Abstract
Introduction
Arterial blood gas (ABG) sampling is routinely performed in major trauma patients to assess the severity of hemorrhagic shock. Compared to venous blood gas (VBG), ABG is an additional procedure with risks of hematoma and pain. We aim to determine if pH, base deficit (BD), and lactate from VBG and ABG in trauma patients are clinically equivalent. If proven, the need for ABG and its associated risks can be eliminated.
Methods
This prospective observational study was conducted in the Emergency Department of National University Hospital, Singapore, between February and October 2016. We correlated paired ABG and VBG results in adult trauma patients. VBG and ABG were obtained within 10 min and processed within 5 min using a point-of-care blood gas analyzer. Bland–Altman plot analysis was used to evaluate the agreement between peripheral VBG and ABG in terms of pH, base deficit and lactate.
Results
There were 102 patients included, with a median age of 34 (interquartile range 28–46) years and male predominance (90.2%). Majority of patients sustained blunt trauma (96.1%), and had injuries of Tier 1 and Tier 2 severity (60/102, 58.8%). Bland–Altman plot analyses demonstrated that only 72.6% of venous pH and 76.5% of venous BD lie within the pre-defined clinically acceptable limits of agreement, whereas 96.0% of venous lactate was within these limits.
Conclusion
Venous and arterial pH and BD are not within clinically acceptable limits of agreement, and ABG should be obtained for accurate acid–base status. However, venous lactate may be an acceptable substitute for arterial lactate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trauma is one of the leading causes of mortality and morbidity, accounting for 9% of global mortality [1]. Among patients with trauma, hemorrhagic shock is the second leading cause of death after traumatic brain injury, responsible for 40% of deaths [2]. Delay in detection and management of hemorrhagic shock can lead to tissue hypoperfusion with resultant end-organ failure and eventual death.
Aside from assessment of hemodynamics, clinical signs and symptoms, arterial blood gas (ABG) sampling is routinely performed in patients with polytrauma to determine the severity of hemorrhagic shock. Initial arterial base deficit (BD), pH and lactate have been shown to be objective and predictive biomarkers for serious injury and tissue hypoperfusion [3,4,5]. In our local context, placement of an arterial line is not routinely done upon arrival of a trauma patient in the emergency department (ED). Obtaining an ABG sample is therefore an additional procedure in the busy trauma resuscitation and is associated with increased morbidity to patients by subjecting them to risks of bleeding, hematoma, thrombosis and pain [6, 7]. This is in contrast to venous blood sampling, which can be easily drawn simultaneously during intravenous cannulation for vascular access to administer fluids and parenteral medications in trauma resuscitation. Furthermore, ABG sampling can be a challenging procedure in hypotensive patients, and exposes healthcare workers to the additional hazard of needlestick injuries, especially in combative and confused patients. The ability to eliminate the need for ABG sampling would eradicate these associated risks, both to patients and healthcare workers.
Previous studies have shown that venous blood gas (VBG) is suitable to replace ABG in conditions like diabetic ketoacidosis [8]. However, there have been limited studies in the trauma population with no general consensus. Arnold and colleagues concluded that venous BD is acceptable to assess trauma patients’ initial metabolic status [9] whereas Rudkin and colleagues demonstrated that ABG samples should still be obtained if accurate acid–base status is required [10]. Although both studies had similar inclusion criteria comprising patients attending the ED with significant mechanism of trauma requiring ABG sampling for evaluation, Rudkin and colleagues compared ABG and VBG samples taken up to 1 h apart [10]. Changes in acid–base status with time and after institution of resuscitative measures may have contributed to the non-equivalent results. On the other hand, Arnold and colleagues’ study only evaluated the agreement between BD in VBG and ABG without other parameters of the blood gases.
The aim of this study is to determine if pH, BD and lactate from ABG and peripheral VBG in trauma patients are clinically equivalent. We defined clinical equivalence as within a threshold of ± 0.05 units for pH, ± 2 units for BD and ± 1.5 mmol/L for lactate in VBG and ABG samples taken within 10 min of one another.
Methods
Study design and participants
The Agreement between arterial and venous blood Gases in trauma REsuscitation in Emergency department (AGREE) study is a prospective observational study, conducted between February and October 2016, in the ED of the National University Hospital, a tertiary trauma center in Singapore. The study was conducted and reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [11].
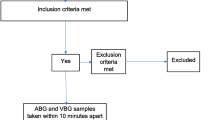
Adult trauma patients aged 21 years and above, seen in the immediate [Patient Acuity Category (PAC) 1] and intermediate (PAC 2) acuity areas, who require an ABG for clinical evaluation based on treating emergency physician’s assessment, were recruited into the study. Once the attending physician decided that an ABG is required, a VBG was obtained at the same setting as peripheral venous cannulation within 10 min of the ABG. Patients with ABG and VBG done more than 10 min apart were excluded.
Triage and scoring systems
Patient acuity categories are assigned based on initial assessment of clinical conditions, vital signs and prioritisation of patients by a triage nurse or physician upon arrival to the ED. PAC 1 are very serious cases such as major trauma with cardiac arrest or shock that require immediate attention. These patients have either suffered cardiovascular collapse or are imminently in danger of collapse. PAC 2 are patients who cannot move on their own and are in distress. They are usually haemodynamically stable, but still require early attention due to the severity of symptoms to prevent deterioration.
The Injury Severity Score (ISS) is calculated based on summation of the squares of injury scores in the top three most severely injured body parts, namely head and neck, face, chest, abdomen, extremity or other external areas [12]. An ISS of 16 and above indicates Tier 1, 9–15 is classified as Tier 2 and an ISS of less than 9 constitutes Tier 3. The higher the ISS, the higher the likelihood of mortality.
Study setting and population
The National University Hospital is a tertiary trauma center with on-site 24-h trauma specialist coverage, catering for an average of 300 Tier 1, 500–600 Tier 2 and close to 30,000 Tier 3 patients annually. Board-certified emergency physicians are on duty every shift 24 h a day, 7 days a week. Evaluation of PAC 1 and PAC 2 trauma patients are supervised and overseen by emergency physicians.
Evaluation of trauma patients is based on recommended guidelines from the Advanced Trauma Life Support Program. The need for an ABG as part of clinical evaluation is decided by emergency physicians after assessment of the individual patient. ABG sampling is routinely obtained in patients presenting with trauma for those with significant mechanism of injury such as penetrating injury, falling from more than standing height, motor vehicle accident with passenger ejection, fatality in same vehicle, involving a pedestrian, or hemodynamic instability.
Study protocol
Patients were recruited into the study if ABG was clinically indicated as per assessment by an emergency physician. ABG and VBG sampling were performed within 10 min apart and analyzed using the i-STAT handheld blood gas analyzer (Abbott Point of Care Inc., Princeton, NJ) within 5 min of blood draw [9, 10]. Samples that exceeded the time limits of collection or i-STAT analysis were excluded from the final analysis.
Informed consent was obtained from patients who have mental capacity before study recruitment. For patients who were unable to provide consent, we obtained waiver of consent at time of enrolment with delayed informed consent after the patient regained mental capacity. The study protocol was approved by the Domain Specific Review Board, National Healthcare Group, Singapore (DSRB 2015/00904).
Sample size
Pre-defined clinically equivalent thresholds for pH, BD and lactate were set at − 0.05 to 0.05 units, − 2 to 2 units and − 1.5 to 1.5 mmol/L, respectively, based on previous studies [9, 10, 13,14,15]. VBG results have to lie within these pre-defined thresholds when compared to ABG to be considered clinically equivalent. Postulating that there is a 100% agreement and allowing a lower 95% confidence interval of 96% (error of 4%), 100 patients will be required. To allow for 10% loss of patients due to missing data, we had planned to enroll 110 patients.
Statistical analysis
Results were analyzed using Stata 14 (StataCorp LP, College Station, TX). Categorical variables [gender, ethnicity, injury mechanism, tier of injury, areas of injuries sustained, need for transfusion or intubation, presence of chest X-ray or focused assessment using sonography in trauma (FAST) abnormalities and disposition from ED] are reported in proportions and non-parametric data is presented as median with interquartile range (IQR). Statistical analyses to evaluate the agreement between peripheral VBG and ABG in terms of pH, BD and lactate were conducted using Bland–Altman plots.
For secondary analysis, unadjusted and adjusted linear regressions of the patients’ demographics, injury severity, clinical findings, interventions and site of arterial draw were performed to evaluate for possible predictors for values of arterial pH, BD and lactate.
Results
A total of 111 patients were recruited during the study period. However, 9 patients had exceeded the pre-set time limits and were thus excluded, leaving 102 patients for final analysis. The median age was 34 (IQR 28–46) years, with a predominance of males (90.2%) and blunt trauma (96.1%) as the main mechanism of injury (Table 1). Majority of patients (60/102, 58.8%) sustained Tier 1 and Tier 2 injuries; 12.8% of patients required endotracheal intubation and 11.8% received blood product transfusion in the ED (Table 1). There were 22.6% of patients (23/102) with tachycardia and 6.9% of patients (7/102) with hypotension at presentation (Table 1).
Agreement between ABG and VBG
Venous pH and BD were not clinically equivalent based on the pre-determined clinically acceptable threshold limits of − 0.05 to 0.05 units for pH and − 2 to 2 units for BD. For pH, only 72.6% of the values were within the clinically acceptable limits while 76.5% of the values for BD lie within the threshold (Fig. 1).
Bland–Altman plots of pH, base deficit and lactate. Top left: Bland–Altman plot of difference between venous and arterial pH (y axis) against average of venous and arterial pH (x axis). top right: Bland–Altman plot of difference between venous and arterial base deficit (BD) (y axis) against average of venous and arterial BD (x axis). Bottom: Bland–Altman plot of difference between venous and arterial lactate (y axis) against average of venous and arterial lactate (x axis). Circle size is proportional to the number of observations for each data point. Green dotted lines indicate mean differences
Conversely, venous lactate was found to be clinically equivalent based on the pre-determined threshold limits of − 1.5 to 1.5 mmol/L where 96.0% of the values were within this acceptable range (Fig. 1).
Secondary analysis
Simple linear regression analyses of variables that may affect the outcomes of arterial pH, BD and lactate was performed (Table 2). None of the variables were significantly associated with arterial pH upon adjusted linear regression. However, heart rate was statistically significantly associated with both arterial BD and lactate (Table 3). After stepwise selection (p < 0.10), GCS was found to be significantly associated with arterial pH; and SpO2 significantly associated with arterial BD. Additional variables that predict lactate were need for intubation in ED, injury severity score, injury mechanism and systolic blood pressure (Table 4).
Discussion
Timely and successful resuscitation of the trauma patient with hemorrhagic shock is limited by the concept of the “golden hour”. The need to accurately and rapidly recognize tissue hypoperfusion from blood loss is of paramount importance. Undertaking fewer procedures with rapid turnaround time to results would greatly aid to achieve this goal. Our study aims to address this issue by studying the agreement between VBG and ABG using a bedside handheld blood gas analyzer, with the objective of eliminating an extra blood-draw procedure and to allow availability of blood gas results within minutes. We have demonstrated that venous pH and BD were not clinically equivalent to arterial pH and BD based on our pre-defined clinically acceptable thresholds. However, venous lactate was found to be clinically equivalent within an acceptable range.
In patients with severe trauma, compromised perfusion may not translate to unstable vital signs due to peripheral vasoconstriction from compensatory mechanisms, particularly in younger patients. Therefore, arterial BD and lactate function as objective indicators of shock, being well studied as markers for oxygen debt and have consistently shown to be predictors of mortality and severity of hemorrhage in patients with trauma [16, 17]. To detect occult hemorrhage and avoid potential delay in recognition of hemorrhagic shock from spuriously normal vital signs, the use of BD and lactate as surrogates for tissue hypoperfusion is therefore imperative in reducing morbidity and mortality [18,19,20,21,22]. As such, rapid and accurate measures of BD and lactate are needed to ensure appropriate institution of resuscitative measures. False-positive results for hemorrhagic shock would induce unnecessary blood products transfusion, which carries risk of blood-borne infections, anaphylaxis or more catastrophic complications such as transfusion-related acute lung injury (TRALI) [23]. On the other hand, false negative results may cause delayed definitive management such as radiological imaging and operative procedures.
Even though VBG has been found to be equivalent to ABG with high degrees of correlation and agreement in evaluating acidosis in other conditions such as diabetic ketoacidosis, the same cannot be concluded for trauma resuscitation [13, 24,25,26,27]. There is currently no consensus with regard to the accuracy of VBG in trauma as existing literature on trauma populations comparing venous and arterial pH and BD produced conflicting results [9, 10, 25]. Our study results are congruent with some of the previous studies, which showed that VBG could not replace ABG in the evaluation of trauma patients [10, 25]. This adds to existing evidence that ABG should still be obtained for accurate acid–base evaluation in resuscitation of trauma patients.
Although traditionally arterial BD has been widely accepted as a reliable early indicator of the magnitude of volume deficit which predicts transfusion requirements and mortality in trauma patients [3, 28], its value can be confounded by a number of factors such as alcohol intoxication, hypoalbuminemia, hypothermia and hypocapnea [29,30,31,32]. Other studies have suggested that initial lactate may be more relevant and a stronger index of blood loss as well as predictor of transfusion requirements and mortality [4, 5, 33]. Comparing lactate and BD in a cohort of trauma and surgical patients in an intensive care unit, Martin and colleagues found that lactate level predicts mortality and longer length of inpatient stay even in normal levels of BD [34]. In contrast, an increase in BD in the setting of a normal lactate level did not have any predictive value on mortality or clinical trajectory [34]. Of importance, BD is a calculated value based on partial pressures of carbon dioxide, pH, and serum bicarbonate, and represents the amount of buffer anions required to be added to normalize the pH [35]. Generally in the acute setting, the assumption for an increase in BD is attributed to lactate acidosis. However, this may not always be reflective of the underlying cause as there are other causes of metabolic acidosis such as uremia and ketonemia that can also result in elevated base deficit. Studies in trauma patients admitted to the surgical ICU have indeed shown that correlation between BD and levels of lactate is weak [5].
Furthermore, pH and BD are affected by regional mixing effects and likely reflect early changes, while lactate is more representative of gradual changes of hypoperfusion over time [34]. Hence, pH and BD from venous samples comprising blood from regional perfusion may vary from arterial blood that originates from the left ventricular outflow tract. With ongoing volume loss in traumatic shock, the perfusion states in such patients may change more rapidly with time. In the Bland–Altman plots of this study, differences in values between arterial and venous pH, BD and lactate were more evident among the sicker patients (lower pH and BD, higher lactate values). With larger discrepancies seen in the critically ill, we further demonstrated that VBG should not be used as a substitute for ABG in such situations.
Additionally from our study results, lactate appears to be better correlated with a greater number of important clinical variables in trauma and perhaps a better biomarker. From regression analyses, tachycardia resulted in a greater BD while a higher systolic blood pressure and peripheral oxygen saturation (SpO2) predicted a lower BD. Comparatively for lactate, increase in injury severity quantified by the Injury Severity Score, the need for intubation in ED, having a penetrating mechanism of injury and tachycardia predicted a higher lactate level whilst higher systolic blood pressure translated to a lower lactate level. If further evidence could support replacing BD with lactate in evaluation of trauma patients, given the good agreement between venous and arterial lactate levels shown by our study, venous lactate alone may be used as an objective biomarker to predict hemorrhagic shock in trauma, thereby reducing additional costs and complications through obviating the need for ABG. Further prospective studies would be required to confirm this.
We have combined the strengths of the previous studies using a handheld blood gas analyzer readily available in the ED and using Bland–Altman plots to analyze the agreement between the venous and arterial pH, BD and lactate rather than looking at correlation. As correlation simply measures the strength of the relation between two variables, there needs to be good agreement within clinically acceptable limits in order to replace one test with another. In addition, we evaluated both BD and lactate in trauma in the same setting, which was also lacking in previous studies.
There are some limitations in our study. First, patients were recruited through convenience sampling due to lack of availability of study investigators in all shifts. Despite that, as our study outcomes involve evaluation of objective biomarkers, convenience sampling is unlikely to have affected the study results. Second, we did not standardize the location of venous and arterial blood draws, allowing peripheral blood draw from the radial artery or centrally from the femoral artery, and any peripheral vein where intravenous cannulation was performed. It would have been impractical to standardize the site of blood draw as any limb injuries sustained by the patients may preclude blood draw from the affected limb. Furthermore, our simple linear regression analysis did not show significant difference for the site of draw between the venous and arterial BD and lactate.
Third, we did not collect data on tourniquet time and hence, was unable to adjust for its effect. Fourth, as this was an observational study and the study team did not interfere with routine clinical care, the decision to perform an ABG was based on the discretion of the attending physician. However, all PAC 1 and PAC 2 trauma cases were managed by emergency physicians as per tenets of the Advanced Trauma Life Support Program.
Lastly, our pre-defined clinically important limits of agreement for lactate levels were based on limited studies on the trauma population [15] and more well-established cut-off values for mortality risk in sepsis [14]. Generally, a lactate level of less than 2.0 mmol/L indicates low mortality risk, 2.0–3.9 mmol/L intermediate risk, and over 4.0 mmol/L carries high risk of mortality in sepsis. Therefore, a difference in lactate level of 1.5 mmol/L transits a patient from one tier to the next, which provided our justification for selection of this limit. Further studies relating these risk stratification levels to outcomes in trauma patients would be required.
Conclusion
In conclusion, an ABG is required to evaluate pH and BD during the initial assessment of a trauma patient. On the contrary, venous lactate is clinically equivalent based on the pre-determined threshold of − 1.5 to 1.5 mmol/L, and may thus be considered as a surrogate for arterial lactate in trauma. Further larger studies would be needed to confirm this.
References
WHO Injuries. WHO. https://www.who.int/topics/injuries/en/. Accessed 25 Jan 2016.
Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93.
Davis JW, Parks SN, Kaups KL, et al. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996;41:769–74.
Moomey CB, Melton SM, Croce MA, et al. Prognostic value of blood lactate, base deficit, and oxygen-derived variables in an LD50 model of penetrating trauma. Crit Care Med. 1999;27:154–61.
Husain FA, Martin MJ, Mullenix PS, et al. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–91.
Mortensen J. Clinical sequelae from arterial needle puncture, cannulation, and incision. Circulation. 1967;35:1118–23.
McGillivray D, Ducharme FM, Charron Y, et al. Clinical decisionmaking based on venous versus capillary blood gas values in the well-perfused child. Ann Emerg Med. 1999;34:58–63.
Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ymem. 1998;31:459–65.
Arnold TDW, Miller M, Van Wessem KJP, et al. Base deficit from the first peripheral venous sample: a surrogate for arterial base deficit in the trauma bay. J Trauma. 2011;71:793–7.
Rudkin SE, Kahn CA, Oman JA, et al. Prospective correlation of arterial vs venous blood gas measurements in trauma patients. Am J Emerg Med. 2012;30:1371–7.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Equator Network. http://www.equator-network.org/reporting-guidelines/strobe/. Accessed 25 Jan 2016.
Baker SP, O’Neill B, Haddon W, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96.
Rang LCF, Murray HE, Wells GA, et al. Can peripheral venous blood gases replace arterial blood gases in emergency department patients? CJEM. 2002;4:7–15.
Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7.
Lavery RF, Livingston DH, Tortella BJ, et al. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000;190:656–64.
Davis JW, Mackersie RC, Holbrook TL, et al. Base deficit as an indicator of significant abdominal injury. Ann Emerg Med. 1991;20:842–4.
Aslar AK, Kuzu MA, Elhan AH, et al. Admission lactate level and the APACHE II score are the most useful predictors of prognosis following torso trauma. Injury. 2004;35:746–52.
van Rein EAJ, Houwert RM, Gunning AC, et al. Accuracy of prehospital triage protocols in selecting major trauma patients. J Trauma Acute Care Surg. 2017;1:328–39.
Liu NT, Holcomb JB, Wade CE, et al. Inefficacy of standard vital signs for predicting mortality and the need for prehospital life-saving interventions in blunt trauma patients transported via helicopter. J Trauma Acute Care Surg. 2017;1:S98–103.
Lechleuthner A, Lefering R, Bouillon B, et al. Prehospital detection of uncontrolled haemorrhage in blunt trauma. Eur J Emerg Med. 1994;1:13–8.
Demetriades D, Chan LS, Bhasin P, et al. Relative bradycardia in patients with traumatic hypotension. J Trauma. 1998;45:534–9.
Parks JK, Elliott AC, Gentilello LM, et al. Systemic hypotension is a late marker of shock after trauma: a validation study of Advanced Trauma Life Support principles in a large national sample. Am J Surg. 2006;192:727–31.
Squires JE. Risks of Transfusion. South Med J. 2011;104:762–9.
Schmelzer TM, Perron AD, Thomason MH, et al. A comparison of central venous and arterial base deficit as a predictor of survival in acute trauma. Am J Emerg Med. 2008;26:119–23.
Malinoski DJ, Todd SR, Slone DS, et al. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg. 2005;140:1122–5.
Malatesha G, Singh NK, Bharija A, et al. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–71.
Byrne AL, Bennett M, Chatterji R, et al. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology. 2014;19:168–75.
Davis JW, Shackford SR, Mackersie RC, et al. Base deficit as a guide to volume resuscitation. J Trauma. 1988;28:1464–7.
Gustafson ML, Hollosi S, Chumbe JT, et al. The effect of ethanol on lactate and base deficit as predictors of morbidity and mortality in trauma. Am J Emerg Med. 2015;33:607–13.
Pascoe S, Lynch J. Management of hypovolaemic shock in the trauma patient. In: Sisson G, Parr M, Sugrue M, editors. Committee NICPG. Sydney: ITIM (Institute of Trauma and Injury Management) NSW Health; 2007.
Davis JW, Kaups KL, Parks SN. Effect of alcohol on the utility of base deficit in trauma. J Trauma. 1997;43:507–10.
McAuliffe JJ, Lind LJ, Leith DE, et al. Hypoproteinemic alkalosis. Am J Med. 1986;81:86–90.
Ohmori T, Kitamura T, Ishihara J, et al. Early predictors for massive transfusion in older adult severe trauma patients. Injury. 2016;48:1006–12.
Martin MJ, FitzSullivan E, Salim A, et al. Discordance between lactate and base deficit in the surgical intensive care unit: which one do you trust? Am J Surg. 2006;191:625–30.
Wilson RF, Sibbald WJ. Approach to acid–base problems in the critically ill and injured. JACEP. 1976;5:515–22.
Acknowledgements
We thank our colleagues at the Emergency Medicine Department, National University Hospital, Singapore, for their contributions to image acquisition, data collection and patient recruitment.
Funding
Junior Pitch for Funds, National University Hospital (Grant No. JPFF-15-2-BYR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from patients who have mental capacity before study recruitment. For patients who were unable to provide consent, we obtained waiver of consent at time of enrolment with delayed written informed consent after the patient regained mental capacity. The study protocol was approved by the Domain Specific Review Board, National Healthcare Group, Singapore (DSRB 2015/00904).
Rights and permissions
About this article
Cite this article
Boon, Y., Kuan, W.S., Chan, Y.H. et al. Agreement between arterial and venous blood gases in trauma resuscitation in emergency department (AGREE). Eur J Trauma Emerg Surg 47, 365–372 (2021). https://doi.org/10.1007/s00068-019-01190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-019-01190-6