Abstract
To diminish the formaldehyde emission, replacement of the formaldehyde by furfural in urea formaldehyde (UF) resin was investigated and its effect on formaldehyde emission and physical–mechanical properties of particleboard panels produced from poplar wood was examined. Resin type: Industrial UF, Laboratory UF, UF-Furfural (in 25 and 50 % replacement levels), and two press temperatures including 170 and 180 °C were considered as variables. Results indicated that formaldehyde emission and modulus of rupture (MOR) of panels reduced thereby replacing the formaldehyde by furfural in UF resin. Internal bonding (IB) of panels made using 50 % replacement-modified resin was superior to others. Water absorption of panels decreased after 2- and 24-h immersion of samples with modified resins, as opposed to thickness swelling. The minimum thickness swelling was observed in panels made by Industrial UF resin. It is noteworthy that formaldehyde emission enhanced by increasing the temperature from 170 to 180 °C, and also all physical–mechanical characteristics developed at 180 °C temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, due to excessive dependence of global need on fossil resources for supplying energy and other various products, sustainable production has become an issue in that its development relies on changing from petro-chemical products to renewable materials. The majority of fossil fuel-based materials have the potential to become environmental pollutants during use and carry end-of-life cycle concerns such as disposal, pollution, and degradation. As a result, the need to decrease pollution caused by petrochemical usage is currently impelling the development of sustainable technologies. Amino-plastic resins are major coupling agents in manufacturing wood composite products. Urea formaldehyde as the major binder in particleboard industry is cured rapidly and provides desired performance in practical uses (Roffael et al. 2010). Although panels manufactured with this resin have a limited strength regarding moisture and temperature, water solubility, non-colored cured polymer, low cost, excellent thermal properties, and compatibility with different curing conditions of this resin offer other advantages (Park et al. 2011; Akyüz et al. 2010; Boran et al. 2011). There are approximately 11 million metric tons of UF resin produced annually throughout the world. Formaldehyde emission is the primary drawback of UF resin application in wood-based composites (Maloney 1993; Pizzi 1994; Conner 1996; Dunky 1998). Formaldehyde emission depends on F/U (formaldehyde to urea) mole ratio, resin dose, amount of catalyst and its proportion, moisture and its distribution in mat, storage time before use (Roffael et al. 2010; Pizzi 1994; Que et al. 2007a, b), and press temperature (Wang et al. 2002; Jiang et al. 2002). The majority of formaldehyde emission originates from: (1) uncondensed or free formaldehyde in resin, and (2) hydrolysis of formaldehyde from UF resin during service life (Ko 1976; Tomita 1980; Park et al. 2009). Industrial application of UF resin was restricted by International Agency Research on Cancer (IARC) because of its harmful side effects that lead to nose and throat cancer in humans (Salem et al. 2011). Thus, there is an urgent need to develop formaldehyde-free wood resins from renewable materials. Formaldehyde emission is a non-negligible factor in evaluation of environmental effects and health safety of wood-based panels. The primary route to reducing the formaldehyde emission is the use of an alternative non-volatile and non-toxic aldehyde in formulation of urea-based resin (Despres et al. 2010). Furfural is a bio-based furan aldehyde obtained through acid hydrolysis followed by acid dehydration of polymeric pentoses from renewable sources like agricultural/industrial wastes (corn cob, rice husk and oat meal) (Garcia et al. 2004). Bagasse and agricultural wastes are the major sources of commercial furfural production. Furfural has been introduced as an excellent aldehyde because of its fair reactivity, capability to form a strong co-polymer, low volatility and it being a low cost bio-based chemical (Schneider and Phillips 2010). Beside the several studies that have been conducted on the possibility of furans application in wood-adhesive formulation, their industrial utilization is still relatively low (Belgacem and Gandini 2003). Partial replacement of formaldehyde by furfural in PF resin formulation for plywood manufacturing has been reported (Belgacem and Gandini 2003).
There is no documented research on the application of furfural in UF resin formulation. Based on that, the present study was aimed at investigating the possibility of replacement of formaldehyde by furfural in UF resin formulation and its effect on formaldehyde emission and physical–mechanical properties of panels produced with modified resin.
2 Materials and methods
2.1 Preparation of resins
Laboratory grade chemicals including formaldehyde, urea and furfural were purchased from Merck Company (Merck-Schuchardt). All other chemicals were purchased as laboratory grade from local markets. UF resin (Laboratory UF, B) was synthesized according to the method presented by Pizzi (2003), with small modifications in which formaldehyde-to-urea mole ratio (F/U) was set to 1.1:1 at the beginning of the reaction. Briefly, urea was added to the formaldehyde with mole ratio F/U of 2.12:1 (pH 8.5) at 70 °C until complete dissolution of urea. The methylolated urea was prepared by methylolation stage at pH 7.6 and 90 °C. Condensation stage was carried out at pH 4.8 and 98 °C. This stage was stopped by adding 22 % NaOH solution to pH 8.7. Vacuum distillation of the reaction water with concomitant cooling was then initiated. After distillation of the amount of water needed to reach a resin content of 60–65 %, the resin was cooled to 40 °C, second urea was added to reach the 1.1 molar ratio, the pH was adjusted to 8.5–8.7.
The resin was kept at ambient temperature for 2 days to fulfill the condensation reaction. Urea formaldehyde furfural (UFF) resin was synthesized approximately the same as synthesis of UF resin by replacement of the formaldehyde by 25 % (C) and 50 % (D) (volume:volume) of furfural. The formaldehyde-furfural:urea (fo-fu:urea) molar ratios of 4.16:1 and 4.1:1 were used in UFF synthesis, respectively. Furfural was added to the UF mixture in the alkaline stage (pH 7) and after methylolation stage at 70 °C. The condensation stage and all other sequences (except for second urea) were conducted in the same manner as sequences of synthesis of UF resin. Industrial UF resin (A) was used as the control sample in the experiments. Physical–chemical characteristics of prepared resins are summarized in Table 1.
2.2 Particleboard preparation
For this purpose, poplar particles (Populus nigra) were prepared using a Pallmann laboratory chipper with 26.03 slenderness ratio, flatness ratio of 3.56, and apparent ratio of 7.7 and dried in a cylinder dryer at 80 °C for 3.5 h to a moisture content of 4–5 %, and then kept in plastic bags until the actual production of panels. The wood particles were placed in a rotating drum-type blender for uniform diffusion of resin and the resin was applied by spraying in the blender. Particleboards with a density of 0.71 g cm–3 and thickness of 12 mm were prepared using press temperature of 170 and 180 °C, homogeneous particle mat moisture of 12 %, resin content of 10 % (based on dry particle weight) along with 1 % (based on solid resin) of catalyst (ammonium chloride), press time of 7 min, and finally press pressure of 35 kg cm–2. Laboratory panels were made using a laboratory press of BURKLE LA160. Prepared panels were kept at conditioned climate for 15 days to reach equilibrium moisture content.
2.3 Characteristics of panels and formaldehyde emission
The test samples were prepared according to EN 310, 317 and 322 standard methods (EN 310 1993; EN 317 1993; EN 322 1993) and kept in the chamber at 20 ± 1 °C and 65 ± 5 rh for 1 week. Modulus of rupture (MOR), internal bonding (IB), thickness swelling (TS), and water absorption (WA) of the samples were measured according to mentioned standard. The formaldehyde emission test was carried out according to Japanese Standard JIS A 5908 test method (JISA 5908 1994), where samples were kept in a water-filled desiccator of 12 × 6 cm2. Absorbed formaldehyde on water was used to determine the gas emission from boards photo-spectroscopically at 410 nm.
2.4 Fourier transform infrared spectroscopy (FT-IR)
IR spectroscopy over the wave number range of 0–4000 cm−1 was used to evaluate the status of bonds and functional groups in synthesized resins. IR spectra were recorded by a Perkin-Elmer Spectrum RXI FT-IR Spectrometer.
2.5 Statistical analysis
Eight treatments were obtained by combination of variables and their level amounts (resin type in four different levels and two press temperature levels). Three panels of 40 × 40 cm2 were made for each treatment, and three samples were prepared (according to cutting pattern) from each panel for tests. Eventually, nine samples (replication) were prepared for each test. Data were statistically analyzed using IBM Statistics Software (SPSS) ver. 16, in a completely randomized design to test the differences among factors and levels. The comparison among the means with 95 % confidence intervals and grouping of data (letters: a–d) was performed using a Duncan’s multiple range test (DMRT) at a 95 % confidence levels.
3 Results and discussion
3.1 Mechanical properties
The results showed that the bending strength (MOR) of panels fabricated with Laboratory UF resin (B) was highest, and modified-resin with 25 % of replaced formaldehyde (C) led to reduced modulus of rupture (Fig. 1a). Average bending strength value of all panels pressed at 180 °C (25.48 N mm–2) was about 20 % higher than that of panels pressed at 170 °C (21.18 N mm–2) (Fig. 1b).
MOR of 25 % furfural modified panels decreased while there was no significant difference between 50 % level of furfural replaced resin (D) and Industrial UF resin (A). The reactivity of furfural is relatively lower than of formaldehyde, and panels made with such resin need more setting time under the same condition as formaldehyde. Belgacem and Gandini (2003) reported that the slow rate of curing in furfural-based resin means higher press time is needed in comparison with ordinary PF resins. The results showed that the panels’ strength was gained by increasing press temperature. In other words, raising the temperature could compensate for the time required for curing of furfural-modified resin.
The highest value of IB strength (1.05 N mm–2) was obtained for samples made by modified resin D which was not significantly different compared to panels made by laboratory UF resin (B) (Fig. 2). The heat transfer improves throughout the panels by increasing press time and temperature. If press time is sufficient for the accumulated water vapor and gases to have the opportunity to leave the panel, panel’s IB strength raises due to better resin curing resulting in stronger bonds. Panels manufactured with D modified resin showed a higher IB compared to other panels. Based on the composition of the prepared resins, it is clear that the free formaldehyde of C modified resin is higher than in D samples. At constant press time, whenever the water vapor pressure and gases into panel are higher, fracture and weakening of bonding among the wood constituents are more likely.
3.2 Physical properties
3.2.1 Water absorption (WA) and thickness swelling (TS)
Water absorption is one of the major issues that limit applications of wood-based materials as it impacts dimensional stability and chemical–mechanical properties. Table 2 represents the percentages of the WA for the panels at different periods of immersion. Based on the test results, weight gain upon exposure to water rose as immersion time increased for all particleboards tested. It must be noted that the maximum WA occurred during the first 2 h of immersion time. This could possibly be due to the hydrogen bonding of the water molecules to the free hydroxyl groups present in the wood cell wall of fibrous materials and the diffusion of water molecules into the microfibrils interfaces. Additionally, resin chemical structures present in wood-based panels can influence the penetration of water via chemical bonding. Most value of water absorption for 2 h (82.03 %) and 24 h (102.86 %) belongs to panels produced using Industrial UF (A), and resin modification diminished water absorption of panels in both C and D groups. From a chemical viewpoint, this result could be explained by the different composition of UF resin compared to furfural-modified resin. Obviously, the water absorption of panels made with modified resin is lower than the conventional panel boards. Introducing new hydroxyl groups upon methylation of urea increases the susceptibility of the structure to water. Therefore, higher water absorption would be expected compared to partial furfural-modified resins (Table 2). The rate of water absorption was reduced upon increasing the press temperature especially for the modified resin. It means that curing of furfural-substituted resin has occurred at 180 °C compared to curing temperature of UF resin that normally takes place at 170 °C.
Thickness swelling (TS) is an important property that represents the stability performance of the wood-based panels. Generally, the panels’ TS increases with the WA and thus has similar manner to WA. As mentioned earlier, the poor absorption resistance of the wood-based materials is mainly due to the presence of polar groups, which attract water molecules through hydrogen bonding. This phenomenon leads to moisture build-up in the fiber cell wall (fiber swelling) and also in the microfibrils interfaces. This is responsible for the changes in the dimension of composites, particularly the thickness and linear expansion due to reversible and irreversible swelling of the composites. As can be seen from Table 2, TS intensively increased during the first 2 h of immersion. A further increment in immersion time showed little change (increment) in the dimensional stability of panels. Results show that panels with furfural-modified resins exhibited inferior dimensional stability compared to panels made with UF resin. For example, the maximum value of TS was 54.86 % for panels made with 50 % furfural (panel D), while the value for conventional UF resin was 22.16 % (panel A) after 24 h of immersion. This is probably due to reasons of chemical compositions. As mentioned, reactivity of formaldehyde is higher than other aldehydes. Thus, the resin adhesion is smaller and weaker in furfural-modified resin because of the lower presence of formaldehyde reacting with urea. Modification of resin with furfural led to increase in TS of panels. Accordingly, modification of resin with furfural increased TS of panels. It is clear that greater molecular weight of furfural rather than formaldehyde makes more space available and leads to more swelling. According to analysis of the data obtained, 24 h TS of particleboards made with A, B and C resin groups was not significantly influenced by press temperature, but particleboards prepared using D resin was considerably affected by press temperature. Also, panels produced at 180 °C and using D resin has better dimensional stability than A resin panels. It was reported that panels prepared with furfuryl alcohol and paraformaldehyde-based resins need much more time for curing than conventional resin systems (Schultz 1990).
3.2.2 Formaldehyde emission
The amount of formaldehyde emission from the panels was generally increased by 10 % when press temperature was enhanced, as can been seen in Fig. 4. The highest amount of formaldehyde emission (2.01 mg L−1) was measured for panels produced using Industrial UF resin. The high emission amount in these panels is probably due to higher molar ratio of formaldehyde to urea in Industrial resin. Modification of UF resin reduced the emission. The lowest formaldehyde emission (0.32 mg L−1) was measured on panels bonded with Laboratory UF resin (Fig. 3).
It was found that, except in panels produced using Industrial UF resin, increasing of press temperature in all manufactured panels had a negative (increasing) impact on formaldehyde emission. It was also found that formaldehyde emission from panels produced using Laboratory resin and 180 °C press temperature was 0.27 mg L−1 (Fig. 4).
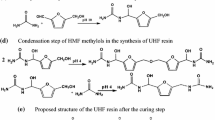
The increased formaldehyde emission from panels produced using UFF indicates that a partial substitution of formaldehyde with furfural led to a higher formaldehyde emission (Fig. 5a). It has been reported that the chemical stability of resins with both formaldehyde and furfural could lead to a higher formaldehyde emission from finished product compared to UF binder (Pizzi 1990). It was supposed that formaldehyde is instantly replaced by furfural when the cross-linking reaction starts and this leads to emission of released formaldehyde. In mixed resins instead, the higher stability to bond hydrolysis of the cross-linking formed by the alternative aldehyde will rapidly displace and drive off formaldehyde from the final product (Fig. 5a). This rapidity in substitution and propulsion of much more free formaldehyde led to increase in formaldehyde emission in a shorter time period and reduced formaldehyde emission rate from boards in use, in comparison with those produced by unmodified resin (Pizzi 1990). When two methylol groups (mono-, di-, or 3- ) in UF resin are combined, they release a water molecule and etheric binding created between them. This is a weak linkage and it tends to break in a warm and humid environment, and a free formaldehyde molecule is released. This is a reason for formaldehyde emission due to hydrolysis of UF resin. However, in the molecular structure of furanic-based resins (Fig. 5b) with higher cross-linked and water-resistant bonds, there is no etheric binding. This is attributed to the stability of existing bonds in modified resin which leads to reduced formaldehyde emission. In the present work, increasing press temperature did not significantly influence the formaldehyde emission from panels, but gradually enhanced formaldehyde emission. Panels produced at higher press temperature and a shorter press time emitted more formaldehyde and VOCs in comparison with panels made at lower temperature and longer press time (Wang et al. 2002).
3.2.3 FTIR analysis
FTIR spectra of prepared resins are shown in Fig. 6. The corresponding assignments of the observed peaks are presented in Table 3. The broad bond located at 3440 cm−1 was attributed to hydroxyl groups of resins and also the released water during condensation of resins. Slight shift of this peak to 3350 cm−1 was attributed to stretch of NH bonds formation in UF contained resins (Zorba et al. 2008). The intensity of the peak at 1663 cm−1 belongs to –C=C– bond that was relatively higher in Laboratory UF resin, while the intensity of CO bonds at 1547 cm−1 was higher in Industrial resin (Fig. 6a). The peak at 1253 cm−1 belonging to CN was broadened to the some extent in Industrial UF resin compared to Laboratory UF (Fig. 6b).
The intensity of CH bond located at 2959.59 cm−1 was found to be higher in UFF 25 % resin (Fig. 6b, c). Furthermore, an increase in intensity of bond at 1651 and 1540 cm−1 belonging to –C=C– was found for the UFF 50 % resin (Fig. 6c).
4 Conclusion
Laboratory UF resin was modified in situ with furfural. Chemical structure of the UF resin and modified UF resin along with Industrial UF resin was investigated by FTIR spectroscopy. Mechanical and physical properties of fabricated particleboards made with different resins were also measured to highlight differences in resin formulations and to evaluate whether the modified resins could replace the conventional UF resin used in typical particleboard manufacturing process. Resin modification with 25 % of furfural decreased the bending strength of panels rather than control samples. Bending strength of panels produced using Laboratory UF resin was superior to other manufactured panels. Modification of resin using 50 % of furfural resulted in maximum IB. Resin modification with both 25 and 50 % of furfural reduced the amount of water absorption and the lowest WA was belonging to panels with 50 % furfural resin. The dimensional stability was noticeably higher in 25 % furfural contained resin samples than those with 50 % of furfural, after 24 h of water immersion. Replacing formaldehyde by furfural in both 25 and 50 % decreased formaldehyde emission. Despite the formaldehyde emission, the quality and physical–mechanical characteristics of panels were improved by increasing of press temperature.
References
Akyüz KC, Nemli G, Baharoğlu M, Zekoviç E (2010) Effects of acidity of the particles and amount of hardener on the physical and mechanical properties of particleboard composite bonded with urea formaldehyde. Int J Adhes Adhes 30(3):166–169
Belgacem MN, Gandini A (2003) Furan-based adhesives. Handbook of adhesive technology. Marcel Dekker, New York, pp 615–634
Boran S, Usta M, Gümüşkaya E (2011) Decreasing formaldehyde emission from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin. Int J Adhes Adhes 31(7):674–678
Conner AH (1996) Urea–formaldehyde adhesive resins. Polym Mater Encycl 11:8496–8501
Despres A, Pizzi A, Vu C, Delmotte L (2010) Colorless formaldehyde-free urea resin adhesives for wood panels. Eur J Wood Prod 68(1):13–20
Dunky M (1998) Urea–formaldehyde (UF) adhesive resins for wood. Int J Adhes Adhes 18(2):95–107
EN 310 (1993) Wood-based panels: determination of modulus of elasticity in bending and of bending strength. CEN European Committee for Standardization
EN 317 (1993) Particleboards and fibreboards: determination of swelling in thickness after immersion in water. CEN European Committee for Standardization
EN 322 (1993) Wood-based panels: determination of moisture content. CEN European Committee for Standardization
Garcia AM, Ortiz M, Martinez R, Ortiz P, Reguera E (2004) The condensation of furfural with urea. Ind Crops Prod 19:99–106
Jiang T, Gardner DJ, Baumann MGD (2002) Volatile organic compound emissions arising from the hot-pressing of mixed-hardwood particleboard. For Prod J 52(11):66–77
JISA 5908 (1994) Standard test methods for evaluation formaldehyde emission from particleboards. UDC 691.14-413:674.817
Ko K (1976) How to control pollution of formaldehyde for formaldehyde series thermosetting resin adhesives. Settyaku Kyokaishi 12(5):160–166
Maloney TM (1993) Modern particleboard and dry-process fiberboard manufacturing. Miller Freeman Publications, San Francisco
Park BD, Lee SM, Roh JK (2009) Effects of formaldehyde/urea mole ratio and melamine content on the hydrolytic stability of cured urea-melamine-formaldehyde resin. Eur J Wood Prod 67(1):121–123
Park BD, Kang EC, Park SB, Park JY (2011) Empirical correlations between test methods of measuring formaldehyde emission of plywood, particleboard and medium density fiberboard. Eur J Wood Prod 69(2):311–316
Pizzi A (1990) Furfural-enhanced formaldehyde emission from UF particleboard. Holz Roh- Werkst 48(10):376
Pizzi A (1994) Advanced wood adhesives technology. Marcel Dekker, New York, p 289
Pizzi A (2003) Urea–formaldehyde adhesives. In: Pizzi A, Mittal KL (eds) Handbook of adhesive technology, revised and expanded. CRC Press, USA, pp 628–645
Que Z, Furuno T, Katoh S, Nishino Y (2007a) Effects of urea–formaldehyde resin mole ratio on the properties of particleboard. Build Environ 42(3):1257–1263
Que Z, Furuno T, Katoh S, Nishino Y (2007b) Evaluation of three test methods in determination of formaldehyde emission from particleboard bonded with different mole ratio in the urea–formaldehyde resin. Build Environ 42(3):1242–1249
Roffael E, Johnsson B, Engström B (2010) On the measurement of formaldehyde release from low-emission wood-based panels using the perforator method. Wood Sci Technol 44(3):369–377
Salem MZM, Böhm M, Beránková J, Srba J (2011) Effect of some manufacturing variables on formaldehyde release from particleboard: relationship between different test methods. Build Environ 46(10):1946–1953
Schneider MH, Phillips JG (2010) Furfural-urea resins and adhesives and their methods of production. US Patent 7,781,521, issued August 24
Schultz TP (1990) Exterior plywood resin formulated from furfuryl alcohol and paraformaldehyde. Holzforschung 44(6):467–468
Tomita B (1980) How chemical structure of UF resin affects formaldehyde emission. Mokuzai Kogyo 35(5):193–199
Wang W, Gardner DJ, Baumann MG (2002) Reviewed articles-volatile organic compound emissions during hot-pressing of southern pine particleboard: panel size effects and trade-off between press time and temperature. For Prod J Index 52(4):24–30
Zorba T, Papadopoulou E, Hatjiissaak A, Paraskevopoulos KM, Chrissafis K (2008) Urea–formaldehyde resins characterized by thermal analysis and FTIR method. J Therm Anal Calorim 92(1):29–33
Acknowledgments
We are using this opportunity to express our gratitude to Mr. Bernard Hasan, Mr. Ehsan Mehrabi, and Mr. Arash Chavooshi who supported us throughout the preparation of this research paper. The authors would also like to thank the Wood and Paper Lab staff at the Faculty of Natural Resources in University of Tehran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghafari, R., DoostHosseini, K., Abdulkhani, A. et al. Replacing formaldehyde by furfural in urea formaldehyde resin: effect on formaldehyde emission and physical–mechanical properties of particleboards. Eur. J. Wood Prod. 74, 609–616 (2016). https://doi.org/10.1007/s00107-016-1005-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-016-1005-6