ugly?
Upon prolonged hyperuricemia, micro-aggregates of MSU crystals occur in all patients with gout, but in some, also large aggregates are present. In these tophi, the pathognomonic structures of gout, the MSU crystals are embedded in an amorphous whitish matrix surrounded by connective tissue and inflammatory cells such as mononuclear phagocytes, osteoclasts, mast cells, and even B and T cells [60]. Tophi are typically a late feature of gout but may also be present in early phases of the disease [61, 62]. Since only subcutaneous tophi are visually apparent, the dark figure of tophi is likely to be higher, and non-invasive detection methods such as dual energy computer tomography (DECT) may reveal additional tophi that cannot be picked up by physical examination.
Tophi can be regarded as a kind of granuloma in which the body tries to wall-off MSU crystals that it cannot eliminate. Deposition of crystals in tophi may continue for months or years without causing clinical symptoms. This silent buildup is however interrupted by occasional acute attacks that are accompanied by the typical signs of strong inflammation, swelling, redness, and pain. Subtle differences in the physical properties, including surface charge and size, coating molecules, the local cytokine milieu, and the cells encountered first are important in determining the magnitude of the inflammatory response to MSU crystals. Crystals exceeding 20 μm may be too large to trigger an inflammatory response, and therefore, microcrystals need to be released from larger crystals or tophi. In support of that, gout flares are often associated with quick changes in urate concentrations (e.g., upon anti-hyperuricemic therapy [63]) and a rapid reduction might release microcrystals from the margins of tophi.
Crystals located within synovial tophi are usually coated with several proteins; IgG attaches through charge interaction and hydrogen bonding and encourages phagocytosis via FcyR. Opsonization of crystals with complement components either promotes inflammation directly or via FcyR signaling. As shown in rabbits, lack of complement components results in a reduced neutrophil inflammatory response to MSU [64]. However, crystals can also be coated with anti-inflammatory proteins, e.g., lysosomal enzymes, the anti-inflammatory cytokine TGF-β, and apolipoprotein E, a component of the LDL fraction in serum that inhibits the stimulation of neutrophils [65]. Progressive dissolution of tophi may thus expose uncoated crystal surfaces which further fuel inflammation.
Less differentiated monocytes show a higher pro-inflammatory mediator response to MSU crystals than macrophages despite equally efficient internalization [66]. Some mature phagocytes residing in the human synovium may be able to ingest MSU crystals without triggering inflammation, whereas the entry of fresh monocytes and neutrophils into the joint is likely eliciting an acute attack.
The observation that acute gout attacks often occur after abundant meals or alcohol consumption can be explained by the release of free fatty acids synergizing with MSU crystals to induce IL-1β release and to enhance inflammation [51, 67].
Aside from the different inflammatory potential of existing MSU crystals, their formation itself is influenced by the presence of particulate seed nuclei (e.g., cartilage debris, collagen, chondroitin, or hyaluronate released by joint trauma or arthritis), local cation concentrations, pH, temperature, and hydration. Damaged joints could thus serve as nidus for crystal formation.
The aggregated structures that form in MSU-stimulated neutrophil cultures at high cell densities show striking resemblance to the crystalline core of tophi; colocalization of extracellular DNA with material from neutrophil granules (neutrophil elastase, myeloperoxidase, anti-microbial peptides) shows that these structures are NETs interspersed with MSU crystals [32]. It is feasible that these aggNETs represent the initial stage of tophus formation and might ripe to the complex multilayered structure that can be found in patients with chronic gout. Tophus formation could therefore be a mechanism to intervene with the self-reinforcing loop of inflammation in gout and stop an acute flare that would otherwise result in dramatic tissue damage and bone remodeling. In line with that, tophi are a way to store MSU crystals in a comparably immunologically silent way. However, in the long run, also tophi cannot completely eliminate the burden of ongoing hyperuricemia.
Although tophi can be clinically silent for a long time, they are associated with radiographic findings [68]. Furthermore, an enhanced Doppler signal is present also in asymptomatic gout patients, indicating hidden or non-clinically apparent inflammation [69]. Tophi contribute to muscosceletal disability through direct contact of the bone with the crystals and through the action of osteoclasts in the peripheral corona zone of the tophus. In vitro, MSU crystals have catabolic effects on stromal cells of the joint, such as chondrocytes and osteoblasts [70, 71]. Typical for the damage caused by tophi are punched-out extramarginal lesions with preservation of the joint space and bone density [72]. Although tophi do not overtly correlate with serum urate levels, they contribute to the total urate pool and total urate pool correlates with flare frequency in long-standing tophaceous gout [61, 73, 74].
Established and novel treatment options of acute and chronic gout
Gout affects 1–2 % of adults in developed countries. Despite the high prevalence, treatment options have remained mainly static for half a century. Long-term treatment relies on reducing the serum urate levels well below the saturation point of MSU (6.8 mg/dl or 408 μM at 37 °C, [75, 76]), so that new crystals cannot form. Serial joint aspiration and DECT studies also confirmed the disappearance of already existing crystals with effective urate-lowering therapy [77, 78]. The xanthine oxidase inhibitor allopurinol has been the standard first-line drug for more than 50 years. During the last years, another xanthine oxidase inhibitor, febuxostat, has become a valuable alternative [79]. However, urate-lowering therapy often suffers from poor adherence. Only 25 % of patients attending general practice keep urate levels on target during follow-up [80], and the maintenance rate of therapy is low [81]. In addition, urate-lowering therapy involves the danger of “mobilization flares” in the first few months, resulting from shedding of microcrystals from pre-existing MSU deposits. An especially high incidence of acute attacks has been observed after initiation of treatment with the polyethylenglycol-coupled recombinant uricase pegloticase, which is used in severe cases of refractory gout and causes a very rapid fall in serum urate [82]. Prophylactic low-dose colchicine or NSAIDs is currently recommended to prevent such flares [83]. Oral colchicine, NSAIDs, and glucocorticoids are also used for the treatment of acute flares.
In the last years, anti-IL therapy has shown its potential for the treatment of acute gout [84]. Treatment with the monoclonal anti-IL-1β antibody canakinumab, the soluble decoy receptor rilonacept, or the IL-1 receptor antagonist anakinra can be important alternatives when NSAIDs, colchicine, or glucocorticoids are contraindicated, which is frequently a problem in gout patients with renal and metabolic comorbidities [84, 85]. Moreover, blocking the IL-1 pathway can also result in the decrease of inflammasome activation in ROS-deficient individuals and therefore has important implications for the treatment of patients with chronic granulomatous disease [86].
Therapeutic intervention exploiting the mechanism of aggNET formation might involve shifting the balance towards resolution of inflammation by promoting aggregation of NETs during acute inflammation. This could be theoretically accomplished by local induction of the oxidative burst or application of the aggNET-accelerator lactoferrin, which triggered aggNET formation even in low cell densities in vitro. If this concept is feasible and how it can be implemented in vivo will be the subject of future studies.
References
Takei H, Araki A, Watanabe H, Ichinose A, Sendo F (1996) Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukocyte Biol 59:229–240
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207:1853–1862
Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA et al (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184:205–213
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191:677–691
Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A (2011) Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117:953–959
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241
Akong-Moore K, Chow OA, von Kockritz-Blickwede M, Nizet V (2012) Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One 7, e42984. doi:10.1371/journal.pone.0042984
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD et al (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13:463–469
Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC et al (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18:1386–1393
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M et al (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185:7413–7425
Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H et al (2010) Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide: Biol Chem / Off J Nitric Oxide Soc 22:226–234
Neeli I, Khan SN, Radic M (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180:1895–1902
Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ (2010) Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 584:3193–3197
Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15:623–625
Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, Laskay T (2014) Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol 193:1954–1965
Kingsbury SR, Conaghan PG, McDermott MF (2011) The role of the NLRP3 inflammasome in gout. J Inflamm Res 4:39–49
Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7, e32366. doi:10.1371/journal.pone.0032366
Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR (2012) Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Investig 122:2661–2671
Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, Thorlacius H (2014) Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol 307:L586–L596
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107:15880–15885
Kienhofer D, Hahn J, Schubert I, Reinwald C, Ipseiz N, Lang SC, Borras EB, Amann K, Sjowall C, Barron AE et al (2014) No evidence of pathogenic involvement of cathelicidins in patient cohorts and mouse models of lupus and arthritis. PLoS One 9, e115474. doi:10.1371/journal.pone.0115474
Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V et al (2011) Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 3:73ra19
Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A (2010) Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107:9813–9818
Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FP Jr, Reiff A, Myones BL, Kaufman KM et al (2012) Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A 109:E59–E67
Barton LL, Johnson CR (1986) Discoid lupus erythematosus and X-linked chronic granulomatous disease. Pediatr Dermatol 3:376–379
Cale CM, Morton L, Goldblatt D (2007) Cutaneous and other lupus-like symptoms in carriers of X-linked chronic granulomatous disease: incidence and autoimmune serology. Clin Exp Immunol 148:79–84
Lee BW, Yap HK (1994) Polyarthritis resembling juvenile rheumatoid arthritis in a girl with chronic granulomatous disease. Arthritis Rheum 37:773–776
Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW (2009) Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol 104:117–124
El Kebir D, Filep JG (2013) Modulation of neutrophil apoptosis and the resolution of inflammation through beta2 integrins. Front Immunol 4:60
Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, Lell M, Manger B, Rech J, Naschberger E et al (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20:511–517
Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R (2004) Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A 101:12646–12651
Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R (2003) Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet 33:25–32
Campbell AM, Kashgarian M, Shlomchik MJ (2012) NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med 4:157ra141
Simchowitz L, Atkinson JP, Spilberg I (1982) Stimulation of the respiratory burst in human neutrophils by crystal phagocytosis. Arthritis Rheum 25:181–188
Chen L, Hsieh MS, Ho HC, Liu YH, Chou DT, Tsai SH (2004) Stimulation of inducible nitric oxide synthase by monosodium urate crystals in macrophages and expression of iNOS in gouty arthritis. Nitric Oxide: Biol Chem / Off J Nitric Oxide Soc 11:228–236
Chu SC, Yang SF, Tzang BS, Hsieh YS, Lue KH, Lu KH (2010) Cathepsin B and cystatin C play an inflammatory role in gouty arthritis of the knee. Clin Chim Acta Int J Clin Chem 411:1788–1792
Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, Gaipl US, Voll RE, Springer E, Munoz LE et al (2011) Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem 286:35–41
Busso N, So A (2012) Microcrystals as DAMPs and their role in joint inflammation. Rheumatology 51:1154–1160
Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW et al (2008) Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29:807–818
Meissner F, Molawi K, Zychlinsky A (2008) Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol 9:866–872
Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A (2010) Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116:1570–1573
Gabelloni ML, Sabbione F, Jancic C, Fuxman Bass J, Keitelman I, Iula L, Oleastro M, Geffner JR, Trevani AS (2013) NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1beta secretion but not in inflammasome activation. Eur J Immunol 43:3324–3335
van de Veerdonk FL, Smeekens SP, Joosten LAB, Kullberg BJ, Dinarello CA, van der Meer JWM, Netea MG (2010) Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A 107:3030–3033
So A, Busso N (2014) The concept of the inflammasome and its rheumatologic implications. Joint Bone Spine: Rev Rhum 81:398–402
Mankan AK, Dau T, Jenne D, Hornung V (2012) The NLRP3/ASC/Caspase-1 axis regulates IL-1beta processing in neutrophils. Eur J Immunol 42:710–715
Harijith A, Ebenezer DL, Natarajan V (2014) Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol 5:352
Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, Nakos K, Tsironidou V, Koffa M, Boumpas DT et al (2014) Neutrophil extracellular traps regulate IL-1beta-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis. doi:10.1136/annrheumdis-2014-205958
Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB (2009) Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum 60:3651–3662
Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ et al (2010) Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum 62:3237–3248
Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A et al (2014) The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 15:727–737
Terkeltaub R, Baird S, Sears P, Santiago R, Boisvert W (1998) The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum 41:900–909
Scanu A, Oliviero F, Gruaz L, Sfriso P, Pozzuoli A, Frezzato F, Agostini C, Burger D, Punzi L (2010) High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res Ther 12:R23
Schorn C, Strysio M, Janko C, Munoz LE, Schett G, Herrmann M (2010) The uptake by blood-borne phagocytes of monosodium urate is dependent on heat-labile serum factor(s) and divalent cations. Autoimmunity 43:236–238
Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K (2011) Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One 6, e29318. doi:10.1371/journal.pone.0029318
Shah K, Spear J, Nathanson LA, McCauley J, Edlow JA (2007) Does the presence of crystal arthritis rule out septic arthritis? J Emerg Med 32:23–26
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286
Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, Rossi AG, Gregory CD (2009) Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Investig 119:20–32
Dalbeth N, Pool B, Gamble GD, Smith T, Callon KE, McQueen FM, Cornish J (2010) Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum 62:1549–1556
Dalbeth N, House ME, Horne A, Taylor WJ (2013) Reduced creatinine clearance is associated with early development of subcutaneous tophi in people with gout. BMC Musculoskelet Disord 14:363
Yu TF, Gutman AB (1967) Principles of current management of primary gout. Am J Med Sci 254:893–907
Yamanaka H, Togashi R, Hakoda M, Terai C, Kashiwazaki S, Dan T, Kamatani N (1998) Optimal range of serum urate concentrations to minimize risk of gouty attacks during anti-hyperuricemic treatment. Adv Exp Med Biol 431:13–18
Tramontini N, Huber C, Liu-Bryan R, Terkeltaub RA, Kilgore KS (2004) Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum 50:2633–2639
Terkeltaub RA, Dyer CA, Martin J, Curtiss LK (1991) Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J Clin Investig 87:20–26
Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, Haskard DO (2002) Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum 46:3026–3033
You M, Fischer M, Deeg MA, Crabb DW (2002) Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277:29342–29347
Perez-Ruiz F, Martin I, Canteli B (2007) Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol 34:1888–1893
Puig JG, de Miguel E, Castillo MC, Rocha AL, Martinez MA, Torres RJ (2008) Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides, Nucleotides Nucleic Acids 27:592–595
Chhana A, Callon KE, Pool B, Naot D, Gamble GD, Dray M, Pitto R, Bentley J, McQueen FM, Cornish J et al (2013) The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol 40:2067–2074
Chhana A, Callon KE, Pool B, Naot D, Watson M, Gamble GD, McQueen FM, Cornish J, Dalbeth N (2011) Monosodium urate monohydrate crystals inhibit osteoblast viability and function: implications for development of bone erosion in gout. Ann Rheum Dis 70:1684–1691
Barthelemy CR, Nakayama DA, Carrera GF, Lightfoot RW Jr, Wortmann RL (1984) Gouty arthritis: a prospective radiographic evaluation of sixty patients. Skelet Radiol 11:1–8
Rajan A, Aati O, Kalluru R, Gamble GD, Horne A, Doyle AJ, McQueen FM, Dalbeth N (2013) Lack of change in urate deposition by dual-energy computed tomography among clinically stable patients with long-standing tophaceous gout: a prospective longitudinal study. Arthritis Res Ther 15:R160
Scott JT, Holloway VP, Glass HI, Arnot RN (1969) Studies of uric acid pool size and turnover rate. Ann Rheum Dis 28:366–373
Fiddis RW, Vlachos N, Calvert PD (1983) Studies of urate crystallisation in relation to gout. Ann Rheum Dis 42(Suppl 1):12–15
Liote F (2011) Treatment of hyperuricemia, gout and other crystalline arthritidies. Reumatismo 63:276–283
Biermann MHC, Araujo EG, Maueröder C, Lell M, Schett G, Manger B, Herrmann M, Rech J, Munoz LE (2014) Dual-energy CT in gout: an update exemplified by selected clinical cases. Gout Hyperuricemia 1:122–126
Araujo EG, Bayat S, Petsch C, Englbrecht M, Faustini F, Kleyer A, Hueber AJ, Cavallaro A, Lell M, Dalbeth N et al. (2015) Tophus resolution with pegloticase– a prospective dual energy computed tomography study. RMD Open in press
Huang X, Du H, Gu J, Zhao D, Jiang L, Li X, Zuo X, Liu Y, Li Z, Li X et al (2014) An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis 17:679–686
Sarawate CA, Patel PA, Schumacher HR, Yang W, Brewer KK, Bakst AW (2006) Serum urate levels and gout flares: analysis from managed care data. J Clin Rheumat: Pract Rep Rheum Musculoskelet Dis 12:61–65
Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M (2004) Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol 31:1575–1581
Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, Hershfield MS (2007) Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum 56:1021–1028
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F et al (2006) EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 65:1312–1324
Schlesinger N (2014) Anti-interleukin-1 therapy in the management of gout. Curr Rheumatol Rep 16:398
Edwards NL, So A (2014) Emerging therapies for gout. Rheum Dis Clin North Am 40:375–387
de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW et al (2014) IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A 111:3526–3531
Acknowledgements
This work was supported by the doctoral training program GRK1660 and the priority program SPP 1468 Osteoimmunology IMMUNOBONE of the German Research Foundation (DFG) and the IMI funded research project BTCure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maueröder, C., Kienhöfer, D., Hahn, J. et al. How neutrophil extracellular traps orchestrate the local immune response in gout. J Mol Med 93, 727–734 (2015). https://doi.org/10.1007/s00109-015-1295-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1295-x