Abstract
Most European hardwoods are nowadays under-utilized due to drawbacks such as low dimensional stability, durability and surface hardness. Seven hardwood species were thermally and/or chemically modified in order to improve these disadvantages. Heat treatment was carried out in air at atmospheric pressure at three temperatures, while two types of chemical modifications were tested, the first being based on furfuryl alcohol with tartaric acid, the second being based on succinic anhydride and glycerol. Then, modified woods were studied to determine weight loss due to thermal treatment, solution uptake, weight percent gain and swelling due to chemical modification processes. After that, main properties of chemically modified woods were characterized including density, equilibrium moisture content, dimensional stability and surface hardness. Effect of thermal pretreatment on subsequent chemical modification and on properties of modified woods obtained have also been evaluated. Combination of thermal modification with chemical modification had led to better improvements than each separately. Furfurylation treatment appeared to be more efficient than polyester modification. Most of the modified samples were denser, more stable and harder than native wood. These modified woods might compete with imported tropical wood for applications requiring high stability and hardness, such as joinery, decking or flooring, music instruments, handles for tools or kitchen utensils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Climate change and the accompanying global warming represent a major challenge for the twenty-first century. The use of renewable materials can help to decrease CO2 emissions, which are acknowledged to be responsible for global warming. In this context, wood is a renewable material coming from trees, which consume CO2 for their growth and stock it into wood components, acting as a CO2 sink. Properties such as ease of machining and conversion with low energy compared to other materials such as steel, concrete or plastic, makes wood a material for the future. In addition, long term use of wood allows carbon storage inside wood products, in varied applications such as construction (sawn timber and wood panels), wood frame, furniture, joinery, decking, which contributes to mitigating the effect of global warming (Cuadrado et al. 2015; Mehr et al. 2018; Parobek et al. 2019; Zhang et al. 2020).
Different wood species available over the world present specific properties, and their applications depend on these properties. European tree species include softwood and hardwood. Softwoods are already well utilized in wood frame and timber construction, while hardwoods can find variety of uses according to their properties, mainly for furniture or interior uses (Zauer et al. 2013; Silva et al. 2014; Nepal et al. 2016). Nowadays, European hardwoods species are underused because of the wide diversity of species, with various physical properties, which complicates their use in standard applications. Moreover, heterogeneity of the resource makes it difficult to use at industrial scale. Indeed, despite their availability, European hardwoods may have some drawbacks such as lack of hardness or dimensional instability under variable air humidity level (Leboucher 2014). These are the reasons why tropical wood species, such as teak (Tectona grandis), ipe (Tabebuia spp.) or massaranduba (Manilkara bidentata), are often chosen for outdoor uses (decking, joinery), due to their exceptional properties such as dimensional stability, hardness and natural durability (de Windt et al. 2014; van Benthem et al. 2018; Haag et al. 2020). According to Németh (2020) and Ninane (2021), improvement of properties such as dimensional stability and hardness of underutilized European hardwood species might lead to new uses (Németh et al. 2020; Ninane et al. 2021).
Different treatments can be considered to improve wood properties (Gérardin 2016; Sandberg et al. 2017). On the one hand, it can be chemical modification using heat treatments, which consist in heating wood in order to modify part of its components, preferably in an oxygen reduced environment to avoid wood combustion. Oxygen reduction can be accomplished using inert atmosphere (N2 or CO2), vegetable oil or even vacuum, while steam can be used to inhibit thermal runaway (Srinivas and Pandey 2012; Todaro et al. 2015; Gérardin 2016; Salman et al. 2017; Sandberg et al. 2017). Heat treatment modifies the structure of wood polymers, especially hemicelluloses and amorphous cellulose regions, which can be depolymerized into organic acids such as acetic, formic and levulinic acids, and sugar degradation products like furfural and hydroxymethylfurfural. Depending on the nature of the wood species, extractive's content can be modified due to evaporation and degradation of initially present molecules or to the formation of new degradation products. Wood hygroscopicity decreases due to degradation of hemicelluloses, which are the most hygroscopic polymers in wood. Durability of heat-treated wood, as well as its dimensional stability are increased (Gérardin 2016). Nano-porosity of wood cell wall may be increased due to degradation of some components, which might make it possible to better impregnate wood thereafter (Monteiro 2018; Jang and Kang 2019), although a decrease of thermally modified poplar porosity was observed by Todaro et al. (2015). However, mechanical properties may be affected, which remains the main drawback of thermal treatment (Srinivas and Pandey 2012; Xu et al. 2019). Ultimately, thermally modified wood properties modification depends mainly on temperature and duration of the thermal treatment. On the other hand, it can be chemical modification of wood involving impregnation of chemicals which react with wood components (acetylation) or polymerize inside wood porosity, for example thermoset resins like furfuryl alcohol, polyester or phenolic resins (Deka and Saikia 2000; Herold et al. 2013; Ibach and Rowell 2013; Damay 2014; Gérardin 2016; Kurkowiak et al. 2021). Physical properties of modified wood can be strongly improved depending on the modification level, notably dimensional stability, hardness, elastic modulus and resistance to biodegradation (decay, insects, marine borers). Many chemicals have been tested to perform wood modification, and the current trend is to move towards renewable products, which includes furfuryl alcohol, tartaric acid, glycerol and succinic anhydride, corresponding to chemicals used in this study. Furfuryl alcohol combined with tartaric acid as catalyst has been used successfully to improve beech wood properties by Sejati et al. (2017), and teak sapwood properties by Martha et al. (2021). In these papers, high improvements of wood properties due to furfurylation were reported, notably dimensional stability, hydrophobicity, mechanical properties and decay resistance (Sejati et al. 2017; Martha et al. 2021). Then, polyester made from organic acids combined with polyols have been tested by impregnation and in-situ polymerization by L’Hostis et al. (2018) and Mubarok et al. (2020) to improve beech wood properties. Kurkowiak et al. (2021, 2022) reported the use of organic acid and polyols to improve softwood properties. Acids previously used to form polyesters in wood were citric acid, tartaric acid and succinic anhydride, while polyols were mainly glycerol and sorbitol, and also, to a lesser extent, glucose or maltodextrin. After curing, polyesters were resistant to water leaching. In addition, the formation of polyester inside wood can imply improvement of dimensional stability and decay resistance (L’Hostis 2017; L’Hostis et al. 2018; Mubarok et al. 2020; Kurkowiak et al. 2021, 2022).
In this study, the combination of thermal pretreatment and chemical modification are investigated to evaluate if a better impregnation of chemicals in wood can be achieved after initial thermal treatment. Indeed, as seen previously, thermal treatment can increase wood porosity, which might be helpful for subsequent chemical impregnation, leading to higher level of modification with higher polymer charge inside wood (higher WPG). Synergies between both thermal and chemical treatments are also expected in order to improve wood properties, the combination of the two treatments being able to lead to better overall properties than each separately. Such synergistic effects have already been observed by Mubarok et al. (2019) using light chemical modifications prior to thermal modification, which resulted in an improvement of dimensional stability and biodegradation resistance, while mechanical properties were affected (Mubarok et al. 2019). Other synergy between chemical pretreatment combined with hot pressing has been demonstrated by Shi et al. (2020) in the case of NaOH/Na2SO3 wood pretreatment followed by compression in a heating press. This combination resulted in wood presenting higher densities, more than doubled, and markedly enhanced mechanical properties (Shi et al. 2020). Synergistic effects are expected between thermal and chemical modification, which might allow hardwood utilization in new areas, under variable moisture conditions, or in case high hardness is necessary, for decking as an example.
To our knowledge, this is the first time in the field of wood modification that three thermal treatment levels were combined with two different chemical modification methods in order to modify different hardwood species—alder (Alnus glutinosa), beech (Fagus sylvatica), birch (Betula pendula), cherry (Prunus avium), hornbeam (Carpinus betulus), maple (Acer pseudoplatanus) and wild service tree (Sorbus torminalis). These seven species have been chosen for their local availability and because they are likely to be easily impregnated. Furthermore, properties of modified woods are compared to control, notably density, dimensional stability and equilibrium moisture content after water immersion, and surface hardness.
2 Materials and methods
2.1 Wood material
Seven different hardwood species have been studied: alder (Alnus glutinosa), beech (Fagus sylvatica), birch (Betula pendula), cherry (Prunus avium), hornbeam (Carpinus betulus), maple (Acer pseudoplatanus) and wild service tree (Sorbus torminalis). Wood samples used in this study were wood blocks of 40 × 40 × 280 mm3 (rad. × tan. × long.), from mature heartwood free of any defect. Wood blocks were purchased from a German company LOGEMANN & WAIBEL (Spitzäcker 2, 74931 Lobbach). Ten replicates were tested for each wood species and thermal modification level. Air dry wood specimens were oven dried at 103 °C for 48 h and weighed to record oven dried mass (m0) before modification. Densities of studied species are presented in Table 1 below, and compared to literature data from Centre Technique du Bois (1972).
2.2 Thermal modification
Thermal modification of wood was performed immediately after drying in an open system laboratory plant of HNEE following conditions detailed in Table 2 in atmospheric oxygen atmosphere (Xu et al. 2019). The process includes a first heating phase at the rate of 10 K h−1 until the targeted temperature inside the oven, followed by a holding phase. Then, heating was stopped and wood material was allowed to cool down. Specimens were weighed after the thermal modification to record the mass of thermally modified samples (mTM).
Weight loss (WL) of each sample was calculated according to the following equation in order to evaluate the thermal modification level.
where m0 is the oven dried mass before modification (g) and mTM is the oven dried mass after thermal modification (g).
2.3 Chemical modification
Wood specimens from previous thermal modification were used in the two chemical modification processes. For this, samples from groups A, B, C, and untreated samples were cut in half so that they could be introduced into the autoclave. For each sample, one half was reserved for furfurylation and the other for polyester modification. Wood sample dimensions used chemical modification process were approximately 140 × 40 × 40 mm3 (long. × rad. × tan.). Samples were dried at 103 °C for 48 h before modification and anhydrous mass (mAnh) and dimensions (length. width and thickness—LAnh. WAnh. TAnh) were recorded.
2.3.1 Furfuryl alcohol modification process—impregnation and polymerization
The impregnation solution was prepared by mixing tartaric acid (TA), a reactive catalyst, with furfuryl alcohol (FA) and water. Tartaric acid (5% w/w) was first solubilized in distilled water (45% w/w). Thereafter, furfuryl alcohol (50% w/w) was added and the mixture was mixed thoroughly (± 15 min) till a homogenous FA solution was obtained.
Impregnation was carried out in a 3.5 L laboratory vacuum pressure reactor. Wood samples (10 replicates) were introduced in the autoclave reactor, the reactor was closed and subjected to a vacuum of 10 mbars for 15 min followed by introduction of the aqueous FA solution, followed by a 15 min waiting period under partial vacuum. After this vacuum step, the reactor was subjected to a pressure of 12 bars and maintained for 40 min at this pressure. The autoclave was finally opened to remove samples, which were then wiped with absorbent paper to remove the solution excess and weighed to record the impregnated sample mass (mImp) in order to check the solution uptake of the specimens. The solution uptake (SU) was calculated according to Eq. (2).
where mImp is the mass after solution impregnation (g) and mAnh is the anhydrous mass before impregnation (g).
The impregnated samples were then air-dried at room temperature for 48 h to evaporate the excess of water avoiding the appearance of cracks during the polymerization step. Samples were wrapped in aluminum foil to avoid furfuryl alcohol evaporation during curing and placed in a ventilated oven for polymerization. The oven temperature was set at 40 °C and maintained for 12 h. After this period. the oven temperature was increased by 0.5 °C min−1 from 40 to 120 °C and the temperature was maintained at 120 °C for 18 h. Heating process was then stopped and wood samples were allowed to cool down to room temperature. Samples were then unwrapped from foil and put back in the oven at 103 °C for 48 h to evaporate residual water. After cooling in a desiccator, samples were weighed (mPol) and their dimensions were measured (LPol. WPol. TPol).
Finally, weight percent gain (WPG) and swelling (ΔV) due to chemical modification were calculated using Eqs. (3) and (4) below:
where mPol is the mass after polymerization (g) and mAnh is the anhydrous mass before impregnation (g).
where VPol is the volume after polymerization (cm3). VAnh is the anhydrous volume before impregnation (cm3) and V = L × W × T (cm3).
2.3.2 Polyester modification process—impregnation and polymerization
The impregnation solution was prepared by mixing thoroughly succinic anhydride (26% w/w) with glycerol (17% w/w) on a heating plate to form a water-soluble ester (Fig. 1), which was dissolved in distilled water after cooling (57% w/w).
Wood samples impregnation was performed following the same cycle as that for furfurylation (see 2.3.1). The solution uptake (SU) was calculated to check the quantity of solution having penetrated the specimens according to Eq. (2). The impregnated samples were then placed in a ventilated oven at 140 °C for 24 h. After this curing period, oven temperature was set at 103 °C for 48 h in order to stabilize samples and evaporate residual water. The samples were weighed to obtain the polymerized mass of chemicals after treatment (mPol) and their dimensions were measured (LPol. WPol. TPol). Weight percent gain (WPG) and swelling (ΔV) were calculated using Eqs. (3) and (4).
2.4 Characterization
2.4.1 Equilibrium moisture content
Control and modified wood samples (10 replicates) with a sample size of 10 × 20 × 20 mm3 (long. × rad. × tan.) cut from the 140 × 40 × 40 mm3 specimens were first dried at 80 °C until they reached constant mass (m0), which means less than 0.5% mass difference between two weight measurements carried out 8 h apart. They were then conditioned at 20 °C and 80% relative humidity (RH) until they have reached constant mass (m80%RH). Equilibrium moisture content (EMC) was calculated according to the following Eq. (5):
where m80%RH is the specimen mass after conditioning at 80% RH and m0 is specimen mass after drying at 80 °C.
2.4.2 Sample swelling/anti-swelling efficiency
Dimensional stability was determined by immersion of wood specimens in water. Samples (10 replicates) with a size of 10 × 20 × 20 mm3 (long. × rad. × tan.) selected randomly per treatment or combination of treatments were put in a beaker with weight above to prevent them from floating. Then, distilled water was added to submerge the wood blocks and the beaker was placed in a desiccator. In order to achieve a high degree of water saturation, the desiccator was placed under vacuum for two 30 min phases spaced by 1 h at atmospheric pressure. Samples were then left submerged in water at atmospheric pressure for 24 h. After the immersion, samples were wiped with absorbent paper and their dimensions were measured (LSat. WSat. TSat). Swelling due to water immersion (ΔVSat) was calculated as follows (Eq. (6)):
where VSat is the water saturated volume after water immersion (cm3). VPol is the volume after polymerization (cm3), and the volume V (cm3) = L (cm) * W (cm) * T (cm).
Anti-swelling efficiency (ASE) was then used in order to evaluate the dimensional stability of modified wood. ASE was calculated according to the following formula (Eq. (7)):
where ΔVControl is the volumetric swelling of untreated wood (controls), and ΔVTreated wood is the volumetric swelling of treated wood.
2.4.3 Brinell hardness
Surface hardness according to Brinell has been measured following DIN EN ISO 6506-1 (2014) using a TIRA test 28025 E22 (International Organization for Standardization (ISO) 2014). Control and modified specimens (10 replicates for each specimens) were conditioned at 20 °C and 65% relative humidity until constant mass before being tested. The steel ball diameter was 10 mm. According to Schwab (1990), a nominal force (F) of 500 N was applied to the specimens in the radial and tangential directions (Schwab 1990). Presented values are the average of radial and tangential measurements. The nominal force was achieved after 15 s and was held for 30 s before being released in 15 s. The deepness (h) of the ball was determined from the deformation path and Brinell hardness (HB) was calculated by the following equation (Eq. (8)):
where F (N) is the target force, D (mm) is the diameter of the steel ball and h (mm) is the deepness of the impact.
3 Results
3.1 Weight loss due to thermal modification
Three thermal treatment levels—160 °C (group A), 180 °C (group B) and 200 °C (group C)—were tested on samples from seven different wood species. Weight loss (WL) due to thermal modification was recorded for each sample as an indicator of efficiency of thermal modification (Hill 2006; Čermák et al. 2021).
Figure 2 shows weight loss for the different wood species/treatment level combinations.
First observation is that thermal treatment temperature is the most influential factor on weight loss, while wood species do not impact a lot. Indeed. For all wood species, increase of weight losses is directly linked to increase in treatment temperature. Regardless of the wood species, samples treated at 160 °C (group A) have average weight losses between 1 and 3%, which correspond more to intensive drying than to thermal degradation due to heat treatment involving degradation of wood’s extractives and of the most sensitive wood components like hemicelluloses and lignin. For samples treated at 180 °C (group B), average weight losses reach 5 to 7% for all species except for birch whose average weight loss is below 2%. Birch samples appear to be more resistant to heat treatment and more thermally stable than other tested wood species at this temperature (180 °C). Weight loss between 5 and 7% corresponds to degradation of more sensitive hemicelluloses (Sivonen et al. 2002). Hornbeam shows a higher variability than other species, which might be explained by natural variability of wood. Treatment at 200 °C (group C) leads to average weight losses between 15 and 24% depending on wood species. Birch and hornbeam are less affected by thermal treatment (WL < 16%), while wild service tree, beech and cherry are the most affected (WL > 22%).
3.2 Chemical modification: solution uptake, weight percent gain, swelling and impact of thermal pretreatment on chemical modification
Two chemical modification methods employing aqueous solution were tested on native wood and heat- treated wood: on the one hand furfurylation using furfuryl alcohol (FA) and tartaric acid, on the other hand polyester modification (PE) involving succinic anhydride with glycerol. Impregnation of the solution inside wood samples was performed in an autoclave following a vacuum-pressure cycle. Impregnated samples were then cured in an oven to polymerize the chemical inside wood while evaporating water. Solution uptake (SU) was recorded to check the success of the impregnation, while weight percent gain (WPG) was recorded after the polymerization step to know the quantity of polymer impregnated in the wood at the end of the process. Swelling of the samples was also measured, because it indicates whether the polymer is located in the vessels or in the cell walls.
As can be seen in Fig. 3, solution uptakes (SU) were relatively high for all species. These are higher than 100 w/w %, except for cherry impregnated with the polyester oligomers solution, for which SU was below 70% for control and the two lower thermal modification levels. Alder had the highest solution uptake (over 178%) compared to other wood species regardless of prior thermal modification and type of impregnation solution. This might be explained by the low density of alder wood, which had the lowest anhydrous density among the seven studied species, below 0.51 g cm−3 before chemical modification (see Fig. 4). In the same way, birch is the second lightest species (initial density = 0.56 g cm−3) and has the second highest SU after alder (SU ≥ 140%). On the contrary, hornbeam is the species presenting the highest initial density (0.75 g cm−3) and the lower solution uptake below 122%. Thermal modification does not change a lot the impregnability of the studied wood species. In the case of furfurylation, the increase of thermal pretreatment severity appears to have some effects on impregnability (for alder, beech, hornbeam, maple, wild service tree), but no effect on birch. For polyester modification, the different thermal pretreatments do not improve the SU for alder, birch and maple. For cherry and hornbeam, increasing thermal pretreatment severity leads to improvement in the SU, while on the contrary, it leads to decrease in the SU for beech and wild service tree. Cherry wood does not behave like other wood species, which might be related to its particular anatomy. Indeed, cherry wood is the only semi-ring-porous wood among the seven studied species, while the six other hardwood species being diffuse porous wood.
Figure 3b shows the weight percent gain (WPG) after the whole process, i.e. after polymerization and post-drying. WPG value takes into account all the phenomena occurring during the treatment and thus depends on the quantity of impregnated reactants and their polymerization degree (polyfurfuryl alcohol and polyester formation occurring by polycondensation, a molecule of water being released for each bond). WPG results follow closely the same trend as SU results. Thus, alder had the highest WPG, between 65 and 70 w/w % for polyester modification and over 72% for furfurylation. Birch and maple WPG come after (WPG between 50 and 66% whatever the treatment), followed by wild service tree (41 to 58% WPG) and beech (42 to 50% WPG). Cherry and hornbeam, which have the two lowest SU, also show the lowest WPG, between 21 and 52%. The initial thermal modifications do not seem to influence the WPG. In some cases, the increase of thermal treatment severity leads to higher WPG (alder and hornbeam with furfurylation. cherry and hornbeam with polyester modification), while for wild service tree, treatment with polyester leads to decrease in WPG. For the other combinations of species and chemical modifications, the impact of thermal modification on WPG is not really significant.
Figure 3 shows the swelling or volume change (ΔV) due to chemical modification processes. Swelling represents the percentage volume change between anhydrous state before impregnation step and anhydrous state after polymerization and post-drying. In most cases, volume change is positive showing that samples have swollen after the chemical modification process. Overall, modification with polyester leads to higher swelling than furfurylation. For native wood, i.e. not thermally modified before chemical modification, swelling for polyester modification ranges from 11 to 22%, while it is limited to – 2 to 12% for furfurylation. This indicates that polyester might have more affinity for cell wall polysaccharides than furfuryl alcohol, leading to higher swelling. Polymers are probably rather more located in the vessels and presented less affinity for wood polymers in case of furfurylation than with polyester modification, reason why swelling is lower for furfurylated samples. Highest swellings are observed with polyester modification for native beech (22%) followed by native hornbeam and native maple (18% for both). On the contrary, in the case of furfurylation, swelling in thermally modified birch and cherry is negative (− 3 to − 4%) except for the highest heat treatment temperature (200 °C). Thermal modification prior to chemical modification induces a decrease in swelling due to thermal degradation reaction. With polyester, the higher the thermal treatment temperature, the lower the swelling for all tested wood species. The same trend is observed for furfurylation with hornbeam and wild service tree. Note that values for hornbeam and wild service tree heat-treated at 200 °C are not available (loss of specimens due to excessive heat treatment). For furfurylation with beech, birch, cherry and maple, increasing the thermal treatment temperature induces a decrease in swelling except at 200 °C. Indeed, specimens treated at 200 °C swell more than others (native or heat-treated at 160 °C or 180 °C) for these four species. It might be possible that for these species, the cell-wall polymers structure was broken due to higher thermal modification level leading to more swelling at the chemical modification step. Swelling in furfurylated alder is between 2 and 7% and unlike other species, increasing thermal treatment temperature leads to higher swelling.
3.3 Density
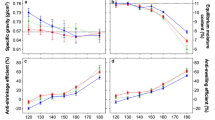
Wood density reflects the effects of wood modification. Wood densities are shown in Fig. 4 for native, thermally treated and chemically modified woods.
Thermal modification leads to lower densities due to degradation of the most sensitive wood components. Thus, as treatment temperature increases, decrease in density is observed in the thermally modified samples. Conversely, chemical modifications imply adding polymer into wood porosity (instead of air), which leads to increased densities compared to native wood. Before any modification, initial density varies from 0.50 g cm−3 for alder to 0.75 g cm−3 for hornbeam. Without thermal pretreatment, furfurylation gave the highest densities between 0.85 g cm−3 (alder and maple) and 0.97 g cm−3 (hornbeam) in best cases, while polyester modifications resulted in moderate increase in density, which reaches 0.68 g cm−3 (cherry) to 0.87 g cm−3 (hornbeam). Effect of combination of thermal pretreatment with chemical modification on density is more important in the case of furfurylation compared to esterification. Thermal pretreatment has no effect on final density for wood modified with polyester, while for furfurylation, increase in treatment temperature induces a decrease in density after chemical modification, especially at 200 °C temperature for alder, beech, birch, cherry and maple (no material for hornbeam and wild service tree pretreated at 200 °C). Concerning density variations after furfurylation, highest density increases are observed for furfurylated alder and birch reaching 68–78% and 58–69% respectively, depending on thermal pretreatment temperature. These two species have the lowest initial densities, which explains such an increase in density. On the contrary, hornbeam and beech, which are the densest woods, display the lowest density change after furfurylation: 31–42% for hornbeam and 30–34% for beech. Looking at the polyester modification, the highest density increase is obtained for alder (lightest species) reaching 49–55%, while it is only 9–32% for cherry, probably due to a lower impregnation of polyester oligomer solution in cherry leading to a lower WPG. The increase in density for the densest species (hornbeam) reaches 13–24% depending on thermal pretreatment temperature.
3.4 Equilibrium moisture content
Specimens from the three processes, i.e. thermal modification, combination of thermal modification with furfurylation or with polyester modifications, were conditioned at 20 °C/80% relative humidity (RH) in order to determine the equilibrium moisture content (EMC) knowing anhydrous mass. This experiment was done to evaluate the decrease in the hydrophilicity of the material resulting from the different modification processes. EMC results after conditioning at 20 °C/80% RH are shown in Fig. 5.
For control samples, EMC is approximately 16% independently of the wood species. Thermal modification leads to decreased EMC for all species, which implies that as treatment temperature increases, EMC decreases. Compared to native wood, hydrophilicity of thermally modified wood at 200 °C decreases more or less according to the wood species: 55% (from 16.1 to 7.3% EMC) for cherry and 66% (from 16.4 to 5.7% EMC) for wild service tree, other species being in this range. Chemical modifications also allow decreasing wood EMC. For not thermally modified wood, EMC decrease is 7% (from 15.6 to 14.5% EMC) for hornbeam and varies from 45% (from 16.1 to 8.8% EMC) for cherry to 58% (from 16.1 to 6.8%) for alder, other species being in this range after furfurylation (no data for birch). For polyester treatment, the decrease in EMC ranges from 29.9 (from 16.1 to 11.3% EMC) for cherry to 52.3% (from 16.1 to 7.7% EMC) for alder. For combinations of thermal pretreatment with chemical modifications, hydrophilicity of modified wood can be further decreased compared to only heat-treated wood, especially for low heat treatment temperature (160 and 180 °C). However, chemical modifications do not significantly improve EMC compared to thermal modification at 200 °C. Finally, higher EMC decreases are obtained for thermal modification at 200 °C or combination of lower temperature heat treatment (160 or 180 °C) with chemical modification. Overall, for the same heat treatment temperature and species, improvement with furfurylation seems to be slightly better than with polyester.
3.5 Dimensional stability: swelling in water and anti-swelling efficiency
Dimensional instability is one of the drawbacks of wood. Dimensional stability test was performed to evaluate the swelling decrease resulting from the modifications processes. After drying, specimens (control, heat-treated, chemically modified) were impregnated by immersion in distilled water under vacuum to reach their maximum swelling. Swelling values were then used to calculate anti-swelling efficiency (ASE). Swelling and ASE results are presented in Fig. 6a and b, respectively.
Swelling in control samples due to water uptake ranges from 15% (alder) to 26% (beech), which agrees with bibliographic data (Benoit 2008; Gérard et al. 2016). Thermal modification leads to a strong decrease in swelling. Moreover, swelling decreases as the treatment temperature increases and this is valid for all tested species. Decrease in swelling is expected since thermal modification is a process known to improve dimensional stability. Chemical modifications, either furfurylation or polyester modification, also induce a strong decrease of swelling except for cherry. Without thermal pretreatment, modification with polyester confers a higher reduction of water swelling than furfurylation. Combination of thermal and chemical modification leads to even higher decrease of swelling in most cases. Thus, increasing thermal pretreatment severity, i.e. the temperature, allows decreasing swelling for furfurylated alder, beech, cherry at each temperature level and for furfurylated birch and maple at 200 °C. Data are not available for hornbeam and wild service tree pretreated at 200 °C. Thermal pretreatment at lower temperature seems to have no effect on these two furfurylated species. Effect of prior thermal modification appears less significant in case of polyester modification at least for alder, beech, birch, hornbeam and maple. It seems significant for cherry and for wild service tree pretreated at 200 °C, for which highest pretreatment temperature leads to lowest swelling.
ASE indicates the reduction percentage of swelling in water due to a treatment compared to untreated control samples. Since thermal modification leads to decrease of swelling, it is logical that thermal treatment has an anti-swelling effect by itself. Depending on thermal treatment temperature, ASE ranges from 12% (cherry) to 34% (maple) at 160 °C and from 16% (hornbeam) to 47% (maple) at 180 °C. The highest ASE is obtained with the 200 °C temperature for the seven tested species and varies from 63% (alder) to 68% (beech and maple). Considering only chemical modifications (applied to native wood), ASE ranges from 46% (alder) to 70% (maple) for furfurylated specimens and from 39% (cherry) and 77% (birch) for polyester modified specimens. Highest ASE values were obtained with 200 °C thermal modification combined with furfurylation for alder (80%), beech (79%), cherry (78%) and maple (80%) and with polyester modification for non-thermally treated birch (76%), hornbeam pretreated at 180 °C (70%), no data for hornbeam pretreated at 200 °C and wild service tree pretreated at 200 °C (73%). Overall, thermal pretreatment allows a gradual increase of ASE values obtained after furfurylation for alder and beech and higher ASE values for birch, cherry and maple at 200 °C, but does not seem significant for hornbeam and wild service tree (no data at 200 °C for these two species). Except for cherry, thermal pretreatment does not impact anti-swelling efficiency.
3.6 Hardness improvement
After conditioning, surface hardness of wood specimens (control, thermally modified and chemically modified) was tested according to Brinell in order to evaluate puncture resistance. Brinell hardness results are shown in Fig. 7.
Brinell hardness of untreated specimens varies from 20 N mm−2 (alder) to 34 N mm−2 (hornbeam). Thermal modification leads to slightly reduce surface hardness of wood, especially at the 200 °C temperature. This is probably due to degradation of wood component occurring during the thermal treatment. Beech and maple are the most affected species, their hardness decreased proportionally from 30 and 28%, respectively, due to thermal modification at 200 °C. Hornbeam hardness is not affected by thermal modification at 200 °C (only − 0.3%), while for other species, hardness decreases due to treatment at 200 °C and ranges between 18% (birch and cherry) and 25% (alder). Interestingly, thermally modified hornbeam at 180 °C presents a higher hardness than native wood (45 N mm−2 + 32%), which was not expected and difficult to explain based on the data of the current study. Regarding chemical modification, furfurylation and polyester do not confer the same increase in hardness. Indeed, furfurylation leads to increase in surface hardness, while polyester modification barely improved it. Without thermal modification, for all species hardness ranges between 61 N mm−2 (beech) and 85 N mm−2 (birch) after furfurylation. Furfurylation allows increasing Brinell hardness with over 97% (furfurylated beech, 61 N mm−2) to over 316% (furfurylated cherry, 85 N mm−2). As for control specimens, furfurylated hornbeam hardness values are the highest and are between 83 and 86 N mm−2 independently of the thermal pretreatment temperature. Only hardness of furfurylated birch without pretreatment also reached 85 N mm−2. When combined with furfurylation, thermal pretreatment does not significantly affect resulting surface hardness even at 200 °C temperature, this latter one being lower for most wood species (except hornbeam). Without thermal pretreatment, hardness of polyester modified wood varies from 23 N mm−2 (alder) to 49 N mm−2 (hornbeam) and increase in hardness ranged between + 7% (beech) and + 43% (hornbeam). Overall, thermal modification prior to polyester modification seems insignificant for alder, beech, birch, hornbeam and maple. For cherry and wild service tree, thermal pretreatment leads to lower hardness. This decrease in hardness is consistent for cherry, while for wild service tree, only the 200 °C pretreatment seems to induce a lower hardness.
4 Conclusion
Seven European hardwood species have been modified using thermal modification, chemical modification (furfurylation or polyester modification) or combination of both thermal and chemical modifications. Different properties of modified woods like solution uptake, weight percent gain, density, equilibrium moisture content, swelling, dimensional stability and surface hardness were characterized to evaluate the interest of the thermal pretreatment before chemical modification.
Thermal modification and chemical modification carried out separately behave as expected. Weight losses due to thermal pretreatment depended on the wood species used and on the treatment temperature; the higher the treatment temperature, the higher is the weight loss recorded. Weight percent gain due to impregnation into the wood is coherent with what can be expected and is also, as for thermal modification, dependent on the wood species used but also on the impregnation solution.
Effect of the thermal pretreatment on wood impregnation during chemical modification is more difficult to evaluate an interpret even if differences may be observed depending on the wood species and on the temperature of thermal pretreatment. Indeed, solution uptake and weight percent gain remain stable, slightly increase or decrease according to the treatment and the wood species. Except for one case, density of all species subjected to thermal pretreatment followed by chemical modification decreased as the temperature of pretreatment increased, whatever the chemical modification carried out. Wood equilibrium moisture is also more reduced after thermal pretreatment at higher temperature followed by chemical modification. This reduction in EMC depends on the nature of the chemical modification envisaged, treatment with polyester presenting higher EMC values compared to furfurylation. In all cases, the EMC obtained for combined treatment are very close to EMC obtained for thermal modification alone. Combination of thermal pretreatment and chemical modification allows to reduce swelling and increase ASE of all species tested comparatively to treatments carried out alone. Brinell hardness was more important in the case of furfurylated samples, while modification with polyester resulted in a lower increase of surface hardness. Pretreatment at 200 °C before chemical modification resulted in a decrease of Brinell hardness comparatively to samples pretreated at lower temperature showing the impact of thermal degradations on wood surface hardness.
Combination of thermal modification pretreatment to subsequent chemical modification treatment allows to obtain materials presenting higher Brinell hardness, lower equilibrium moisture content and higher dimensional stability, especially in the case of furfurylation treatment. Further investigations implying thermal modification resulting in 5–10% weight loss, combined with furfurylation using a more concentrated furfuryl alcohol impregnation solution would lead to even greater improvements in terms of density, dimensional stability and surface hardness, which would allow the use of modified hardwood in applications such as joinery, decking or flooring, music instruments, and handles for tools or kitchen utensils.
References
Benoit Y (2008) Le guide des essences de bois. Deuxième édition. FCBA : Forêt. Cellulose, Bois,Construction, Ameublement, Paris [The guide to wood species. Second edition. FCBA: Forest, Cellulose, Wood, Construction, Furniture. Paris] ISBN13 978-2-212-67634-1
Čermák P, Hess D, Suchomelová P (2021) Mass loss kinetics of thermally modified wood species as a time–temperature function. Eur J Wood Prod 79:547–555. https://doi.org/10.1007/s00107-020-01634-6
Cuadrado J, Zubizarreta M, Pelaz B, Marcos I (2015) Methodology to assess the environmental sustainability of timber structures. Constr Build Mater 86:149–158. https://doi.org/10.1016/j.conbuildmat.2015.03.109
Centre Technique du Bois (1972) Principaux Bois Indigènes et Exotiques Utilisés en France. Caractéristiques sommaires et emplois. [Main indigenous and exotic woods used in France. Principal Characteristics and uses.-] 4th Edition, Paris
Damay J (2014) Développement de nouveaux traitements du bois basés sur le procédé d’imprégnation axiale [Development of new wood treatments based on the axial impregnation process] PhD Université de Lorraine http://docnum.univ-lorraine.fr/public/DDOC_T_2014_0178_DAMAY.pdf. Accessed 9th Oct 2023
De Windt I, Van den Bulcke J, Wuijtens I, Coppens H, Van Acker J (2014) Outdoor weathering performance parameters of exterior wood coating systems on tropical hardwood substrates. Eur J Wood Prod 72:261–272. https://doi.org/10.1007/s00107-014-0779-7
Deka M, Saikia CN (2000) Chemical modification of wood with thermosetting resin: effect on dimensional stability and strength property. Bioresour Technol 73:179–181
Gérard J, Guibal D, Paradis S, Cerre JC (2016) Atlas des bois tropicaux : Caractéristiques technologiques et utilisations. [Atlas of tropical woods: technological characteristics and uses] Editions Quae. Versailles
Gérardin P (2016) New alternatives for wood preservation based on thermal and chemical modification of wood—a review. Ann for Sci 73:559–570. https://doi.org/10.1007/s13595-015-0531-4ï
Haag V, Koch G, Melcher E, Welling J (2020) Characterization of the wood properties of cedrelinga cateniformis as substitute for timbers used for window manufacturing and outdoor applications. Maderas: Ciencia y Tecnologia 22:23–36. https://doi.org/10.4067/S0718-221X2020005000103
Herold N, Dietrich T, Grigsby WJ, Franich RA, Winkler A, Buchelt B, Pfriem A (2013) Effect of maleic anhydride content and ethanol dilution on the polymerization of furfuryl alcohol in wood veneer studied by differential scanning calorimetry. BioResources 8:1064–1075
Hill CAS (2006) Thermal modification of wood. In: Stevens C (eds) Wood modification: chemical. Thermal and other processes. John Wiley & Sons. Ltd. Chichester. UK, pp 99–127
Ibach RE, Rowell RM (2013) 16 Lumen Modifications. In: Rowell RM (ed) Handbook of wood chemistry and wood composites. Second. CRC Press, Boca Raton, pp 599–625
International Organization for Standardization (ISO) (2014) Metallic materials—Brinell hardness test—Part 1 : test method. DIN EN ISO 6506-1 1-22
Jang ES, Kang CW (2019) Changes in gas permeability and pore structure of wood under heat treating temperature conditions. J Wood Sci 65(37):2–9. https://doi.org/10.1186/s10086-019-1815-3
Kurkowiak K, Emmerich L, Militz H (2021) Wood chemical modification based on bio-based polycarboxylic acid and polyols-status quo and future perspectives. Wood Mater Sci Eng 17(5):1–15
Kurkowiak K, Emmerich L, Simmering C, Militz H (2022) Wood modification with citric acid and sorbitol—a review and future perspectives. In: Proceedings of the Tenth European Conference on Wood Modification. Université de Lorraine. Nancy. pp 32–39
Leboucher B. (2014) Fabriquer en bois massif : anticiper les variations. Le Bouvet 21–33 [Solid wood manufacturing: anticipating variations. Le Bouvet 21–33]
L’Hostis C (2017) Développement de nouveaux traitements non-biocides de protection du bois basés sur la formation in-situ de polyesters bio-sourcés. [Development of new non-biocidal wood protection treatments based on the in-situ formation of bio-sourced polyesters] PhD, Université de Lorraine. http://docnum.univ-lorraine.fr/public/DDOC_T_2017_0319_L_HOSTIS.pdf. Accessed 9th Oct 2023
L’Hostis C, Thévenon MF, Fredon E, Gérardin P (2018) Improvement of beech wood properties by in situ formation of polyesters of citric and tartaric acid in combination with glycerol. Holzforschung 72:291–299. https://doi.org/10.1515/hf-2017-0081
Martha R, Mubarok M, Batubara I, Rahayu IS, Setiono L, Darmawan W, Obounou AF, George B, Gérardin C, Gérardin P (2021) Effect of furfurylation treatment on technological properties of short rotation teak wood. J Market Res 12:1689–1699. https://doi.org/10.1016/j.jmrt.2021.03.092
Mehr J, Vadenbo C, Steubing B, Hellweg S (2018) Environmentally optimal wood use in Switzerland—investigating the relevance of material cascades. Resour Conserv Recycl 131:181–191. https://doi.org/10.1016/j.resconrec.2017.12.026
Monteiro G (2018) Structural characterization of heat-treated poplar wood. J Eng 4:9–26. https://doi.org/10.24840/2183-6493_004.001_0002
Mubarok M, Dumarcay S, Militz H, Candelier K, Thévenon MF, Gérardin P (2019) Comparison of different treatments based on glycerol or polyglycerol additives to improve properties of thermally modified wood. Eur J Wood Prod 77:799–810. https://doi.org/10.1007/s00107-019-01429-4
Mubarok M, Militz H, Dumarçay S, Gérardin P (2020) Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci Technol 54:479–502. https://doi.org/10.1007/s00226-020-01172-7
Németh R, Horváth N, Fodor F, Báder M, Bak M (2020) Wood Modification for Under-Utilised Hardwood Species. In: IOP Conference Series: Earth and Environmental Science. 505. https://doi.org/10.1088/1755-1315/505/1/012017
Nepal P, Skog KE, Mc Keever DB, Bergman RD, Karen L, Robert C (2016) Carbon mitigation impacts of increased softwood lumber and structural panel use for nonresidential construction in the United States. For Prod J 66:77–87. https://doi.org/10.13073/FPJ-D-15-00019
Ninane M, Pollet C, Hébert J, Jourez B (2021) Physical, mechanical and decay resistance properties of heat-treated wood by Besson® process of three European hardwood species. Biotechnologie, Agronomie, Société et Environnement 25:129–139. https://doi.org/10.25518/1780-4507.19050
Parobek J, Paluš H, Moravčík M, Kovalčík M, Dzian M, Murgaš V, Šimo-Svrček S (2019) Changes in carbon balance of harvested wood products resulting from different wood utilization scenarios. Forests 10(7):590. https://doi.org/10.3390/f10070590
Salman S, Thévenon MF, Pétrissans A, Dumarçay S, Candelier K, Gérardin P (2017) Improvement of the durability of heat-treated wood against termites. Maderas: Ciencia y Tecnologia 19:317–328. https://doi.org/10.4067/S0718-221X2017005000027
Sandberg D, Kutnar A, Mantanis G (2017) Wood modification technologies—a review. Iforest 10:895–908
Schwab E (1990) Die Härte von Laubhölzern für die Parkettherstellung. Holz Als Roh- Und Werkstoff 48:47–51. https://doi.org/10.1007/BF02610703
Sejati PS, Imbert A, Gérardin-Charbonnier C, Dumarçay S, Fredon E, Masson E, Nandika D, Priadi T, Gérardin P (2017) Tartaric acid catalyzed furfurylation of beech wood. Wood Sci Technol 51:379–394. https://doi.org/10.1007/s00226-016-0871-8ï
Shi J, Peng J, Huang Q, Huang Q, Cai L, Shi SQ (2020) Fabrication of densified wood via synergy of chemical pretreatment. Hot-pressing and post mechanical fixation. J Wood Sci 66:1–9. https://doi.org/10.1186/s10086-020-1853-x
Silva C, Branco JM, Camões A, Lourenço PB (2014) Dimensional variation of three softwood due to hygroscopic behavior. Constr Build Mater 59:25–31. https://doi.org/10.1016/j.conbuildmat.2014.02.037
Sivonen H, Maunu SL, Sundholm F, Jämsä S, Viitaniemi P (2002) Magnetic resonance studies of thermally modified wood. Holzforschung 56:648–654
Srinivas K, Pandey KK (2012) Effect of heat treatment on color changes. Dimensional stability. and mechanical properties of wood. J Wood Chem Technol 32:304–316. https://doi.org/10.1080/02773813.2012.674170
Todaro L, Liuzzi S, Pantaleo AM, Lo GV, Moretti N, Stefanizzi R (2015) Thermo-modified native black poplar (Populus nigra l.) wood as an insulation material. Iforest 14:268–273. https://doi.org/10.3832/ifor3710-014
Van Benthem M, Kremers J, Oldenburger J, Stam N, Sleurink N (2018) Les importations de bois tropicaux en Europe : à quel point sont-elles durables ? [Tropical wood imports into Europe: how sustainable are they?] https://www.idhsustainabletrade.com/uploaded/2018/08/EU-market-share-of-verified-sustainable-tropical-timber_IDH_STTC_Probos-report_June_2018_FR.pdf
Xu J, Zhang Y, Shen Y, Li C, Wang Y, Ma Z, Sun W (2019) New perspective on wood thermal modification: Relevance between the evolution of chemical structure and physical-mechanical properties and online analysis of release of VOCs. Polymers 11:1145. https://doi.org/10.3390/polym11071145
Zauer M, Pfriem A, Wagenführ A (2013) Toward improved understanding of the cell-wall density and porosity of wood determined by gas pycnometry. Wood Sci Technol 47:1197–1211. https://doi.org/10.1007/s00226-013-0568-1
Zhang X, Chen J, Dias AC, Yang H (2020) Improving carbon stock estimates for in-use harvested wood products by linking production and consumption—a global case study. Environ Sci Technol 54:2565–2574. https://doi.org/10.1021/acs.est.9b05721
Funding
The authors gratefully acknowledge the Chambre Franco-Allemande de Commerce et d’Industrie (CFACI). Central Innovation Program for SMEs (ZIM) in Germany and Banque Publique d'Investissement (BPI) in France for their financial support.
Author information
Authors and Affiliations
Contributions
MJ, AP, and PG define and supervise the research program. JD, TB and CM did experimental work. JD write the first draft of the paper. MC. PJM, RR, EF, TB, CM, MJ, AP, reviewed the article. PG and JD reviewed and validated the last draft of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Damay, J., Bender, T., Munk, C. et al. Properties improvement of seven hardwood species by combination of thermal and chemical modifications. Eur. J. Wood Prod. 82, 93–106 (2024). https://doi.org/10.1007/s00107-023-02000-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-023-02000-y