Abstract
Purpose
High ratios of Plasma to Packed Red Blood Cells (FFP:PRBC) improve survival in massively transfused trauma patients. We hypothesized that non-trauma patients also benefit from this transfusion strategy.
Methods
Non-trauma patients requiring massive transfusion from November 2003 to September 2011 were reviewed. Logistic regression was performed to identify independent predictors of mortality. The population was stratified using two FFP:PRBC ratio cut-offs (1:2 and 1:3) and adjusted mortality derived.
Results
Over 8 years, 29 % (260/908) of massively transfused surgical patients were non-trauma patients. Mortality decreased with increasing FFP:PRBC ratios (45 % for ratio ≤1:8, 33 % for ratio >1:8 and ≤1:3, 27 % for ratio >1:3 and ≤1:2 and 25 % for ratio >1:2). Increasing FFP:PRBC ratio independently predicted survival (AOR [95 % CI]: 1.91 [1.35–2.71]; p < 0.001). Patients achieving a ratio >1:3 had improved survival (AOR [95 % CI]: 3.24 [1.24–8.47]; p = 0.016).
Conclusion
In non-trauma patients undergoing massive transfusion, increasing FFP:PRBC ratio was associated with improved survival. A ratio >1:3 significantly improved survival probability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of hemostatic resuscitation, which has focused on the empiric replacement of blood components has changed the management of trauma patients with ongoing hemorrhage. The foundation of this strategy is the early and aggressive utilization of fresh frozen plasma (FFP) for patients requiring massive packed blood red cell (PRBC) transfusion. The emerging literature suggesting improved survival for patients receiving high FFP:PRBC ratios both in the military and in the civilian populations has resulted in the widespread adoption of this strategy [1–18].
Although the research targeting resuscitation and coagulopathy reversal during exsanguination has been carried out predominantly in the trauma setting, the bleeding non-trauma patient presents similar challenges. Whether the concept of hemostatic resuscitation with balanced blood product replacement can be extrapolated to patients with major blood loss from non-traumatic etiologies is unclear and the impact of this strategy in massively transfused non-trauma patients remains unclear. We hypothesized that high FFP:PRBC transfusion ratios would be associated with improved survival in this population.
Methods
This study received IRB approval and has been performed in accordance with the ethical standards defined in the 1964 Declaration of Helsinki and its later amendments. All non-trauma patients treated by a surgical service receiving a massive transfusion at the Los Angeles County + University of Southern California (LAC + USC) Medical Center from November 2003 to September 2011 were identified. For the purpose of this study, massive transfusion was defined as 10 or more units of PRBC during the first 24 h after initiation of transfusion. Patient variables extracted included age, gender, need for uncrossmatched blood transfusion, time of initiation of transfusion, heart rate, systolic blood pressure, hematocrit, hemoglobin, INR, pH, pO2, pCO2 and base deficit at initiation of transfusion, total units of PRBCs and FFP received and time of transfusion of each blood component. The time to completion of massive transfusion was carefully documented. Ratios of FFP:PRBC transfused within 24 h of initiation of blood transfusion were subsequently calculated. In order to minimize the possibility of survival bias, which has been a significant concern when analyzing this time-dependent variable [14, 16, 19, 20], all patients that died in the first 24 h after initiation of transfusion were excluded. The primary outcome measure was in-hospital mortality.
Continuous variables were dichotomized using clinically relevant cut points: age (older than 55 versus 55 years or younger), hematocrit (<24 versus ≥24 %), hemoglobin (<8 versus ≥8 g/dL), INR (<1.3 versus ≥1.3) and base deficit (<−6 versus ≥−6). Continuous variables were compared using Student’s t test or Fisher’s exact test and differences between proportions were compared using Chi square test or Mann–Whitney U test. Patients were subsequently classified into four categories according to their FFP:PRBC ratio using the same criteria previously published for trauma patients [3]: low ratio (≤1:8), medium ratio (>1:8 and ≤1:3), high ratio (>1:3 and ≤1:2) and highest ratio (>1:2). To identify if the ratio was independently associated with mortality, a stepwise logistic regression model was created using all variables that were found on univariate analysis to be associated with mortality at p < 0.2. The FFP:PRBC ratio was entered into the regression model as an ordinal variable based on the classification previously described.
Plotting the mortality rates for each of the FFP:PRBC ratio groups, a significant trend towards decreased mortality was observed and confirmed with regression analysis reaching a plateau at a ratio of 1:3. Chi square was used to compare the different FFP: PRBC groups. When 1:3 was compared with higher ratios, no statistical difference was noted. Based on this finding, the study population was dichotomized into two groups according to this cut-off ratio. To explore the association of FFP:PRBC ratio and mortality, logistic regression analysis was performed adjusting for all factors that on univariate analysis were different between the two groups p < 0.05 as well as the independent predictors previously identified. All analyses were performed using SPSS for Windows 17 (Chicago, IL).
Results
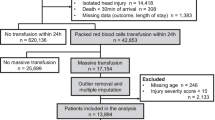
During the 8-year study period, 13,682 surgical patients required a blood transfusion. Massive transfusion occurred in 908 (7 %) of the transfused patients. Among the massively transfused patients, 343 (38 %) were non trauma patients and 260 survived greater than 24 h, meeting the inclusion criteria (Fig. 1). The majority of patients were male (72 %) with a mean age of 44 ± 17 years. On admission, 17 % were hypotensive and 26 % were tachycardic (Table 1). The reason for transfusion was gastrointestinal (GI) bleeding in 56 % (145) of cases, followed by obstetric and postpartum bleeding in 23 % (60), unexpected bleeding during elective or emergency surgery in 11 % [29], and other causes in 10 % [26]. The overall mortality was 30 %. Out of a total of 145 patients that had a GI bleed, only 6 required surgery due to inability to control the bleeding endoscopically or with interventional radiology catheter based therapy. The majority (88 %, 53) of the postpartum patients required return to the operating room for definitive bleeding control.
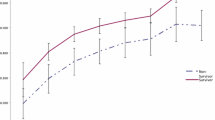
After the study population was classified into four different groups according to their FFP:PRBC ratio, a statistically significant decrease in mortality was observed with increasing ratios, reaching a plateau after a ratio >1:3 was achieved (Fig. 2).
After stepwise logistic regression to identify factors associated with mortality in this population, the ratio of FFP:PRBC was found to be the second strongest independent predictor of mortality, with an odds ratio (OR) [95 % confidence interval (CI)] of 0.52 [0.37, 0.74], p < 0.001 (Table 2). The area under the ROC curve for this regression model was 0.76 (95 % CI 0.69, 0.82). A scatterplot using the FFP:PRBC ratio and the probability of survival of each patient according to this regression model was built and demonstrated a linear increase in the probability of survival as the ratio of FFP:PRBC increased.
The study population was then dichotomized into two groups (FFP:PRBC ratio ≤1:3 versus >1:3). The two groups were compared for differences in baseline characteristics using univariate analysis and the results are displayed in Table 1. Crude mortality was significantly lower for patients with ratio >1:3 (26 versus 38 %, p = 0.050). After multivariable analysis, adjusted mortality remained significantly lower for those patients with a ratio >1:3 [Adjusted OR (95 % CI) = 0.308 (0.118, 0.803), p = 0.016] (Table 3).
In order to evaluate if achieving the highest ratio (FFP:PRBC > 1:2) would provide additional survival benefit, the study population was subsequently dichotomized using this value as a cutoff. After multivariate analysis, survival was significantly higher for those receiving a ratio >1:2 [Adjusted OR (95 % CI) = 3.984 (1.345, 11.764), p = 0.012].
Discussion
This analysis demonstrated that increasing ratios of FFP:PRBC were associated with a significantly improved survival for non-trauma patients undergoing massive transfusion. Patients that received an FFP:PRBC transfusion ratio of more than 1:3 were significantly more likely to survive, with an adjusted odds ratio for survival of 3.24 (95 % CI 1.24, 8.47, p = 0.016). Patients in the highest FFP:PRBC ratio (>1:2) group had a 44 % relative reduction in mortality compared to those in the low ratio (<1:8) group.
These findings are consistent with the data emerging from the trauma literature suggesting that aggressive use of FFP in injured patients undergoing massive transfusion is associated with improved outcomes [1–17, 21]. The past decade has been marked by a significant paradigm shift regarding resuscitation strategies for injured patients with massive blood loss. The primary therapeutic goals for an exsanguinating patient, whether the etiology of the bleeding is traumatic or not, is surgical or endovascular control, which should be pursued concomitantly with an evidence based resuscitation strategy. This includes the restoration of blood volume and coagulation profile, while optimizing tissue oxygen delivery. Traditionally, crystalloid and PRBC infusion have been the main interventions used to achieve this goal, resulting in worsening of coagulopathy secondary to pro-inflammatory immunomodulation and dilution of clotting factors [22]. For crystalloids, recent evidence suggests a beneficial role for limited and goal directed crystalloid infusion strategy [23, 24].
For blood components, the evolving concept of hemostatic resuscitation strategy originated from a shift in transfusion practices during recent conflicts, with the use of fresh whole blood [25, 26] and the demonstration of improved survival with high FFP:PRBC ratios transfusion [2]. Multiple studies investigating the impact of FFP utilization in the outcomes of injured patients undergoing massive transfusion both in the military and in the civilian sector have been published and suggest that high FFP:PRBC ratios are associated with improved survival [1–17, 21]. The optimal blood component ratios for patients undergoing massive blood transfusion however remains subject of debate and whether or not the use of fixed-ratio blood component resuscitation is beneficial is still unresolved. Holcomb et al. compared two high-ratio transfusion strategies in a prospective randomized trial and found no significant early or delayed mortality in massively transfused patients with 1:1:1 or 1:1:2 ratios [18]. Nascimento et al. demonstrated that although feasible, the institution of a fixed-ratio protocol resulted in increased waste of plasma units [27]. More recently, the use of viscoelastic assay thrombelastography (TEG) to guide the use of blood products during massive transfusion for traumatic hemorrhage was found to be associated with a survival benefit compared to conventional coagulation studies [28]. This finding suggests that goal-directed transfusion therapy using TEG may be a superior benchmark against which to compare fixed-ratio blood component resuscitation.
The issue of hemostatic resuscitation for non-trauma patients requiring massive transfusion has been previously investigated in the subset of patients with ruptured abdominal aortic aneurysms (AAA). In a retrospective review of 128 patients requiring massive transfusion for ruptured AAA, Mell et al. demonstrated a statistically significant survival benefit for patients receiving FFP:PRBC ratios greater than 1:2, with an odds ratio of 4.23 (95 % CI 1.23–14.49, p < 0.003) [29]. Similarly, Johansson et al. found that for patients with ruptured AAA, a transfusion protocol that included proactive transfusion of FFP and platelets, with a 1:1 ratio of FFP:PRBC, was associated with improved survival and reduced the need for PRBC transfusion [30.] Those findings are in contrast to a study from Kauvar et al. investigating the impact of FFP utilization in the outcomes of 89 patients receiving massive transfusion for ruptured AAA, which demonstrated that the use of autotransfusion but not FFP was associated with increased survival [31.] In that study, patients with FFP:PRBC ratio >1:2 had comparable mortality compared to those with FFP:PRBC ≤1:2 (49 versus 40 %, p = 0.39) [31]. As data accumulates supporting the empiric and more aggressive utilization of FFP both in trauma and non-trauma patients with major bleeding, shifting to such a resuscitation strategy may increase the risk of potential complications associated with plasma transfusion, as has been demonstrated in non massively transfused trauma patients [32–34]. The optimal ratios, trigger for initiation and timing of replacement are unknown and warrant further investigation.
This study shares the limitations observed in many of the studies investigating the issue of plasma utilization in the resuscitation of injured patients. The retrospective nature of the study limits our ability to fully evaluate the relationship of these dynamic time-dependent variables and the outcome, as well as the possible contribution of ratio variability that may occur during the transfusion process. Several studies have highlighted the possibility of survival bias when investigating the FFP:PRBC ratios during massive transfusion [14, 19, 20]. The potential of survival bias exists because patients who survive longer are more likely to eventually receive higher quantities of FFP transfusion. This happens because traditionally FFP transfusion is started only after several units of PRBCs have been given. As a result, those patients that die early receive comparatively less FFP overall. Time-dependent covariate analysis has been used with success to address the issue of survival bias [14]. That type of analysis is only possible if hourly FFP:PRBC ratios are available, which was not the case in our population. Despite data suggesting that the association with survival is stronger than the potential survival bias [16], we attempted to minimize this possibility using a conservative approach and only including patients who survived the initial 24 h. Additional limitations to the present study include the inability to account for other unmeasured possible confounders such as the presence of liver cirrhosis, withdrawal of care, and ratio variation during the resuscitation period. More importantly, the study included a broad and heterogeneous group of patients with bleeding from gastrointestinal as well as gynecological and obstetrical bleeding. It is also important to highlight that cardiac surgery patients, which frequently require massive transfusion, were not included in the study as this is not a group of patients frequently treated at our institution.
Conclusion
In non-trauma patients undergoing a massive transfusion, an increasing plasma to red blood cell ratio was associated with improved survival. Achieving an FFP:PRBC ratio greater than 1:3 significantly improved the probability of survival. Further prospective validation is warranted.
References
Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma Acute Care Surg. 2007;62(1):112–9.
Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma Acute Care Surg. 2007;63(4):805–13.
Teixeira PGR, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma Acute Care Surg. 2009;66(3):693–7.
Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, et al. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang. 2008;95(2):112–9.
Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58.
Duchesne JC, Islam TM, Stuke L, Timmer JR, Barbeau JM, Marr AB, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma Acute Care Surg. 2009;67(1):33–7–discussion37–9.
Gunter OL, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma Acute Care Surg. 2008;65(3):527–34.
Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197(5):565–70 (discussion 570).
Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang Y-Z, Weintraub SE, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma Acute Care Surg. 2008;65(2):272–6 (discussion 276–8).
Davenport R, Curry N, Manson J, De’Ath H, Coates A, Rourke C, et al. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma Acute Care Surg. 2011;70(1):90–5 (discussion 95–6).
Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50(2):493–500.
Lier H, Böttiger BW, Hinkelbein J, Krep H, Bernhard M. Coagulation management in multiple trauma: a systematic review. Intensive Care Med. 2011;37(4):572–82.
Brown LM, Aro SO, Cohen MJ, Trauma Outcomes Group, Holcomb JB, Wade CE, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma Acute Care Surg. 2011;71(2 Suppl 3):S358–63.
Lustenberger T, Frischknecht A, Brüesch M, Keel MJB. Blood component ratios in massively transfused, blunt trauma patients—a time-dependent covariate analysis. J Trauma Acute Care Surg. 2011;71(5):1144–50 (discussion 1150–1).
Kautza BC, Cohen MJ, Cuschieri J, Minei JP, Brackenridge SC, Maier RV, et al. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg. 2012;72(1):106–11.
Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73(2):358–64 (discussion 364).
Guidry C, Dellavope J, Simms E, Heaney JB, Guice J, McSwain N, et al. Impact of inverse ratios on patients with exsanguinating vascular injuries: should more be the new paradigm? J Trauma Acute Care Surg. 2013;74(2):403–9 (discussion 409–10).
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82.
Magnotti LJ, Zarzaur BL, Fischer PE, Williams RF, Myers AL, Bradburn EH, et al. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma Acute Care Surg. 2011;70(1):97–102.
Snyder CW, Weinberg JA, McGwin G, Melton SM, George RL, Reiff DA, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma Acute Care Surg. 2009;66(2):358–62 (discussion 362–4).
Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36.
Cohen MJ, West M. Acute traumatic coagulopathy: from endogenous acute coagulopathy to systemic acquired coagulopathy and back. J Trauma Acute Care Surg. 2011;70(5 Suppl):S47–9.
Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient. J Trauma Acute Care Surg. 2012;72(4):892–8.
Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Goal-directed resuscitation in the prehospital setting. J Trauma Acute Care Surg. 2013;74(5):1207–14.
Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma Acute Care Surg. 2006;60(6 Suppl):S59–69.
Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma Acute Care Surg. 2009;66(4 Suppl):S69–76.
Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ. 2013;185(12):E583–9.
Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2015 [Epub ahead of print].
Mell MW, O’Neil AS, Callcut RA, Acher CW, Hoch JR, Tefera G, et al. Effect of early plasma transfusion on mortality in patients with ruptured abdominal aortic aneurysm. Surgery. 2010;148(5):955–62.
Johansson PI, Stensballe J, Rosenberg I, Hilsløv TL, Jørgensen L, Secher NH. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47(4):593–8.
Kauvar DS, Sarfati MR, Kraiss LW. Intraoperative blood product resuscitation and mortality in ruptured abdominal aortic aneurysm. J Vasc Surg. 2012;55(3):688–92.
Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PGR, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210(6):957–65.
Sambasivan CN, Kunio NR, Nair PV, Zink KA, Michalek JE, Holcomb JB, et al. High ratios of plasma and platelets to packed red blood cells do not affect mortality in nonmassively transfused patients. J Trauma Acute Care Surg. 2011;71(2 Suppl 3):S329–36.
Johnson JL. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145(10):973–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
Pedro G. Teixeira, Kenji Inaba, Efstathios Karamanos, Peter Rhee, Ira Shulman, Dimitra Skiada, Konstantinos Chouliaras, Demetrios Demetriades declare that they have no conflict of interest.
Compliance with ethical requirements
This study received IRB approval and has been performed in accordance with the ethical standards defined in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Teixeira, P.G., Inaba, K., Karamanos, E. et al. The survival impact of plasma to red blood cell ratio in massively transfused non-trauma patients. Eur J Trauma Emerg Surg 43, 393–398 (2017). https://doi.org/10.1007/s00068-016-0674-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-016-0674-5